Abstract
Background
Rectum-preservation for locally advanced rectal cancer has been proposed as an alternative to total mesorectal excision (TME) in patients with major (mCR) or complete clinical response (cCR) after neoadjuvant therapy. The purpose of this study was to report on the short-term outcomes of ReSARCh (Rectal Sparing Approach after preoperative Radio- and/or Chemotherapy) trial, which is a prospective, multicenter, observational trial that investigated the role of transanal local excision (LE) and watch-and-wait (WW) as integrated approaches after neoadjuvant therapy for rectal cancer.
Methods
Patients with mid-low rectal cancer who achieved mCR or cCR after neoadjuvant therapy and were fit for major surgery were enrolled. Clinical response was evaluated at 8 and 12 weeks after completion of chemoradiotherapy. Treatment approach, incidence, and reasons for subsequent TME were recorded.
Results
From 2016 to 2019, 160 patients were enrolled; mCR or cCR at 12 weeks was achieved in 64 and 96 of patients, respectively. Overall, 98 patients were managed with LE and 62 with WW. In the LE group, Clavien–Dindo 3+ complications occurred in three patients. The rate of cCR increased from 8- to 12-week restaging. Thirty-three (94.3%) of 35 patients with cCR had ypT0–1 tumor. At a median 24 months follow-up, a tumor regrowth was found in 15 (24.2%) patients undergoing WW.
Conclusions
LE for patients achieving cCR or mCR is safe. A 12-week interval from chemoradiotherapy completion to LE is correlated with an increased cCR rate. The risk of ypT > is reduced when LE is performed after cCR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Neoadjuvant therapy followed by total mesorectal excision (TME) is the standard of care for mid-low locally advanced rectal cancer. This approach results in lower rates of local recurrence and increased survival rates compared with TME surgery alone or TME surgery followed by adjuvant chemoradiotherapy (CRT).1,2,3 Neoadjuvant CRT in locally advanced rectal cancer also has the potential to downstage the primary tumor, sometimes inducing a pathological complete response (pCR), which occurs in 10–30% of patients.4,5,6 These patients show better oncological outcomes than patients with residual tumor.7 A recent meta-analysis also has shown that the rate of pCR can be increased prolonging the interval between the completion of preoperative CRT and the surgical intervention.8
On the other hand, TME is associated with relatively high rates of morbidity (26–45%) and mortality (2–5%) and usually requires the creation of a temporary or permanent stoma, with a negative impact on patients’ quality of life (QoL).9,10,11,12 Therefore, growing interest has developed for rectum-sparing strategies, based on the hypothesis that patients with major (mCR) or complete (cCR) clinical response to neoadjuvant therapy may avoid TME surgery. In past years, three prospective phase 2 trials and one phase 3 trial have shown encouraging outcomes of transanal local excision (LE) in terms of local failure, disease-free survival (DFS), and overall survival (OS).13,14,15,16 Alternatively, the “Watch and Wait” approach (WW), as proposed by Habr-Gama et al. has been shown to be comparable to the TME in terms of long-term oncological outcomes in patients who achieve a cCR.17,18,19
While the findings of these studies are encouraging, the evidence on the efficacy of these approaches is still limited. Most of the studies are small in size, have a single-center design, and differ in patients’ selection and definition of clinical response. Moreover, no studies include both rectal sparing strategies: LE or WW. Because LE is usually performed for patients both with mCR and cCR, while WW is only indicated for patients with cCR, the two approaches are potentially complementary.
The purpose of the present study was to analyze the short-term outcomes (clinical response to neoadjuvant chemoradiotherapy, correspondence between clinical and pathological response, postoperative morbidity, and rate of completion TME surgery) of the ReSARCh (Rectal Sparing Approach after preoperative Radio- and/or Chemo-therapy) trial which was designed to investigate the role of both transanal LE and WW approaches in patients who underwent neoadjuvant therapy for rectal cancer.20
Materials and Methods
Study Design
The ReSARCh trial (Protocol registered as NCT02710812, https://clinicaltrials.gov/ct2/show/NCT02710812) is a prospective, multicenter observational study with the principal goal to assess the rate of rectal-preservation at 2 years. Secondary end-points include both long-term (overall survival, disease-free survival, local recurrence-free survival, the rate of patients without stoma, bowel function, and quality of life) and short-term outcomes. The study protocol was described elsewhere.20 It was approved by the Institutional Review Board of Padova Hospital. Moreover, each participating institution obtained a specific approval.
Inclusion and Exclusion Criteria
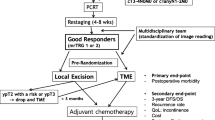
The trial enrolled patients with histologically confirmed adenocarcinoma of the rectum up to 12 cm from the anal verge, who received any neoadjuvant therapy and were considered fit for radical TME surgery. Patients who showed a mCR or cCR at restaging were eligible for the trial, whereas those achieving poor clinical response were excluded, because it is widely accepted that organ-sparing approaches are unsafe for patients who are poor responders after neoadjuvant treatments. Before signing the informed consent, patients were fully informed that any rectum-sparing approach (WW and transanal LE) was not the standard of care.21 Patients younger than age 18 years and those considered unfit for TME surgery were excluded. For the purpose of the present study, patients with a follow-up less than 1 year were excluded. A CONSORT Flow Diagram of the present trial is provided (Fig. 1).
Neoadjuvant Treatments Details
Neoadjuvant treatments were classified in three groups: chemotherapy only, radiotherapy only, and chemoradiotherapy. The latter included patients who underwent total neoadjuvant therapy. Most of the enrolled patients underwent a fluoropyrimidine-based chemotherapy (capecitabine or 5-FU bolus or continuous infusion) concomitantly with external beam radiotherapy at a total dose of 50.4 Gy, given in 28 fractions of 1.8-Gy each.
Clinical Evaluation and Staging
Clinical staging and pathological TNM staging were reported according to the American Joint Committee on Cancer 8th Edition.22 Clinical staging before neoadjuvant treatment included clinical history and physical examination, routine laboratory tests, digital rectal examination (DRE), proctoscopy and complete colonoscopy, serum carcinoembryonic (CEA) level, chest and abdomen computed tomography (CT) scan, and pelvic magnetic resonance imaging (MRI). With the exclusion of colonoscopy, the same tests were used to restage the patients 7–8 weeks after the completion of neoadjuvant treatment (first restaging). Patients showing poor clinical response underwent immediate radical surgery, whereas those with a mCR o cCR were recommended to undergo a further proctoscopy at least 11–12 weeks after the completion of neoadjuvant treatment (second restaging). LE was recommended for patients with a mCR, whereas in those with a cCR, both LE and WW were allowed at surgeon’ and patients’ discretion.
After LE, a completion TME surgery was recommended in patients showing at least one of the following histological features: ypT ≥ 2, high grade ypT1, positive margins, lymphovascular or perineural invasion, and TRG ≥ 3 according to the Mandard’s classification.20,23
Surgical Treatment and Histopathology
LE was performed either with the traditional transanal approach (TAE) or using transanal minimally invasive approaches as transanal endoscopic microsurgery (TEM) or transanal endoscopic operations (TEO) or transanal minimally invasive surgery (TAMIS). A gross margin of at least 0.5 cm was recommended and a full-thickness excision, including mucosa, submucosa, muscularis propria, and perirectal fat was obtained in all patients. Surgical specimens were oriented using a cardboard.
Definition of Response
As previously reported, cCR was defined as the absence of palpable mass at DRE, no mucosal abnormalities at endoscopy and no metastatic nodes at MRI.20 mCR was defined as the absence of palpable mass at DRE, the presence of small mucosal irregularity or superficial ulcer no more than 2 cm in diameter at endoscopy, and no metastatic nodes at MRI.
pCR was defined as the absence of any viable tumor cell in the specimen (ypT0NX) following LE, and an ypT0N0 following TME. Metastatic nodes at MRI were defined as mesorectal nodes with a diameter > 0.5 cm along the short axis.
Follow-Up
Follow-up strategies following LE or WW have been previously described.20 Recurrence was defined as local if confined to the true pelvis or distant if located outside. A rectal regrowth of the tumor following WW was defined as “local regrowth.” The diagnosis of recurrence was determined on the basis of clinical examination, radiological findings, or biopsy.
Statistical Analysis
Descriptive statistics were reported as median with interquartile range (IQR) for continuous variables and absolute numbers (percentages) for categorical variables.
Variables distribution in the two intervention groups was evaluated by using Wilcoxon test for continuous variables and a simplified Monte Carlo significance test procedure (2000 replicates) for categorical variables.24
The association between the clinical response at second restaging and the pathological response was evaluated using a logistic regression approach. Results were reported as odds ratio (OR), 95% confidence interval (CI), and p value. The performance of the model was evaluated by assessing discrimination with the Harrell’s C-index. The estimates have been corrected for overoptimism via bootstrap resampling with 10,000 replicates. Sensitivity, specificity, positive predictive value, and negative predictive value were calculated to test diagnostic accuracy. The analyses were performed by using R-software (4.0.2) with the package rms.25,26
Results
Clinicopathological Characteristics of the Study Group
From April 2016 to December 2019, a total of 160 patients from 17 Italian institutions were enrolled. Clinical baseline characteristics, treatments, and postneoadjuvant restaging characteristics are summarized in Table 1. The majority of patients was Caucasian. The median (IQR) age of patients was 65 (60–72) years, and 104 (65%) of them were males. Only 22 (17%) patients were classified as ASA III–IV, and 115 (72.3%) had clinical T3 tumor at baseline staging. The median (IQR) distance of the tumor from the anal verge was 5 (3–7) cm.
LE was performed in 98 (61.25%) patients (LE group), whereas 62 (38.75%) were observed (WW group). The patients’ characteristics were similar between the two groups, with the exclusion of the ycT stage, which was significantly lower in patients who underwent WW than in those who underwent LE (p = 0.0453; Table 1).
Clinical Response
Clinical response at restaging, after neoadjuvant therapy, is summarized in Table 2. cCR was found in 61 of 62 (98.2%) patients of the WW group and in 35 of 98 (35.7%) patients of the LE group (p < 0.001). There was a significant downstaging, with cCR rate increasing from 40% at the first restaging to 60% at the second restaging (Fig. 2).
Local Excision
The median (IQR) interval time between the completion of neoadjuvant therapy and LE was 14.7 (range 12.5–17.2) weeks. The median (IQR) length of hospital stay was 3 (range 2–4) days. The local approach was the following: TAE (n = 36, 36.7%), TAMIS (n = 13, 13.3%), TEO (n = 12, 12.2%), and TEM (n = 37, 37.8%). Postoperative complications were found in 20 patients. According to the Clavien–Dindo score, only three (3.1%) of them were classified as grade III–IV: one patient showed postoperative rectal bleeding and required blood transfusions and endoscopic hemostatic treatment, and two patients showed a rectovaginal fistula and a pelvic sepsis from rectal suture dehiscence, respectively.27 Both required a temporary stoma, which at the last follow-up has been reversed in only one patient.
At histopathology, the following ypT stage were found: ypT0 (n = 57, 58.2%), ypT1 (n = 22, 22.4%), ypT2 (n = 17, 17.3%), and ypT3 (n = 2, 2%). Twenty-six patients showed the following unfavorable histopathologic features: positive margin (n = 1), lymphovascular invasion (n = 2), perineural invasion (n = 1), TRG > 2 (n = 21), ypT2 (n = 17), ypT3 (n = 2).
The relationship between clinical and pathological response in patients who underwent LE is summarized in Table 3. Interestingly, an ypT0–1 stage was found in 33 of 35 (94.3%) patients who had cCR and in 46 of 63 (73%) patients who had mCR. The accuracy in predicting an ypT0–1 was poor (specificity 42%). However, in patients with cCR, the negative predictive value (probability to exclude an ypT > 1 tumor) was 94% (95% confidence interval [CI] 81–99%) (Table 4).
At logistic regression analysis, patients with cCR at second restaging were found to have a significant reduced probability to have ypT > 1 residual tumor than those who had mCR (odds ratio [OR] 0.16, 95% CI 0.04–0.66, p = 0.02). The Concordance Index of Harrell was 0.66 (95% CI 0.57–0.75).
Reoperations
Overall the median (IQR) follow-up time was 24 (17–30) months. Among 26 (26.5%) patients requiring a completion radical surgery after LE, only 11 agreed to undergo TME surgery. At definitive histopathology, 10 of them were free from residual cancer and one had positive mesorectal lymph nodes (Table 5). As previously reported, two patients had a stoma construction due to surgical complications. Fifteen patients refused the surgical treatment for unknown reasons and despite being adequately informed on the risks of treatment refusal. In the WW group, 15 (24.2%) patients had a regrowth.
Discussion
The major findings of the present observational, multicenter trial were: the low incidence of postoperative severe complications (3.1%) after LE; the significant increase of cCR rate when the restaging was delayed to 12 weeks; the high rate (94.3%) of ypT0–1 stage in patients who underwent LE after a cCR; and finally, the absence of residual cancer in the majority of patients who underwent a completion TME surgery after LE.
The rate of major complication after LE is consistent with the available literature, which shows rates of severe complication ranging between 4.8 and 10.6%.14,16 The low rate of major complications in the present study might be related to the adoption of minimally invasive techniques, such as TEM, TEO, and TAMIS, as well as the increased expertise of the participating centers and surgeons. Minimally invasive techniques are exposed to a learning curve effect, which is well visible even when we compare our first results with actual ones.14
The increasing rate of cCR (Fig. 2) from the first to the second restaging is in line with the literature and strongly suggests that, in patients showing a major or complete response at the first-restaging, a 12-week interval between the completion of neoadjuvant therapy and the LE seems preferrable.28,29
In the present study, ypT1 tumors with favorable histopathologic features were considered similar to ypT0 tumors and amenable of local excision. This assumption is based on the findings of the study by D’Alimonte at al., showing less than 5% of local recurrence and more than 95% of rectum preservation in ypT0–T1 patients.14,30 In their systematic review, Hallam et al. found that local recurrence in ypT1 patients treated with LE was approximately 12%.31 However, this review included any ypT1 tumors. Our hypothesis is that, similar to native pT1 tumors, ypT1 will likely be split into two categories with low metastatic risk manageable with LE and high metastatic risk requiring completion TME surgery. The long-term results of the ReSARCh trial will contribute to answer this question.
Completion TME surgery was required in 17–46% of cases managed with rectal-sparing approaches, depending on patients characteristics and study protocols.15,32 In the present study, 26 patients (26.5%) showed unfavorable histological features after LE, requiring a completion TME. The main reason for performing TME in these patients is to avoid local mesorectal recurrence. In a previous study, analyzing the relationship between ypT stage and node positivity after TME, we found that the rate of positive nodes was 17% for ypT2, and more than 30% for ypT3–4 tumors.33 Similar findings have been reported in other studies.34 Moreover, high rates of local recurrence following LE have been found in patients with ypT2, ranging from 6 to 20% depending on whether tumor was low- or high-risk.35 Based on these findings, we recommended TME for ypT ≥ 2.
Noteworthy, among patients who agreed to undergo completion TME surgery, only 1 of 11 patients showed residual cancer (lymph node metastasis) at final histopathology. The clinical implication is relevant as four patients underwent an abdominoperineal resection despite the absence of residual cancer at final histopathology.
Different from previous studies, we included patients who underwent either LE or WW. LE was found to be a potentially definitive treatment in 33 (94.3%) patients with cCR and in 46 (73.0%) patients with a mCR. From this preliminary analysis, compared with WW, LE increases the spectrum of organ preservation by including patients with cCR (and eventual residual pT1 cancer) and mCR not eligible for WW. In the subgroup of patients with cCR who underwent LE, our definition of cCR was able to exclude (negative predictive value) an ypT > 1 cancer in more than 90% of patients (Table 4). Moreover, at logistic regression analysis, a cCR at second restaging was associated with a reduced probability to have ypT > 1 residual tumor compared with a mCR.
In this clinical scenario, both approaches show pros and cons. While the WW approach allows to avoid surgery in the first place, it is associated with approximately 20% risk of regrowth. The LE approach extends the rate of organ preservation by including patients with mCR but is not free from morbidity and exposes patients (almost exclusively mCR patients) to two surgeries. Concerning the relationship between clinical and pathologic response, the findings of the present study overlap those reported by Smith et al.36 who found that most (75%) patients with pCR were near complete clinical responders. As the WW approach is indicated only in patients with cCR, it will miss the majority of patients with pCR.
At the moment, the definition of cCR differs between study groups.35,37,38,39 Our findings ulteriorly confirm the difficulty to select patients for a rectal-sparing approach and the need of more reliable strategies to better predict the pCR.
Most authors prefer the WW over LE approach, because the latter bears a high rate of surgical complications, worse anorectal function, difficulty in case of completion, or salvage TME.40,41 The preliminary results of the ReSARCh trial show that the advantage of performing a LE relies on the possibility to offer an adequate treatment in approximately 94% (ypT0–1) of patients with a cCR and in approximately 75% of patients with a mCR. Given these observations, it seems justified to consider both LE and WW as feasible and complementary organ-sparing approaches. Unfortunately, at the present moment, no clinical exams are able to predict accurately the pCR and the risk of residual cancer in the WW approach.
The strengths of this trial are the multicenter, prospective design and a large sample size, including both LE and WW. Furthermore, the inclusion criterion of “fit for TME surgery” at the time of enrollment prevented a selection bias. In fact, in previous studies LE often was recommended in older patients with comorbidities.40
The main limitations of the present study are the short follow-up in order to make assumptions on long-term outcomes (local regrowth rate, local recurrence rate, disease free survival, stoma-free survival, rectum preservation rate, overall survival, reinterventions rate due to local recurrence or local regrowth), the absence of randomization, the presence of different neoadjuvant regimens, the absence of a functional, and quality of life analyses, which will be reported later.
Conclusions
The LE after neoadjuvant therapy for rectal cancer was found to be safe. An interval of 12 weeks instead of 8 weeks from the completion of chemoradiotherapy to LE increases the rate of complete clinical responders. The risk to find an advanced ypT stage is reduced when LE is performed in patients with a complete clinical response; however, the majority of patients with pathological complete response show a major clinical response. Further investigations are needed to find better criteria to select patients with pCR to be managed with a tailored approach.
References
Bosset J-F, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006. https://doi.org/10.1056/NEJMoa060829.
Kapiteijn E, Marijnen CAM, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001. https://doi.org/10.1056/NEJMoa010580.
Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004. https://doi.org/10.1056/NEJMoa040694.
Smith FM, Reynolds JV, Miller N, Stephens RB, Kennedy MJ. Pathological and molecular predictors of the response of rectal cancer to neoadjuvant radiochemotherapy. Eur J Surg Oncol. 2006. https://doi.org/10.1016/j.ejso.2005.09.010.
Beddy D, Hyland JMP, Winter DC, et al. A simplified tumor regression grade correlates with survival in locally advanced rectal carcinoma treated with neoadjuvant chemoradiotherapy. Ann Surg Oncol. 2008. https://doi.org/10.1245/s10434-008-0149-y.
Mignanelli ED, Campos-Lobato LF, Stocchi L, et al. Downstaging after chemoradiotherapy for locally advanced rectal cancer: Is there more (tumor) than meets the eye? Dis Colon Rectum. 2010. https://doi.org/10.1007/DCR.0b013e3181bcd3cc.
Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010. https://doi.org/10.1016/S1470-2045(10)70172-8.
Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer: a meta-analysis of published studies. Ann Surg. 2016. https://doi.org/10.1097/SLA.0000000000000368.
Luna-Pérez P, Rodríguez-Ramírez S, Vega J, Sandoval E, Labastida S. Morbidity and mortality following abdominoperineal resection for low rectal adenocarcinoma. Rev Investig Clin. 2001;53(5):388–95.
Petrelli NJ, Nagel S, Rodriguez-Bigas M, Piedmonte M, Herrera L. Morbidity and mortality following abdominoperineal resection for rectal adenocarcinoma. Am Surg. 1993;59(7):400–4.
Lezoche E, Baldarelli M, Lezoche G, Paganini AM, Gesuita R, Guerrieri M. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg. 2012. https://doi.org/10.1002/bjs.8821.
Pucciarelli S, Giandomenico F, De Paoli A, et al. Bowel function and quality of life after local excision or total mesorectal excision following chemoradiotherapy for rectal cancer. Br J Surg. 2016. https://doi.org/10.1002/bjs.10318.
Bujko K, Richter P, Smith FM, et al. Preoperative radiotherapy and local excision of rectal cancer with immediate radical re-operation for poor responders: a prospective multicentre study. Radiother Oncol. 2013. https://doi.org/10.1016/j.radonc.2012.12.005.
Pucciarelli S, De Paoli A, Guerrieri M, et al. Local excision after preoperative chemoradiotherapy for rectal cancer: results of a multicenter phase II clinical trial. Dis Colon Rectum. 2013. https://doi.org/10.1097/DCR.0b013e3182a2303e.
Rullier E, Rouanet P, Tuech J-J, et al. Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open-label, multicentre, phase 3 trial. Lancet. 2017. https://doi.org/10.1016/S0140-6736(17)31056-5.
Verseveld M, de Graaf EJR, Verhoef C, et al. Chemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery (CARTS study). Br J Surg. 2015. https://doi.org/10.1002/bjs.9809.
Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004. https://doi.org/10.1097/01.sla.0000141194.27992.32.
Habr-Gama A, Santinho B, de Souza PM, Ribeiro U, et al. Low rectal cancer: impact of radiation and chemotherapy on surgical treatment. Dis Colon Rectum. 1998. https://doi.org/10.1007/BF02239429.
Habrgama A, Perez R, Proscurshim I, et al. Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg. 2006. https://doi.org/10.1016/j.gassur.2006.09.005.
Barina A, De Paoli A, Delrio P, et al. Rectal sparing approach after preoperative radio- and/or chemotherapy (RESARCH) in patients with rectal cancer: a multicentre observational study. Tech Coloproctol. 2017. https://doi.org/10.1007/s10151-017-1665-1.
Benson AB, Venook AP, Cederquist L, et al. Colon cancer, Version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2017. https://doi.org/10.6004/jnccn.2017.0036.
Amin MB, Gress DM. AJCC cancer staging manual. 8th edn. New York: Springer; 2017.
Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994. https://doi.org/10.1002/1097-0142(19940601)73:11%3c2680::AID-CNCR2820731105%3e3.0.CO;2-C.
Hope ACA. A simplified Monte Carlo significance test procedure. J R Stat Soc Ser B. 1968. https://doi.org/10.1111/j.2517-6161.1968.tb00759.x.
R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. 2020. Available at: https://www.R-project.org/. Accessed 11 May 2021.
Jr FEH. rms: Regression modeling strategies. 2020. Available at: https://CRAN.R-project.org/package=rms. Accessed 11 May 2021.
Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
Hupkens BJP, Maas M, Martens MH, et al. Organ preservation in rectal cancer after chemoradiation: should we extend the observation period in patients with a clinical near-complete response? Ann Surg Oncol. 2018. https://doi.org/10.1245/s10434-017-6213-8.
Sloothaak DM, Geijsen DE, van Leersum NJ, et al. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg. 2013. https://doi.org/10.1002/bjs.9112.
D’Alimonte L, Bao QR, Spolverato G, et al. Long-term outcomes of local excision following neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Ann Surg Oncol. 2020. https://doi.org/10.1245/s10434-020-09243-6.
Hallam S, Messenger DE, Thomas MG. A systematic review of local excision after neoadjuvant therapy for rectal cancer: Are ypt0 tumors the limit? Dis Colon Rectum. 2016. https://doi.org/10.1097/DCR.0000000000000613.
Stijns RCH, de Graaf EJR, Punt CJA, et al. Long-term oncological and functional outcomes of chemoradiotherapy followed by organ-sparing transanal endoscopic microsurgery for distal rectal cancer: the CARTS study. JAMA Surg. 2019. https://doi.org/10.1001/jamasurg.2018.3752.
Pucciarelli S, Capirci C, Emanuele U, et al. Relationship between pathologic T-stage and nodal metastasis after preoperative chemoradiotherapy for locally advanced rectal cancer. Ann Surg Oncol. 2005. https://doi.org/10.1245/ASO.2005.03.044.
Read TE, Andujar JE, Caushaj PF, et al. Neoadjuvant therapy for rectal cancer: histologic response of the primary tumor predicts nodal status. Dis Colon Rectum. 2004. https://doi.org/10.1007/s10350-004-0535-x.
Lezoche G, Baldarelli M, Guerrieri M, et al. A prospective randomized study with a 5-year minimum follow-up evaluation of transanal endoscopic microsurgery versus laparoscopic total mesorectal excision after neoadjuvant therapy. Surg Endosc. 2008. https://doi.org/10.1007/s00464-007-9596-y.
Smith FM, Wiland H, Mace A, Pai RK, Kalady MF. Clinical criteria underestimate complete pathological response in rectal cancer treated with neoadjuvant chemoradiotherapy. Dis Colon Rectum. 2014. https://doi.org/10.1097/DCR.0b013e3182a84eba.
Benzoni E, Cerato F, Cojutti A, et al. The predictive value of clinical evaluation of response to neoadjuvant chemoradiation therapy for rectal cancer. Tumori. 2005. https://doi.org/10.1177/030089160509100504.
Zmora O, Dasilva GM, Gurland B, et al. Does rectal wall tumor eradication with preoperative chemoradiation permit a change in the operative strategy? Dis Colon Rectum. 2004. https://doi.org/10.1007/s10350-004-0673-1.
Maas M, Lambregts DMJ, Nelemans PJ, et al. Assessment of clinical complete response after chemoradiation for rectal cancer with digital rectal examination, endoscopy, and MRI: selection for organ-saving treatment. Ann Surg Oncol. 2015. https://doi.org/10.1245/s10434-015-4687-9.
Habr-Gama A, Lynn PB, Jorge JMN, et al. Impact of organ-preserving strategies on anorectal function in patients with distal rectal cancer following neoadjuvant chemoradiation. Dis Colon Rectum. 2016. https://doi.org/10.1097/DCR.0000000000000543.
Morino M, Allaix ME, Arolfo S, Arezzo A. Previous transanal endoscopic microsurgery for rectal cancer represents a risk factor for an increased abdominoperineal resection rate. Surg Endosc. 2013. https://doi.org/10.1007/s00464-013-2911-x.
Acknowledgments
Mariasole Bigon helped as data manager. Valentina Chiminazzo performed the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marchegiani, F., Palatucci, V., Capelli, G. et al. Rectal Sparing Approach After Neoadjuvant Therapy in Patients with Rectal Cancer: The Preliminary Results of the ReSARCh Trial. Ann Surg Oncol 29, 1880–1889 (2022). https://doi.org/10.1245/s10434-021-11121-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-11121-8