Abstract
Background
The expression of solute carrier (SLC) 7 family genes is reportedly associated with several malignancies. Here, we focused on SLC7A9 and investigated its expression, function, and clinical significance in esophageal squamous cell carcinoma (ESCC).
Methods
SLC7A9 transcription levels were evaluated in 13 ESCC cell lines, and polymerase chain reaction (PCR) array analysis was conducted to detect coordinately expressed genes with SLC7A9. SLC7A9 contributions to proliferation, invasion, and migration were evaluated in ESCC cells subjected to siRNA-mediated gene knockdown and pCMV6-entry plasmid-mediated overexpression. SLC7A9 expression was detected in 189 ESCC tissues by quantitative reverse-transcription (qRT)-PCR and correlated with clinicopathological parameters.
Results
The expression levels of SLC7A9 varied widely in ESCC cell lines and correlated with FGFBP1 expression. Knockdown of SLC7A9 significantly suppressed the proliferation, invasion, and migration of the ESCC cell lines. Moreover, overexpression of SLC7A9 enhanced cell proliferation and migration. In analyses of clinical specimens, SLC7A9 mRNA was overexpressed in the ESCC tissues compared with the adjacent normal esophageal tissues. High mRNA expression was significantly associated with high levels of squamous cell carcinoma-related antigen and carcinoembryonic antigen, advanced disease stage, and lymph node metastasis. High SLC7A9 expression was also significantly associated with poor disease-specific and disease-free survival, and lymph node recurrence after radical surgery, but not with the other recurrence patterns. On multivariate analysis, high SLC7A9 expression was an independent predictor of lymph node recurrence.
Conclusions
SLC7A9 influences the malignant behavior of ESCC cells. Tumor SLC7A9 expression may serve as a novel biomarker for predicting lymph node metastasis and recurrence in ESCC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Esophageal cancer is the eighth most common cancer and the sixth leading cause of cancer-related mortality globally.1 Esophageal cancer is histopathologically divided into two main subtypes: adenocarcinoma and esophageal squamous cell carcinoma (ESCC), and the latter is the more common subtype in Asia and developing countries compared with the Western countries.2 Despite current improvements in multimodal therapeutic strategies for ESCC, the disease is generally associated with a poor outcome due to its high metastatic potential. In particular, lymphatic metastasis is observed in a relatively early phase of tumor progression because of its well-developed connection to the lymphatic system. The lymph node (LN) metastasis rate is reported as 10–20% even in pT1 ESCC.3,4,5 In addition, the 5-year overall survival rate is < 40%, even for ESCC patients undergoing radical treatment,6 and LN recurrence is the main cause of treatment failure after radical surgery.7 Although there is no available biomarker specific for metastatic patterns in ESCC, it can be helpful for better clinical outcomes to precisely predict the risk of LN metastasis or recurrence after radical treatment.
Recently, the solute carrier (SLC) 7 gene family has been focused on as oncogenes in a variety of cancers. The SLC7 gene family, comprising 14 genes, encodes amino acid transporters, which are essential for the maintenance of amino acid nutrition and survival of tumor cells.8 The expression pattern of SLC7 family genes depends on the type of cancer, and several genes of this family have been reported to function as oncogenes in a variety of cancers.9 For example, SLC7A3 is overexpressed and associated with a poor prognosis in papillary thyroid cancer, SLC7A5 in gastric, pancreatic, and prostatic cancers,10,11,12 and SLC7A11 in gliomas.13,14 However, the expression and oncogenic function of SLC7 family genes in ESCC are unknown.
In the present study, we aimed to identify a novel oncogene for ESCC among the SLC7 gene family. By analyzing a large set of ESCC patient data from The Cancer Genome Atlas (TCGA) database,15 we selected SLC7A9 as a candidate gene and investigated its expression, function, and clinical significance in ESCC. We evaluated the biological function of SLC7A9 in a panel of human ESCC cell lines subjected to small interfering RNA (siRNA)-mediated SLC7A9 knockdown and pCMV6-entry plasmid-mediated SLC7A9 overexpression. In addition, we measured SLC7A9 expression level in clinical samples and its relationship to clinicopathological characteristics.
Methods
Ethics
Our study conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki–Ethical Principles for Medical Research Involving Human Subjects and has been approved by the institutional review board (approval no. 2014–0044) at Nagoya University, Japan. We obtained written informed consent for the use of clinical samples and data from all patients, as required by the institutional review board.
TCGA Dataset
To select a candidate gene from the SLC7 family of genes, we analyzed a dataset of 96 ESCC patients from the TCGA database.15 We analyzed the mRNA expression levels of accessible 13 SLC7 family genes, except SLC7A12, in ESCC and normal esophageal tissues.
Cell Lines
A panel of 13 human ESCC cell lines was obtained as follows: NUEC2 and WSSC were established at Nagoya University.16 TE1, TE2, TE3, TT, and TTn were obtained from the American Type Culture Collection (Manassas, VA, USA); KYSE1170, KYSE1260, KYSE1440, KYSE510, KYSE590, and KYSE890 were obtained from the Japanese Collection of Research Bio Resources Cell Bank (Osaka, Japan). All the cell lines were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) with 10% fetal bovine serum at 37 °C and 5% CO2.
Patients and Clinical Samples
For mRNA expression analysis by quantitative reverse-transcription polymerase chain reaction (qRT-PCR), esophageal tissues (primary ESCC and adjacent noncancerous mucosa) were collected from 189 patients who underwent radical esophagectomy at Nagoya University Hospital between 2001 and 2016. Tissue samples were directly frozen and stored at −80 °C upon resection. Specimens were confirmed to be ESCC by histological classification based on the eighth edition of the Union for International Cancer Control (UICC) staging system for esophageal cancer.17 Of the 118 patients with stage II or III ESCC, 98 underwent fluorouracil combined with platinum-based neoadjuvant chemotherapy (NAC), according to standard recommendations since 2006. This cohort does not contain patients who received preoperative radiation therapy.
qRT-PCR Analysis and PCR Array Analysis
Expression levels of SLC7A9 mRNA in 13 ESCC cell lines and tissue samples from the 189-patient cohort were analyzed by qRT-PCR as described previously18 with the specific primers listed in Supplementary Table 1. We used glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA as an internal standard to calculate the relative SLC7A9 mRNA level in each sample.
To identify genes coordinately expressed with SLC7A9 in ESCC cell lines, we conducted PCR array analysis with the Human Epithelial to Mesenchymal Transition (EMT) RT2 Profiler PCR Array (Qiagen, Hilden, Germany). This array includes 84 “key” genes encoding proteins with the following functions: extracellular matrix protein, transcription factors, and proteins involved in EMT, growth, proliferation, migration, cytoskeleton, morphogenesis, cell differentiation, and signaling pathways.19,20
siRNA-Mediated Knockdown of SLC7A9
To evaluate the biological function of SLC7A9, we performed siRNA-mediated SLC7A9 knockdown experiment. KYSE590 cells were plated at 1 × 105 cells/mL in 24-well plates, incubated overnight, and then transiently transfected with 20 nM siRNA specific for SLC7A9 or a control siRNA (Supplementary Table 1) with LipoTrust EX Oligo (Hokkaido System Science, Sapporo, Japan). KYSE890 cells (1 × 105 cells/mL) were also transfected with siRNAs by an electroporation method using Neon System (Thermo Fisher Scientific, Waltham, MA, USA). After transfection, the cells were cultured in RPMI-1640 medium without antibiotics for 48 h before use in functional assays.
Forced Expression of SLC7A9
To further evaluate the biological function of SLC7A9 in ESCC, we also performed the forced expression experiment with the control C-terminal Myc and DDK-tagged, destination vector (pCMV-entry control) and pCMV6-entry SLC7A9 expression vector (RC205055) (OriGene Technoloqies, Rockville, MD, USA). Each vector was transfected into 1 × 105 cells/ml of NUEC2 cells with LipoTrust EX Gene (Hokkaido System Science, Sapporo, Japan), and cells were incubated for 24 h.
Functional Assays
Cell proliferation was determined using Cell Counting Kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan); migration was measured using a wound-healing assay (ibidi GmbH, Martinsried, Germany), and invasion was determined using BioCoat Matrigel invasion chambers (BD Biosciences, Bedford, MA, USA) as described previously.21,22 For the migration assay, the wound width in each well was measured 20 times at 100-mm intervals. These cell functional assays were performed with KYSE590 and KYSE890, and NUEC2 cells (see Results section). Migration was determined with KYSE590 and NUEC2 cells because the migration speed of KYSE890 was too slow to analyze, and invasion was determined with KYSE890 cells because KYSE590 and NUEC2 cannot penetrate the Matrigel layer.23
Clinical Significance of SLC7A9 mRNA Expression Level
Patients were divided into two groups with the median SLC7A9 mRNA expression level obtained from all 189 analyzed patients as a cutoff for low (≤ median) and high (> median) SLC7A9 expression. Correlations between low/high SLC7A9 mRNA expression, clinicopathological parameters, and long-term outcomes, including disease-specific survival (DSS), disease-free survival (DFS), and recurrence pattern-specific survival, were evaluated.
Statistical Analysis
Data were compared between the two groups using the Mann–Whitney U test or the χ2 test. SLC7A9 mRNA expression levels were compared between ESCC tissues and adjacent noncancerous tissues from the 189-patient cohort using the Wilcoxon signed-rank test. DSS, DFS, and recurrence pattern-specific survival rates were calculated by the Kaplan–Meier method and analyzed with a Cox proportional hazards model. Univariate regression analysis of prognostic factors was performed with a Cox proportional hazards model, and variables with p < 0.05 were included in the final multivariate model. All statistical analyses were performed with JMP 14 software (SAS Institute Inc., Cary, NC, USA). p < 0.05 was considered statistically significant.
Results
Selection of Candidate Gene from SLC7 Family Genes
To select a candidate gene from the SLC7 family of genes, we compared the mRNA expression levels of 13 SLC7 family genes between ESCC tissues and normal esophageal tissues from the TCGA dataset including 96 ESCC patients. As a result, we selected SLC7A9 for the subsequent analyses because it was the most overexpressed in ESCC tissues with a significant difference (Table 1; Supplementary Fig. 1a).
Expression of SLC7A9 and Cancer-Related Genes in ESCC Cell Lines
Expression of SLC7A9 mRNA in 13 human ESCC cell lines was analyzed by qRT-PCR. The expression levels varied widely in the ESCC cell lines, with no significant differences between cell lines with different degrees of differentiation (p = 0.584) or between lines derived from primary tumors and metastases (TTn, TT, KYSE1260, and KYSE1170) (p = 0.165) (Fig. 1a). PCR array analysis revealed that FGFBP1 was expressed at a level that was significantly and inversely correlated with that of SLC7A9 in ESCC cell lines. Interestingly, FGFBP1 also showed the most negative correlation with SLC7A9 among 20,104 genes in ESCC tissues from the TCGA dataset24,25 (correlation coefficient −0.504, p < 0.001; Supplementary Fig. 2).
Expression of SCL7A9 and effects of SLC7A9 knockdown in ESCC cells. a mRNA levels of SLC7A9 and FGFBP1 in 13 ESCC cell lines. b siRNA-mediated knockdown efficiency of SLC7A9 in KYSE590 and KYSE890 cells. c Proliferation of KYSE590 and KYSE890 cells with and without siRNA-mediated SLC7A9 knockdown. d Wound-healing assay with KYSE590 cells. The panels on the left show representative images of cells, and the graph on the right shows the mean migration distance at the indicated times. e Invasion assay with KYSE890 cells. The panels on the left show representative images of stained cells (×200 magnification). The graph on the right shows the mean number of invading cells in eight randomly selected fields. *p < 0.05
Effect of SLC7A9 Knockdown and Forced Expression on Biological Activities of ESCC Cells
Then, we investigated the transfection efficiency of siRNA-mediated knockdown of SLC7A9 in the fifth cell line with the highest SLC7A9 mRNA expression levels (KYSE1260, KYSE890, KYSE590, TTn, and TE3). qRT-PCR analysis indicated that knockdown efficiency of > 50% was observed in KYSE590 and KYSE890 cells (Fig. 1b). Therefore, cell functional assays were performed with KYSE590 and KYSE890 cells expressing control siRNA or SLC7A9-specific siRNA. Cell proliferation was significantly decreased in KYSE590 cells as well as KYSE890 cells by SLC7A9 knockdown from 72 h to 120 h compared with the controls (Fig. 1c). Moreover, SLC7A9 knockdown significantly decreased the migration of KYSE590 cells (Fig. 1d), and significantly inhibited the invasion of KYSE890 cells (Fig. 1e) compared with the control cells.
Next, we performed forced expression of SLC7A9 in NUEC2 cells, which had the lowest SLC7A9 mRNA expression level. SLC7A9 plasmid successfully overexpressed SLC7A9 in NUEC2 cells (Supplementary Fig.3a). Cell proliferation was significantly increased in NUEC2 cells by the forced expression of SLC7A9 from 24 h to 72 h compared with pCMV-entry control cells (Supplementary Fig. 3b). In addition, the forced expression of SLC7A9 significantly increased the migration of NUEC2 cells compared with pCMV-entry control cells (Supplementary Fig. 3c).
ESCC Patients and SLC7A9 mRNA Expression in ESCC Tissues
Next, we examined SLC7A9 mRNA expression in primary ESCC tissues and adjacent normal tissues from 189 ESCC patients. The 189 patients consisted of 147 men and 42 women with a median age of 66 years (range, 44–84 years). The majority of the patients (162, 86%) were diagnosed with differentiated ESCC, and the rest with undifferentiated ESCC. Based on the eighth edition of the UICC classification, 37, 43, 75, and 34 patients were in pathological stages I, II, III, and IV, respectively. NAC was administered to 98 patients (52%). The median follow-up duration was 37.7 months, during which time 85 patients (45%) experienced recurrence and 66 patients (35%) succumbed to the disease.
SLC7A9 mRNA levels were higher in ESCC tissues than in normal adjacent esophageal tissues in 96 ESCC patients (51%), and SLC7A9 mRNA expression levels were significantly higher in ESCC tissues than in corresponding normal adjacent tissues (p = 0.034; Supplementary Fig. 1b).
Prognostic Value of SLC7A9 mRNA Level in ESCC Tissue
The 189 ESCC patients were divided into high/low SLC7A9 mRNA expression groups using the median value as the cutoff and correlations between SLC7A9 mRNA expression levels, and the relationships between expression and clinicopathological parameters were analyzed (Table 2). High SLC7A9 mRNA expression in ESCC tissues was significantly associated with tumor location, high serum levels of carcinoembryonic antigen (CEA) and squamous cell carcinoma-related antigen (SCC), advanced disease stage, and LN metastasis. High SLC7A9 expression was also significantly associated with LN metastasis even on multivariable analysis (Supplementary Table 2).
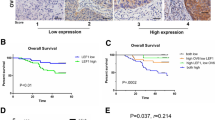
Next, we performed survival analyses of SLC7A9 mRNA expression. DSS was significantly lower in patients with high SLC7A9 expression than in those with low SLC7A9 expression (5-year DSS rates, 54% and 73%, respectively, p = 0.004; Fig. 2a). DFS was also significantly lower in patients with high SLC7A9 expression than in those with low SLC7A9 expression (5-year DFS rates, 47% and 63%, respectively, p = 0.012; Fig. 2b). Multivariable analyses showed that high SLC7A9 expression was an independent predictive factor both for DSS and DFS (Supplementary Tables 3, 4). Interestingly, high SLC7A9 mRNA expression was significantly associated with LN recurrence (p = 0.010) as well as overall recurrence (p = 0.049), but not with local or hematogenous recurrence patterns (Fig. 2c). Of 189 patients, 38 patients experienced LN recurrence, including 19 patients with multiple recurrence patterns. The cumulative incidence of LN recurrence was significantly higher in patients with high SLC7A9 expression compared with those with low expression (p = 0.006, Fig. 2d). Multivariable Cox regression analysis revealed that high SLC7A9 mRNA expression in ESCC tissues was an independent predictive factor for LN recurrence after radical surgery (hazard ratio 2.55, 95% confidence interval 1.28–5.08, p = 0.007; Table 3).
Prognostic value of SLC7A9 mRNA in ESCC tissues. a, b Kaplan–Meyer analyses of disease-specific a and disease-free survival b of 189 patients who underwent radical surgery for ESCC. c, d Frequency of the sites of initial recurrence c and the cumulative incidence of lymph node recurrence d in 189 patients after radical surgery. HR hazard ratio, CI confidence interval
Prognostic Value of Tumor SLC7A9 mRNA Level in Patient Subgroups
Next, we performed subgroup analyses stratified by age, sex, UICC stage, LN metastatic status, tumor differentiation status, and NAC to determine the predictive value of SLC7A9 mRNA expression for LN recurrence. We found no significant interactions among any of the subgroups analyzed (Fig. 3). On the other hand, it is possible that patients in the LN metastasis group may have stronger predictive effects of SLC7A9 mRNA expression compared with those in the LN metastasis absent group.
Discussion
In the present study, we focused on SLC7A9 as a candidate oncogene and investigated the expression, function, and clinical significance of SLC7A9 in ESCC. We showed that knockdown of SLC7A9 in ESCC cell lines significantly reduced malignant abilities such as cell proliferation, migration, and invasion in vitro. We also showed that overexpression of SLC7A9 enhanced malignant potential in ESCC cells. On analyses of clinical specimens, we demonstrated that high SLC7A9 mRNA expression in ESCC tissue was significantly associated with LN metastasis and recurrence, as well as poor prognosis. These results implicate SLC7A9 in the malignant potential of ESCC and indicate that tumor SLC7A9 expression could be a useful biomarker for predicting LN metastasis and recurrence.
The SLC7A9 gene, a member of the SLC7 gene family, is located on human chromosome 19p13.11 and encodes a light subunit of amino acid transporters, playing a role in the high-affinity and sodium-independent transport of cystine and cationic amino acids.26 SLC7A9 has been well studied as a gene responsible for cystinuria,27 but has never been focused on from the oncological perspective. Therefore, we first revealed the relationship between SLC7A9 and cancer in ESCC. Our in vitro analyses showed that the knockdown of SLC7A9 directly attenuated, and the overexpression of SLC7A9 potentiated, the aggressiveness of ESCC cell lines, suggesting that SLC7A9 is more likely to act as a driver gene rather than a passenger gene in ESCC. Currently, the oncogenic mechanism of SLC7A9 is unfortunately unclear. On the other hand, cystine is essential for cancer cells to maintain their antioxidant system with glutathione; thus, the cystine transport system reportedly plays a malignant role in several cancers.28 Indeed, SLC7A11, the cystine/glutamate transporter, is upregulated in various types of cancer and has been recently found to influence tumor growth, progression, and metastasis.9 In colorectal cancer, high tumor SLC7A11 expression is reported to be an independent predictor for LN metastasis and disease recurrence.29 SLC7A9 may also act as a tumor promoter via abnormal uptake of cystine. In addition, our study showed that FGFBP1 was expressed in concert with the expression of SLC7A9 both from PCR array analysis with ESCC cell lines and TCGA’s genome dataset. FGFBP1 is an important molecule in EMT and is reportedly downregulated during EMT.30,31 Although the pathway involved with SLC7A9 and FGFBP1 is currently unknown, FGFBP1 may play a key role in the oncogenic mechanism of SLC7A9 and promote metastatic ability via EMT.
In the present study, we showed the potential of SLC7A9 as a prognostic biomarker for ESCC. What was particularly unique was that high tumor SLC7A9 expression was highly associated with LN metastasis and recurrence, but not with other metastatic patterns. Even in the modern era with the development of radiological diagnosability, radiological N-staging accuracy for ESCC is less than 80%.32,33 In a clinical setting, underestimation of N-staging could lead to skipping of NAC or reductive extent of LN dissection, which sometimes worsens the prognosis.34 Thus, the development of surrogate markers to predict the likelihood of LN metastasis is desired, and SLC7A9 could be a candidate biomarker. High tumor SLC7A9 expression in biopsy samples might be indicative of strong consideration for NAC and extended LN dissection, even when clinically diagnosed as early-stage ESCC. In addition, patients with high SLC7A9 levels in surgical specimens might be expected to benefit from frequent follow-ups to enable early detection of postoperative recurrence, particularly LN recurrence. Although LN recurrence of ESCC after radical surgery infers a poor prognosis, a recent study indicated that salvage chemoradiotherapy for postoperative LN recurrence is more effective for patients with a single LN or a single regional recurrence than those with multiple LNs or multiple regional recurrences.35 Therefore, early detection of LN recurrence with this biomarker could expand the possibilities for additional treatment and prolong survival after recurrence. Previous studies have reported a few possible biomarkers for LN metastasis of ESCC, such as MUC1,36 VEGF-C,37 and TNFAIP8;38 however, these biomarkers, including SLC7A9, do not have sufficient detectability. Therefore, further examination focusing on combining SLC7A9 and other biomarkers would be informative, as this can enhance the performance of SLC7A9 as a biomarker.39 Also, the prognostic power of SLC7A9 for LN recurrence was not much different from those of known risk factors, such as pN category and lymphatic involvement. On the other hand, the subgroup analysis showed that the prognostic power of SLC7A9 may be stronger in patients with LN metastasis than those without LN metastasis. Therefore, the risk stratification with SLC7A9 expression and known risk factors may be useful to determine the treatment policy.
In addition to its potential as a biomarker, our results of knockdown assays suggest that SLC7A9 might be a promising target molecule for chemotherapy for ESCC. Indeed, SLC7A11, the cystine transporter mentioned above, has been focused on as a therapeutic target, and the efficacy of SLC7A11-targeted therapy has been validated in various cancers.40,41 Considering that NAC did not affect the prognostic value of SLC7A9 in our subgroup analysis, the expression control of SLC7A9 could show an antitumor effect in a manner different from existing chemotherapeutic regimens. Therefore, SLC7A9-targeted therapy in combination with conventional chemotherapy might contribute to the improvement of oncological outcomes for ESCC.
This study has several limitations. First, we did not investigate the metabolic profile of the antioxidant system or signaling pathways of ESCC cells, which would have shed light on the molecular mechanisms explaining the malignant activity of SLC7A9 shown in our in vitro experiment. Second, our clinical analyses were retrospectively examined. Third, we used the median expression level as a cutoff for the stratification of patients into high/low SLC7A9 expression groups. An optimal cutoff value needs to be calculated from larger-scale studies for clinical application. In addition, we could not examine fully the prognostic value of tumor overexpression of SLC7A9 compared with adjacent normal tissues. Moreover, normal adjacent tissues themselves can have some genetic abnormality, and the expression analysis and comparison of SLC7A9 with esophageal tissue samples of non-ESCC patients is desired for further study.
In conclusion, our results show that SLC7A9 is related to the malignant potential in ESCC and suggest that tumor SLC7A9 expression may serve as a novel prognostic marker for LN metastasis and recurrence in ESCC patients.
References
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-386.
Malhotra GK, Yanala U, Ravipati A, Follet M, Vijayakumar M, Are C. Global trends in esophageal cancer. J Surg Oncol. 2017;115:564–79.
Dubecz A, Kern M, Solymosi N, Schweigert M, Stein HJ. Predictors of lymph node metastasis in surgically resected T1 esophageal cancer. Ann Thorac Surg. 2015;99:1879–85.
Duan XF, Tang P, Shang XB, Jiang HJ, Yu ZT. The prevalence of lymph node metastasis for pathological T1 esophageal cancer: a retrospective study of 143 cases. Surg Oncol. 2018;27:1–6.
Wu J, Chen QX, Shen DJ, Zhao Q. A prediction model for lymph node metastasis in T1 esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg. 2018;155:1902–8.
Chapman BC, Weyant M, Hilton S, et al. Analysis of the National Cancer Database esophageal squamous cell carcinoma in the United States. Ann Thorac Surg. 2019;S0003–4975:31006–9.
Yamashita K, Watanabe M, Mine S, et al. Patterns and outcomes of recurrent esophageal cancer after curative esophagectomy. World J Surg. 2017;41:2337–44.
Bhutia YD, Babu E, Ramachandran S, Ganapathy V. Amino acid transporters in cancer and their relevance to “glutamine addiction”: novel targets for the design of a new class of anticancer drugs. Cancer Res. 2015;75:1782–8.
Bhutia YD, Ganapathy V. Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim Biophys Acta. 2016;1863:2531–9.
Ichinoe M, Mikami T, Yoshida T, et al. High expression of L-type amino-acid transporter 1 (LAT1) in gastric carcinomas: comparison with non-cancerous lesions. Pathol Int. 2011;61:281–9.
Yanagisawa N, Ichinoe M, Mikami T, et al. High expression of L-type amino acid transporter 1 (LAT1) predicts poor prognosis in pancreatic ductal adenocarcinomas. J Clin Pathol. 2012;65:1019–23.
Sakata T, Ferdous G, Tsuruta T, et al. L-type amino-acid transporter 1 as a novel biomarker for high-grade malignancy in prostate cancer. Pathol Int. 2009;59:7–18.
Robert SM, Buckingham SC, Campbell SL, et al. SLC7A11 expression is associated with seizures and predicts poor survival in patients with malignant glioma. Sci Transl Med. 2015; 7: 289ra86.
Takeuchi S, Wada K, Toyooka T, et al. Increased xCT expression correlates with tumor invasion and outcome in patients with glioblastomas. Neurosurgery. 2013;72:33–41.
National Cancer Institute: The Cancer Genome Atras Program. https://www.cancer.gov/tcga. Accessed on 8 January 2021.
Tsunoo H, Komura S, Ohishi N, et al. Effect of transfection with human interferon-beta gene entrapped in cationic multilamellar liposomes in combination with 5-fluorouracil on the growth of human esophageal cancer cells in vitro. Anticancer Res. 2002;22:1537–43.
TNM Classification of Malignant Tumours, 8th Edition. James DB, Mary KG, Christian W, eds. New York: Wiley, 2016: 57-62.
Kanda M, Shimizu D, Tanaka H, et al. Significance of SYT8 for the detection, prediction, and treatment of peritoneal metastasis from gastric cancer. Ann Surg. 2018;267:495–503.
Umeda S, Kanda M, Miwa T, et al. Expression of sushi domain containing two reflects the malignant potential of gastric cancer. Cancer Med. 2018;7:5194–204.
Baba H, Kanda M, Sawaki K, et al. PRAME expression as a potential biomarker for hematogenous recurrence of esophageal squamous cell carcinoma. Anticancer Res. 2019;39:5943–51.
Kanda M, Tanaka H, Shimizu D, et al. SYT7 acts as a driver of hepatic metastasis formation of gastric cancer cells. Oncogene. 2018;37:5355–66.
Kanda M, Shimizu D, Sawaki K, et al. Therapeutic monoclonal antibody targeting of neuronal pentraxin receptor to control metastasis in gastric cancer. Mol Cancer. 2020;19:131.
Baba H, Kanda M, Sato Y, et al. Expression and malignant potential of B4GALNT4 in esophageal squamous cell carcinoma. Ann Surg Oncol. 2020;27:3247–56.
LinkedOmics: analyzing multi-omics data within and across 32 cancer types. http://www.linkedomics.org. Accessed on 19 January 2021.
Vasaikar SV, Straub P, Wang J, Zhang B. LinkedOmics: analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018;46:D956–63.
Chillarón J, Font-Llitjós M, Fort J, Zorzano A, Goldfarb DS, Nunes V, Palacín M. Pathophysiology and treatment of cystinuria. Nat Rev Nephrol. 2010;6:424–34.
Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12:931–47.
Liu J, Xia X, Huang P. xCT: a critical molecule that links cancer metabolism to redox signaling. Mol Ther. 2020;28:2358–66.
Sugano K, Maeda K, Ohtani H, Nagahara H, Shibutani M, Hirakawa K. Expression of xCT as a predictor of disease recurrence in patients with colorectal cancer. Anticancer Res. 2015;35:677–82.
Minafra L, Bravatà V, Forte GI, Cammarata FP, Gilardi MC, Messa C. Gene expression profiling of epithelial-mesenchymal transition in primary breast cancer cell culture. Anticancer Res. 2014;34:2173–83.
Thomson S, Petti F, Sujka-Kwok I, et al. A systems view of epithelial-mesenchymal transition signaling states. Clin Exp Metastasis. 2011;28:137–55.
Foley KG, Christian A, Fielding P, Lewis WG, Roberts SA. Accuracy of contemporary oesophageal cancer lymph node staging with radiological-pathological correlation. Clin Radiol. 2017;72(693):e1-693.e7.
Aoyama J, Kawakubo H, Mayanagi S, et al. Discrepancy between the clinical and final pathological findings of lymph node metastasis in superficial esophageal cancer. Ann Surg Oncol. 2019;26:2874–81.
Akutsu Y, Kato K, Igaki H, et al. The prevalence of overall and initial lymph node metastases in clinical T1N0 thoracic esophageal cancer: from the results of JCOG0502, a prospective multicenter study. Ann Surg. 2016;264:1009–15.
Kawamoto T, Nihei K, Sasai K, Karasawa K. Clinical outcomes and prognostic factors of chemoradiotherapy for postoperative lymph node recurrence of esophageal cancer. Jpn J Clin Oncol. 2018;48:259–64.
Sun ZG, Wang Z. Clinical study on lymph node metastatic recurrence in patients with N0 esophageal squamous cell cancer. Dis Esophagus. 2011;24:182–8.
Sun ZG, Wang Z, Liu XY, Liu FY. Mucin 1 and vascular endothelial growth factor C expression correlates with lymph node metastatic recurrence in patients with N0 esophageal cancer after Ivor-Lewis esophagectomy. World J Surg. 2011;35:70–7.
Sun Z, Liu X, Song JH, et al. TNFAIP8 overexpression: a potential predictor of lymphatic metastatic recurrence in pN0 esophageal squamous cell carcinoma after Ivor Lewis esophagectomy. Tumour Biol. 2016;37:10923–34.
Kanda M, Murotani K, Tanaka H, et al. Integrated multigene expression panel to prognosticate patients with gastric cancer. Oncotarget. 2018;9:18775–85.
Cobler L, Zhang H, Suri P, Park C, Timmerman LA. xCT inhibition sensitizes tumors to γ-radiation via glutathione reduction. Oncotarget. 2018;9:32280–97.
Okazaki S, Umene K, Yamasaki J, et al. Glutaminolysis-related genes determine sensitivity to xCT-targeted therapy in head and neck squamous cell carcinoma. Cancer Sci. 2019;110:3453–63.
Acknowledgment
We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baba, H., Kanda, M., Sawaki, K. et al. SLC7A9 as a Potential Biomarker for Lymph Node Metastasis of Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 29, 2699–2709 (2022). https://doi.org/10.1245/s10434-021-11001-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-11001-1