Abstract
Background
It is important to determine the effect of clinical factors on several domains (symptoms, living status, and quality of life [QOL]) after gastrectomy to establish individualized therapeutic strategies. This study was designed to determine the factors—particularly surgical method—that influence certain domains after gastrectomy for proximal gastric cancer by using the Postgastrectomy Syndrome Assessment Scale-45 (PGSAS-45) questionnaire.
Methods
We conducted a nationwide study of PGSAS-45 questionnaire responses retrieved from 1950 (82.5%) patients from 70 institutions who had undergone gastrectomy for gastric cancer. Of these, 1,538 responses for proximal gastric cancer (1020 total gastrectomies and 518 proximal gastrectomies [PGs]) were examined.
Results
PG significantly and favorably affected four main outcome measures (MOMs): elderly affected 10 MOMs, male sex affected 4 MOMs, longer postoperative period affected 8 MOMs, preservation of the vagus nerve affected 1 MOM, adjuvant chemotherapy affected 1 MOM, clinical stage affected 2 MOMs, and more extensive lymph node dissection affected 2 MOMs. However, the laparoscopic approach had an adverse effect on MOMs and combined resection of other organs had no favorable effect on any MOMs.
Conclusions
This PGSAS NEXT study showed that it is better to perform PG for proximal gastric cancer, even for patients with advanced cancer, to obtain favorable postoperative QOL if oncological safety is guaranteed. Because the MOMs of PGSAS-45 are positively and negatively influenced by various background factors, it also is necessary to provide personalized care for each patient to prevent deterioration and further improve symptoms, living status, and QOL postoperatively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
With the decreased incidence in Helicobacter pylori infection, advances in the effective treatment for H. pylori eradication, and Westernization of diet and lifestyle, the prevalence of gastric cancer changed in Japan.1,2 Specifically, the incidence of proximal gastric cancer, including esophagogastric junction cancer, has increased.3 The goals of gastric cancer treatments include achieving oncological safety and better quality of life (QOL). Therefore, appropriate gastrectomy with lymph node dissection and chemotherapy, if necessary, are warranted to obtain satisfactory outcomes. Moreover, optimal gastrectomy and reconstruction methods are necessary for better QOL. It also is important to ensure a balance between oncological safety and QOL in patients with proximal gastric cancer—the prevalence of which has been rapidly increasing.
Proximal gastrectomy (PG) is listed as one of the function-preserving gastrectomies for proximal gastric cancer in the Japanese Gastric Cancer Treatment Guidelines (ver. 5).4
Recently, some studies have reported the superiority of PG for proximal gastric cancer, including advanced gastric cancer, over total gastrectomy (TG) in terms of postoperative QOL.5,6 However, these two studies did not employ a questionnaire module suitable for postoperative status and did not evaluate a sufficient number of patients and important clinical factors other than surgical method. Therefore, a more reliable questionnaire module is necessary. As the incidence of proximal gastric cancer has been increasing, laparoscopic gastrectomy has been more widely used even for high-risk patients, such as the elderly.
This study, i.e., the PGSAS NEXT study, was conducted to identify various factors that influence the main outcome measures (MOMs) after surgery for proximal gastric cancer in a large population with a greater number of explanatory factors obtained from multiple institutions.
Methods
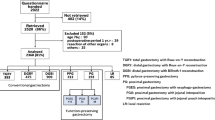
In total, 70 institutions participated in this study from July 2018 through December 2019. The PGSAS-45 questionnaire form was administered to 2,364 patients.7,8 Responses were retrieved from 1950 (82.5%) patients, of which 41 were excluded for the following reasons: < 6 months after chemotherapy in 22 patients, failure of R0 gastrectomy in 6, ineligible surgical procedure in 5, ineligible disease in 2, recurrence of gastric cancer in 2, second gastrectomy in 2, < 6 months after gastrectomy in 1, and withdrawal of consent in 1. Data from 1909 (80.8%) patients were analyzed. A total of 1,685 patients had gastric cancer in the upper-third of their stomachs, and 224 had esophagogastric junction cancer. In this study, we analyzed the outcomes in the upper-third of the stomach of 1538 patients, of which 1020 patients underwent TG and 518 underwent PG (Fig. 1).
Patients’ Eligibility Criteria
The patients’ eligibility criteria were as follows: (1) gastric cancer in the upper-third of the stomach regardless of pathological stage and pathological type; (2) first-time gastrectomy; (3) age >20 years for both sexes; (4) R0 gastrectomy; (5) no recurrence or distant metastasis; (6) >6 months after gastrectomy; (7) regardless of chemotherapy (>6 months after completion of chemotherapy); (8) performance status (PS) 0 or 1 based on the Eastern Cooperative Oncology Group scale; (9) sufficient capacity to understand and respond to the questionnaire; (10) no history of other diseases or operations that might influence the responses to the questionnaire; (11) no organ failure or mental illness; and (12) provision of written, informed consent.
Additionally, patients with dual malignancy or concomitant resection of other organs (with exception of resection or extraction of perigastric organs to accomplish gastrectomy or lymph node dissection and ones equivalent to cholecystectomy) and patients considered inadequate by the attending physician were excluded from this study.
QOL Assessment
The PGSAS-45 questionnaire was developed as a multidimensional QOL questionnaire (QLQ) based on the Short-Form Health Survey (SF-8) and the Gastrointestinal Symptom Rating Scale (GSRS). The PGSAS-45 questionnaire comprises 45 questions: with 8 items from the SF-8, 15 from the GSRS, and 22 clinically important items selected by members of the Japan Postgastrectomy Syndrome Working Party. The PGSAS-45 questionnaire includes 23 items pertaining to postgastrectomy symptoms (items 9–33), including 15 items from the GSRS and 8 additionally selected items. Moreover, 12 questionnaire items relating to dietary intake, work, and level of satisfaction with daily life are included. Twenty-three symptom items were consolidated into seven symptom subscales by factor analysis. The 19 MOMs were refined through consolidation and a selection process and classified into three domains: symptoms, living status, and QOL.7,8
Study Methods
This study employed continuous sampling from a central registration system for participant enrollment. The questionnaire was distributed to all eligible patients. The patients were instructed to return completed forms to the data center. All QOL-related data from the questionnaires were matched with individual patient data collected via case report forms. This study was registered with the University Hospital Medical Information Network’s Clinical Trials Registry (UMIN-CTR; registration number 000032221). This study was approved by the ethics committees of all institutions and conformed to the principles of the Declaration of Helsinki. Written, informed consent was obtained from all included patients.
Statistical Analysis
The patient characteristics and MOMs were compared by performing the t-test and Fisher’s exact test. All MOMs were further analyzed using multiple regression analysis. A total of 10 factors (i.e., type of gastrectomy, age, sex, postoperative period, operative approach, vagus celiac-branch preservation, chemotherapy, cStage, extent of lymph node dissection, and combined resection) were included in the multiple regression analysis as explanatory variables. These factors were selected according to their clinical importance and based on the results of previous PGSAS studies. A p-value of <0.05 was considered to be indicative of statistical significance. In the case of p-values of <0.1 by univariate analysis, Cohen’s d was calculated. In the case of p-values of <0.1 in multiple regression analysis, the standardized coefficient of regression (β), and p-value were calculated and shown in a table. Cohen’s d, β, and R2 measured effect sizes, which were interpreted as small for ≥0.2 to < 0.5, medium for ≥ 0.5 to < 0.8, and large for ≥0.8 in Cohen’s d; small for ≥0.1 to < 0.3, medium for ≥ 0.3 to < 0.5, and large for ≥ 0.5 in β; and small for ≥ 0.02 to < 0.13, medium for ≥ 0.13 to < 0.26, and large for ≥ 0.26 in R2. JMP 12.0.1 (SAS Institute Inc., Cary, NC) was used for all statistical analyses.
Results
Patient Characteristics
The patient characteristics are presented in Table 1. Age was significantly younger (p = 0.005). Open surgery (p < 0.001), tumors extending to the middle-third of the stomach (p < 0.001), D2 or more lymph node dissection (p < 0.001), combined resection (p < 0.001), and division of the vagal nerve celiac branch were significantly more frequent in the TG group than in the PG group (p < 0.001). The extent (p = 0.003) and length of esophageal resection (p < 0.001) were greater and the anastomotic site was observed at a more cranial mediastinum position in the TG group than in the PG group (p < 0.001). Clinical stage was more advanced and postoperative period longer (p < 0.001) in the TG group.
QOL Assessment
MOMs were assessed using univariate analysis (Table 2). In the PG group, the constipation subscale was significantly higher (TG vs. PG, 2.2 vs. 2.3, p = 0.023, Cohen’s d = 0.12) and dumping subscale significantly lower (TG vs. PG, 2.2 vs. 2.1, p = 0.022, Cohen's d = 0.13) than those in the TG group, although the total symptom score did not differ between the two groups among the symptoms domain. The esophageal reflux subscale and diarrhea subscale tended to have lower values in the PG group than in the TG group, although the differences were not significant. In the living status domain, the change in body weight (TG vs. PG, − 14.3% vs. − 12.0%, p < 0.001, Cohen’s d = 0.26) and the necessity for additional meals (TG vs. PG, 2.4 vs. 2.2, p < 0.001, Cohen’s d = 0.20) were significantly better and the ability to work was more favorable (TG vs. PG, 2.4 vs. 2.2, p = 0.001, Cohen’s d = 0.18) in the PG group than in the TG group. In the QOL domain, the dissatisfaction with working (TG vs. PG, 2.1 vs. 1.9, p < 0.001, Cohen’s d = 0.19) and dissatisfaction with daily life (TG vs. PG, 2.3 vs. 2.2, p = 0.020, Cohen’s d = 0.13) subscales were significantly suppressed in the PG group relative to those in the TG group. However, the physical and mental component summaries of the SF-8 did not significantly differ between the two groups.
Multiple Regression Analysis of MOMs
Multivariate analysis was performed to adjust for the influence of confounding factors, including age, sex (male, female), postoperative period, surgical approach (laparoscopic, open), vagal nerve celiac branch (preserved, divided), chemotherapy (yes, no), clinical stage (I/II, III/IV), lymph node dissection (D0/D1, D1+, D2/D2+), and combined resection (yes, no) as explanatory variables (Table 3). Although the effect size of the advantages in the two groups was relatively small, change in body weight (β = 0.199; p < 0.001), necessity for additional meals (β = − 0.102, p = 0.002), ability to work (β = − 0.095; p = 0.003), and dissatisfaction with working (β = − 0.082; p = 0.012) were significantly better in the PG group than in the TG group. Additionally, the constipation subscale (β = 0.061; p = 0.063) tended to be worse in the PG group than in the TG group.
In addition to the type of gastrectomy, several clinical factors, such as age, sex, postoperative period, and operative approach, significantly affected several MOMs of the PGSAS-45 either positively or negatively. For older patients, for example, 75 years or older, all MOMs belonging to the symptoms domain and most of the MOMs related to dissatisfaction with daily life were alleviated; in contrast, several MOMs belonging to the living status domain and physical component summary (PCS) of the SF-8 worsened. Several MOMs were worse for women; in contrast, diarrhea SS was worse for men. Longer postoperative period significantly alleviated several MOMs related to symptoms, living status, and dissatisfaction with daily life. Unexpectedly, laparoscopic approach worsened several MOMs related to the domains of symptoms, living status, and dissatisfaction with daily life.
In contrast to the above clinical factors, the celiac branch of the vagal nerve preservation and clinical factors related to cancer progression (i.e., clinical stage, extent of lymph node dissection, combined resection of other organs, and chemotherapy) had rather small effects on the MOMs of PGSAS-45.
Discussion
In this study, proximal gastrectomy and several factors were identified as independent factors for favorable QOL for proximal gastric cancer by analyzing a sufficient number of patients and factors. In cancer treatments, QOL and oncological outcomes are important. Several studies have reported that poor QOL induced discontinuation of additional treatments after surgical resection and resulted in worse therapeutic outcomes.9,10 Therefore, the assessment and improvement of QOL are imperative to achieve satisfactory oncological outcomes. It is essential to have a common assessment scale to compare and improve the ability to understand the results. Particularly, an assessment for proximal gastric cancer, which has been rapidly increasing, is warranted.
Previously, many questionnaire studies have assessed QOL after gastrectomy, although few studies have used a questionnaire established to assess the surgical method for gastric cancer.11,12,13,14,15,16,17 It appears to be difficult to perform satisfactory assessment for postgastrectomy QOL without a gastrectomy-specific questionnaire. Accordingly, we established the PGSAS-45 to evaluate postgastrectomy daily living and reported a series of studies focusing on QOL assessed by the PGSAS-45 after various types of gastrectomy and reconstruction procedures.7,8 The obtained results of the PGSAS are easily understood by surgeons because of their daily practice, so we assumed that the previous PGSAS-45 questionnaire could be a reliable tool to assess postgastrectomy QOL, including for proximal gastric cancer.
The current study, named the PGSAS NEXT study, revalidated the usefulness of PG over TG in the current clinical setting regarding postoperative QOL in a nationwide, large cohort. Our previous PGSAS study also compared postoperative QOL between TG and PG by using the PGSAS-45, in which multivariate analysis was used to adjust for the influences of confounding factors, such as age, sex, postoperative period, surgical approach, and vagal nerve celiac branch preservation.18 We concluded that PG was better than TG in several MOMs of the PGSAS-45. However, the participants enrolled in the previous PGSAS study were limited to age ≤ 75 years, with pathologically confirmed stage IA or IA, no history of chemotherapy, and no combined resection except ones equivalent to cholecystectomy. One decade has passed since the initial PGSAS study, and the clinical circumstances surrounding proximal gastric cancer have been changing, as shown by the increased prevalence of proximal gastric cancer, wider application of PG, the increased age of gastric cancer patients undergoing surgery, and the increased popularity of the laparoscopic approach. Therefore, we conducted the PGSAS NEXT study to obtain more realistic and reliable findings that apply to the current clinical settings surrounding proximal gastric cancer by eliminating several restrictions with many patients.
The present study showed that PG had some advantages over TG for proximal gastric cancer in terms of postoperative QOL. First, MOMs were assessed using univariate analysis (t-test). We detected significant differences between total gastrectomy and proximal gastrectomy in constipation subscale and dumping subscale in symptoms domain, change in body weight, necessity for additional meals and ability for working in living status domain, dissatisfaction at working, and dissatisfaction for daily subscale in QOL domain in this analysis. However, p-value generally becomes smaller despite no substantial difference according to the greater sample size. Therefore, we employed Cohen’s d to clearly show the effect size.19 Cohen’s d of 5 main outcome measures with significant differences were less than 0.2, which indicates small effect size. Therefore, the clinical impact of these measures may low. We have to interpret these outcomes carefully.
Nest, postoperative QOLs were assessed by the next PGSAS-45 after adjustment by multiple regression analysis at patient enrollment for the influences of many clinical factors that might affect proximal gastric cancer outcomes. The results of this study validated those of the previous study.18 Therefore, PG for proximal gastric cancer should be considered an appropriate option even for advanced proximal gastric cancer if oncological safety can be assured.
Regarding the patients’ background factors, all MOMs related to symptoms and most of the MOMs related to dissatisfaction with daily life were alleviated by PG in the elderly (> 75 years old) patients. In contrast, several MOMs related to living status and the PCS of the SF-8 worsened, women experienced worse QOL in several MOMs, men experienced more diarrhea, and longer postoperative period was associated with significant improvement in several MOMs related to the domains of symptoms, living status, and dissatisfaction with daily life.
Although the patients’ backgrounds influenced postoperative QOL, we cannot actually change those factors. Therefore, it is necessary for clinicians to understand the influence of various kinds of factors on postoperative QOL so that they can keep a close watch on the patients’ postoperative status and suggest appropriate postoperative guidance to patients, as necessary.
Regarding the surgical intervention, unexpectedly, the laparoscopic approach worsened several MOMs related to the domains of symptoms, living status, and dissatisfaction with daily life. Laparoscopic gastrectomy is considered a minimally invasive surgery.20,21 Many surgeons commonly observe that many factors associated with QOL after laparoscopic gastrectomy must be more comfortable or equal to those resulting from open gastrectomy. Although some randomized, controlled trials have compared short- and long-term outcomes between laparoscopic and open groups and reported similar surgical outcomes between the two groups, these studies did not exactly compare long-term QOL after gastrectomies.22,23 Moreover, meticulous and proficient skills are necessary to perform the laparoscopic procedures of TG and PG. It is sometimes difficult to set the delicate dividing line of the stomach and adjust the shape of the remnant stomach particularly in the reconstruction in these laparoscopic surgeries. Even if the incidence of the postoperative complication is equal between the open surgery and the laparoscopic surgery groups, subtle hand manipulation may contribute to the favorable postoperative QOL in these complex procedures. Therefore, it is mandatory to conduct a prospective, randomized, controlled trial to compare QOL after surgery for proximal gastric cancer between open surgery and laparoscopic surgery.
Preservation of the celiac branch of the vagal nerve slightly increased food intake and is important for preserving gastric function, providing good motility of the remnant stomach after PG, and normal function of the intestine after TG. Therefore, it is meaningful to preserve the celiac branch after gastrectomy for proximal gastric cancer if oncologically acceptable. The results also validated those of the previous study conducted by our group.24
Regarding the clinical factors related to cancer progression, the clinical stage, extent of LN dissection, combined resection of other organs, and chemotherapy had rather small effects on the PGSAS-45 MOMs. Because surgical interventions are thought to alleviate the postgastrectomy burden of patients but have not shown positive effects and because the clinical factors related to cancer progression had a rather small effect on postgastrectomy QOL, curability of the cancer and surgical safety should be prioritized.
Recently, some studies have shown either the superiority or equivalence of PG relative to TG for proximal gastric cancer. These outcomes are consistent with those of the present study, and therefore, it may be clinically possible to perform PG for patients with advanced gastric cancer.
There were some limitations to this study. First, this was a retrospective, questionnaire study. Second, more advanced tumors were frequently observed, more likely to receive adjuvant chemotherapy, and likely to have higher risk for disease recurrence in the TG group. Therefore, postoperative QOL could be greatly affected by their chemotherapy, cancer recurrence, etc. Third, although this was a cross-sectional study with a single-observation timepoint during different postoperative times, the effect of these differences was minimized by employing multiple regression analysis. A strength, however, is that there has been no multivariate study that used a gastrectomy-specific questionnaire module to estimate postgastrectomy QOL in a sufficient number of patients. Consequently, this study provides clinically useful recent information.
Conclusions
This PGSAS NEXT study principally showed that it is better to perform PG to obtain favorable QOL after surgery for proximal gastric cancer, even for advanced cancer if oncological safety is guaranteed. Given that the MOMs of PGSAS-45 were positively and negatively influenced by each background factor, it also is necessary to consider the factors relevant to each specific patient to prevent deterioration and further improve symptoms, living status, and QOL after gastrectomy by providing personalized guidance and care.
References
Asaka M, Kobayashi M, Kudo T, et al. Gastric cancer deaths by age group in Japan: Outlook on preventive measures for elderly adults. Cancer Sci. 2020;111:3845–53.
Tokunaga M, Hiki N, Fukunaga T, Ohyama S, Yamaguchi T, Nakajima T. Better 5-year survival rate following curative gastrectomy in overweight patients. Ann Surg Oncol. 2009;16:3245–51.
Li Y, Li J, Li J. Two updates on oesophagogastric junction adenocarcinoma from the fifth WHO classification: alteration of definition and emphasis on HER2 test. Histol Histopathol. 2021;36:339–46.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines. Gastric Cancer. 2018;24:1–21.
Tsumura T, Kuroda S, Nishizaki M, et al. Short-term and long-term comparisons of laparoscopy-assisted proximal gastrectomy with esophagogastrostomy by the double-flap technique and laparoscopy-assisted total gastrectomy for proximal gastric cancer. PLOS ONE. 2020;15:e0242223. https://doi.org/10.1371/journal.pone.0242223.
Asaoka R, Irino T, Makuuchi R, et al. Changes in body weight, skeletal muscle and adipose tissue after gastrectomy: a comparison between proximal gastrectomy and total gastrectomy. ANZ J Surg. 2019;89:79–83.
Nakada K, Ikeda M, Takahashi M, et al. Characteristics and clinical relevance of postgastrectomy syndrome assessment scale (PGSAS)-45: newly developed integrated questionnaires for assessment of living status and quality of life in postgastrectomy patients. Gastric Cancer. 2015;18:147–58.
Nakada K, Takahashi M, Ikeda M, et al. Factors affecting the quality of life of patients after gastrectomy as assessed using the newly developed PGSAS-45 scale: a nationwide multi-institutional study. World J Gastroenterol. 2016;22:8978–90.
Bernhardt D, Adeberg S, Bozorgmehr F, et al. Outcome and prognostic factors in patients with brain metastases from small-cell lung cancer treated with whole brain radiotherapy. J Neurooncol. 2017;134:205–12.
Bozec A, Majoufre C, De Boutray MD, et al. Oral and oropharyngeal cancer surgery with free-flap reconstruction in the elderly: factors associated with long-term quality of life, patient needs and concerns A GETTEC cross-sectional study. Surg Oncol. 2020;35:81–8.
Lee SS, Chung HY, Kwon OK, Yu W. Long-term quality of life after distal subtotal and total gastrectomy: symptom- and behavior-oriented consequences. Ann Surg. 2016;263:738–44.
Tanaka C, Kanda M, Murotani K, et al. Long-term quality of life and nutrition status of the aboral pouch reconstruction after total gastrectomy for gastric cancer: a prospective multicenter observational study (CCOG1505). Gastric Cancer. 2019;22:607–16.
Wei M, Wang N, Yin Z, et al. Short-term and quality of life outcomes of patients using linear or circular stapling in esophagojejunostomy after laparoscopic total gastrectomy. J Gastrointest Surg. 2021;25:1667–76. https://doi.org/10.1007/s11605-020-04806-0.
Youn SI, Son SY, Lee K, et al. Quality of life after laparoscopic sentinel node navigation surgery in early gastric cancer: a single-center cohort study. Gastric Cancer. 2021;24:744–51. https://doi.org/10.1007/s10120-020-01145-6.
Ronellenfitsch U, Najmeh S, Andalib A, et al. Functional outcomes and quality of life after proximal gastrectomy with esophagogastrostomy using a narrow gastric conduit. Ann Surg Oncol. 2015;22:772–9.
Nakamura M, Nakamori M, Ojima T, et al. Randomized clinical trial comparing long-term quality of life for Billroth I versus Roux-en-Y reconstruction after distal gastrectomy for gastric cancer. Br J Surg. 2016;103:337–47.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70.
Takiguchi N, Takahashi M, Ikeda M, et al. Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): a nationwide multi-institutional study. Gastric Cancer. 2015;18:407–16.
Cumming Geoff. The new statistics: why and how. Sychological Sci. 2014;25:7–29.
Kunisaki C, Makino H, Kimura J, et al. Application of reduced-port laparoscopic total gastrectomy in gastric cancer preserving the pancreas and spleen. Gastric Cancer. 2015;18:868–75.
Nakauchi M, Vos E, Janjigian YY, et al. Comparison of long- and short-term outcomes in 845 open and minimally invasive gastrectomy’s for gastric cancer in the United States. Ann Surg Oncol. 2021;28:3532–44. https://doi.org/10.1245/s10434-021-09798-y.
Katai H, Mizusawa J, Katayama H, et al. Single-arm confirmatory trial of laparoscopy-assisted total or proximal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan Clinical Oncology Group study JCOG1401. Gastric Cancer. 2019;22:999–1008.
Katai H, Mizusawa J, Katayama H, et al. Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5:142–51.
Kinami S, Takahashi M, Urushihara T, Nakada K. Background factors influencing postgastrectomy syndromes after various types of gastrectomy. World J Clin Cases. 2018;6:1111–20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kunisaki, C., Yoshida, K., Yoshida, M. et al. Effects of Proximal Gastrectomy and Various Clinical Factors on Postoperative Quality of Life for Upper-third Gastric Cancer Assessed using the Postgastrectomy Syndrome Assessment Scale-45 (PGSAS-45): A PGSAS NEXT Study. Ann Surg Oncol 29, 3899–3908 (2022). https://doi.org/10.1245/s10434-021-11136-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-11136-1