Abstract
Background
The oncologic advantage of anatomic resection (AR) for primary hepatocellular carcinoma (HCC) remains controversial. This study aimed to evaluate the clinical advantages of AR for primary HCC by using propensity score-matching and by assessing treatment strategies for recurrence after surgery.
Methods
The study reviewed data of patients who underwent AR or non-anatomic resection (NAR) for solitary HCC (≤ 5 cm) in two institutions between 2004 and 2017. Surgical outcomes were compared between the two groups in a propensity score-adjusted cohort. The time-to-interventional failure (TIF), defined as the elapsed time from resection to unresectable/unablatable recurrence, also was evaluated.
Results
The inclusion criteria were met by 250 patients: 77 patients (31%) with AR and 173 patients (69%) with NAR. In the propensity score-matched populations (AR, 67; NAR, 67), the 5-year recurrence-free survival (RFS) for AR was better than for NAR (62% vs 35%; P = 0.005). No differences, however, were found in the 5-year overall survival between the two groups (72% vs 78%; P = 0.666). The 5-year TIF rates for the NAR group (60%) also were similar to those for the AR group (66%) (P = 0.413). In the cohort of 67 patients, curative repeat resection or ablation therapy was performed more frequently for the NAR patients (42%) than for the AR patients (10%) (P < 0.001).
Conclusion
For solitary HCC, AR decreases recurrence after the initial hepatectomy. However, aggressive curative-intent interventions for recurrence compensate for the impaired RFS, even for patients undergoing NAR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hepatocellular carcinoma (HCC) is among the leading causes of cancer-related death and is estimated to be the fourth most common cause of death worldwide.1 Liver resection currently is accepted as an initial treatment for small HCC in patients with preserved hepatic function.2 Percutaneous ablation also is a curative treatment for single and multinodular HCC (up to three lesions smaller than 3 cm in diameter).3
Advances in surgical techniques and perioperative management have transformed the resection of HCC into a relatively safe operation with a low mortality rate.4 Because HCC has a high propensity to invade intrahepatic vascular structures and spreads mainly via the closest portal veins,5 anatomic resection (AR), including systemic removal of the tumor-bearing portal territories, was proposed in the 1980s as a theoretically curative procedure for HCC to eradicate potential micrometastases surrounding tumors.6
The prognostic superiority of AR over non-anatomic resection (NAR) has long been controversial. Recently, several authors have published comparative studies using propensity score-matched (PSM) analysis.7,8,9,10,11,12,13 However, the conclusions of these studies lacked consensus. Some studies found that AR improved survival for patients with HCC,7,8,9,10,11 whereas others did not show any prognostic benefit of AR compared with NAR.12,13
The major limitation of the previous PSM studies is that they did not consider treatment for tumor recurrence after initial hepatectomy in their analyses. The cumulative 5-year recurrence rate remains as high as 70–80%, even after radical surgery, and curative-intent repeat hepatectomy or radiofrequency ablation (RFA) significantly affects survival for patients who have recurrence after surgery for HCC.14
The current study aimed to evaluate the potential prognostic superiority of AR over NAR for patients with solitary HCC using PSM analysis. Recurrence pattern and recurrence treatment also were reviewed to assess the impact of initial AR or NAR on survival.
Methods
Study Population
The study identified patients who underwent initial hepatectomy for HCC between January 2004 and December 2017 at two Japanese institutions [the Department of Hepato-biliary Pancreatic Surgery, Juntendo University Hospital (JUH), and the Department of Surgery, Cancer Institute Hospital, Japanese Foundation for Cancer Research (CIH)]. This study was approved by the ethics committees of the two institutions (JHS 18-060 for JUH and 2019-1028 for CIH).
The study population was composed of Asian patients who underwent AR of Couinaud’s segment and NAR for solitary HCC (≤ 5 cm). The exclusion criteria ruled out history of treatment for HCC, other malignancy, and AR larger than Couinaud’s segmentectomy (sectionectomy, right or left hepatectomy).
Surgical Procedures
The detailed surgical procedures for HCC at JUH and CIH have been described previously.15,16 Segmentectomy was defined as complete resection of one Couinaud’s segment identified by dye-staining. Segmental staining was performed by indocyanine green (ICG) fluorescence and Sonazoid to indicate the segmental section.
At CIH, the indication whether to perform AR or NAR was based on an algorithm that included the presence or absence of ascites, the serum total bilirubin level, and the results from a test of ICG retention at 15 min (ICGR15; i.e., Makuuchi’s criteria).17 All the patients at JUH underwent NAR, defined as incomplete resection of the portal tributaries of the tumor-bearing segment, which included partial resection or enucleation of the liver.15
Patient Follow-Up Evaluation
Peri- and postoperative complications or death were recorded to assess the morbidity and mortality of the procedures. Major complication was defined as a Clavien-Dindo classification of grade 3a or higher.18
In-hospital and 90-day mortality also were assessed.19 Patients were routinely followed by checking tumor markers such as alpha-fetoprotein concentration (AFP) and prothrombin induced by vitamin K (PIVKA-II) and computed tomography or magnetic resonance imaging every 3 months. Recurrence was defined as the appearance of a new lesion with radiologic features compatible with HCC. When a recurrence was detected, the patient was treated further by repeat hepatectomy, ablation therapies [including RFA or transcatheter arterial chemoembolization (TACE)], or other treatment methods (including systemic therapy).
In both institutions, the resectability and ablatability of the recurrent lesions were initially determined based on the indication criteria for surgery in a multidisciplinary discussion by physicians, including hepatobiliary surgeons. Then, a treatment plan was discussed that considered the resectability/ablatability of the tumors, the recommended treatments from the multidisciplinary discussion, the physical status of the patient, the patient’s preference of treatment, and other socioeconomic factors.
In the current study, the following survival outcomes were recorded. Recurrence-free survival (RFS) was defined as the interval between the date of operation and the date the first recurrence was diagnosed or death occurred. Overall survival (OS) was defined as the interval between the date of operation and the date of death due to any cause. Local recurrence was defined as any recurrence observed in the residual part of the tumor-bearing third-order portal branches or recurrence adjacent to the cut surface of the liver. The time-to-interventional failure (TIF), defined as the elapsed time from resection to unresectable/unablatable recurrence, also was evaluated to assess the prognostic impact of interventional treatment for recurrent HCC.
Propensity Score Analysis
To avoid confounding differences due to baseline variation between the AR and NAR groups, we established a propensity score-matched subset of the original data. The propensity scores were generated using a logistic regression model, and the following perioperative characteristics were included in the model: sex, age, underlying liver disease [hepatitis B surface antigen (HBsAg) and anti-hepatitis C virus antibody (HCV Ab) positivity], preoperative serum total bilirubin concentration, aspartate aminotransferase (AST), alanine aminotransferase (ALT) concentration, albumin concentration, platelet count, ICGR15, serum AFP, serum PIVKA-II concentration, image of maximum tumor size, and image of macroscopic vascular invasion in the portal and/or hepatic veins. After calculation of propensity scores, a matched subset of patients was extracted by a one-to-one greedy nearest-matching algorithm without replacement, with a caliper width equal to 0.2 of the standard deviation of the logit of the propensity score.
Statistical Analysis
To summarize patient characteristics, medians and 25th to 75th percentiles were used for continuous variables, whereas frequencies and proportions were calculated for categorical variables. The clinical characteristics of the two groups were compared by either the chi-square test or Fisher’s exact test for categorical variables, and by the Wilcoxon rank-sum test for continuous variables. The RFS, OS and TIF rates after hepatectomy were calculated by the Kaplan–Meier product-limit method and compared by the log-rank test. Both PSM and statistical analysis were performed using SAS (version 9.4; SAS institute, Cary, NC, USA). Statistical analyses other than PSM were performed with IBM SPSS software (version 26.0; SPSS Inc., Chicago, IL, USA).
Results
During the study period, 1217 patients underwent initial hepatic resection for HCC at the two institutions. The study excluded patients who had multiple tumors (n = 397), a tumor larger than 5 cm (n = 296), another malignancy, or an unknown follow-up period (n = 211), which left 313 patients. From these patients, 63 who underwent sectionectomy or a right or left hepatectomy were excluded. This left 77 patients who underwent AR of Couinaud’s segment and 173 patients who underwent NAR to be enrolled in the study.
After PSM, 134 patients were divided into the AR group (n = 67) and the NAR group (n = 67). Figure 1 outlines the patient selection. Table 1 summarizes the characteristics of both groups before and after PSM.
Before PSM, platelet count, albumin level, prothrombin time, and PIVKA-II level were lower in the NAR group than in the AR group. After PSM, all the baseline characteristics except for platelet count were matched.
Surgical Outcomes
Table 2 summarizes the surgical outcomes for the two groups before and after PSM. The amount of blood loss was lower in the NAR group than in the AR group. After PSM, no differences were found in pathologic findings between the two groups.
Long-Term Outcomes
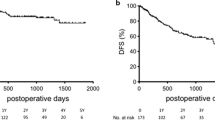
The median follow-up period was 53 months [interquartile range IQR, 27–79 months] in the AR group and 75 months (IQR, 45–110 months) in the NAR group. Figure 2 shows the survival curves for the two groups before PSM. The 5-year RFS was better in the AR group than in the NAR group (63% vs 42%; P = 0.023). However, no significant difference was found in the 5-year OS between the two groups (74% vs 79%; P = 0.61).
After PSM, the 5-year RFS in the AR group was better than in the NAR group (62% vs 35%; P = 0.005; Fig. 3a). However, no significant difference was found in the 5-year OS between the two groups (72% vs 78%; P = 0.666; Fig. 3b).
Recurrence After Initial Hepatectomy
Table 3 summarizes the recurrence mode and treatment for recurrence after initial hepatectomy. Among the PSM patients, intrahepatic recurrence after the initial hepatectomy was observed in 17 AR patients (25.3%) and 41 NAR patients (61.2%). The incidence of recurrence in the same segment after initial hepatectomy in the NAR group was 15% (6/41). The rate of the recurrence within the adjacent portal veinous territories was 41% (17/41) in the NAR group and 29% (5/17) in the AR group (P = 0.389].
Although the incidence of curative-intent interventions for recurrence was similar between the two groups, among the cohort of 67 patients, repeat hepatectomy or RFA for recurrence was performed more frequently in the NAR group (n = 28, 42%) than in the AR group (n = 7, 10%) (P < 0.001). The prevalence of curative-intent interventions for recurrence differed between CIH (43%, 9/21) and JUH (70%, 26/37) (P = 0.043).
The TIFs for the two groups are presented in Fig. 3c, which shows that the TIFs of the curative intent treatment, including repeat hepatectomy or RFA, did not differ significantly from those of TACE (P = 0.413).
Discussion
The current study investigated the prognostic impact from anatomic resection of Couinaud’s segments by comparing surgical outcomes between patients who underwent AR and those who had NAR for a solitary HCC of 5 cm or smaller. We found that AR decreased recurrence after initial hepatectomy, but that OS did not differ between the AR and NAR groups. Assessment of recurrence mode and treatment for the recurrence showed that aggressive curative-intent interventions for intrahepatic recurrence was performed more frequently in the NAR group than in the AR group, which led to the comparable TIF and OS between the two groups.
The current PSM analysis demonstrated that curability of AR as an initial treatment outweighs NAR for patients with solitary HCC by showing better RFS in the AR group than in the NAR group. Previous PSM studies analyzing the prognostic impact of AR for HCC failed to reach robust conclusions.7,8,9 The major cause of the incoherence was that most of the studies included hepatectomies larger than Couinaud’s segmentectomy, which may have introduced bias into the selection of surgical procedures influenced by tumor characteristics such as size, location, and vascular infiltration. In addition, the inclusion of large hepatectomies, such as sectionectomy or hemihepatectomy, posed a risk for overestimation of the prognostic impact of AR because a large hepatectomy removes a greater amount of “at-risk” liver parenchyma in which future recurrences may occur.
To minimize these biases, two PSM studies previously limited the procedure to Couinaud’s segmentectomy when comparing AR with NAR.10,11 These two studies and the current study agree that RFS was better in the AR group than in the NAR group, which reinforces the theory that AR improves local control of the disease by eradicating potential micrometastases via the portal veins.10,11
In addition, tumor exposure at the surgical margin was found in 6% of the NAR group. On the other hand, no patients in the AR group had a positive surgical margin. This result indicates that NAR poses a risk for exposure of the tumor during resection. However, in the current analysis, the incidence of early recurrence that the tumor exposure might have caused did not differ between the AR and NAR groups. In our clinical practice, surgical procedures that also consider the radicality and the hepatic functional reserve need to be selected because the parenchymal-sparing NAR can be the only choice for surgical treatment of patients with impaired liver function.
A difference in RFS was found, but our study found no difference in the 5-year OS between the two groups. This paradoxical result may have been due to specific characteristics of HCC treatment. Similar to colorectal liver metastases, the survival outcomes for patients with HCC can be improved by optimal repeated interventions for recurrence.20,21,22,23,24
A major strength of the current study was its detailed analysis of recurrence treatment, which showed that aggressive curative-intent interventions for recurrence compensate for the impaired RFS even for patients undergoing NAR. Shindoh et al.14 found that treatment choice for recurrence significantly affects the survival outcomes for patients with resectable/ablatable HCC recurrence. Their report, which first introduced the concept of TIF, showed that the survival of patients undergoing curative-intent treatment (repeat resection or RFA) for recurrence was better than that of the patients who had non-curative-intent treatment (e.g., TACE, radiotherapy, chemotherapy).
The current study found that optimal treatment for recurrence can salvage the impaired RFS of NAR patients. Conversely however, repeated interventions are needed to achieve comparable OS for patients who underwent NAR as an initial treatment.
In our analysis, as many as 42% of the patients in the NAR group underwent interventional treatment for recurrence, compared with 10% in the AR group. Although the institutional difference in treatment approach to the recurrence cannot be ignored, the parenchymal-sparing nature of NAR may have improved salvageability for the recurrence by saving room for future aggressive treatment even in patients with well-preserved liver function.25 In addition, the necessity of exposing the Glissonean sheath and major hepatic veins during AR may have made surgeons reluctant to perform repeat resection because exposure of the Glissonean sheath and the major vessels is reportedly a risk to increase postoperative bile leakage.26
The necessity of frequent interventions raises two concerns about choosing NAR as an initial surgical procedure. First, repeated surgery or RFA can compromise physical and mental quality of life (QOL) during the entire course of treatment. Previous studies have demonstrated temporary deterioration of QOL after either hepatectomy or RFA in patients who have an initial treatment for HCC.27,28,29,30 Although no evidence is available regarding QOL change after repeated surgery or RFA, the recurrence treatment must affect the patient’s mental and emotional well-being because the anxiety associated with the tumor still is present. Further investigations are needed to confirm the clinical benefits of AR from a viewpoint of QOL in HCC patients who need to undergo repetitive treatments.
Second, institutional differences in aggressiveness in performing repeated interventions may directly affect the survival outcomes for patients undergoing NAR. Although several studies have demonstrated the feasibility of repeated hepatectomy it reportedly is technically demanding in terms of the increased operation time or the higher incidence of bile leakage compared with the initial hepatectomy.26,31,32,33,34,35,36 Additionally, repeated RFA after hepatic resection raises the possibility of several complications. Bowel damage is more likely with the initial hepatic resection than with the initial treatment of HCC due to fibrotic adhesions between the liver and bowel.37
Abscess formation is another potential complication for patients who undergo repeat RFA after hepatic resection. The RFA makes connections between the biliary duct and the ablation zones through a thermal injury to the bile ducts, which causes enteric bacterial contamination around the ablation zones when combined with hepatic resection.38,39 Conflicting results of OS in previous comparative studies may well be explained by the institutional differences in treatment policies for recurrence.
There current study had several limitations, mainly associated with the retrospective data derived from two different institutions. Selection bias could not be completely eliminated even after PSM analysis because the treatment policies for primary and recurrent HCC differed between the two institutions. Particularly, as discussed earlier, differences in aggressiveness in performing repeated interventions for the recurrence must have strongly affected OS outcomes. A previous comparative PSM study of 54 institutions demonstrated a better OS in the AR group than in the NAR group, which may have been attributable to the comparable rates of repeated interventions for recurrence between the two groups (AR, 31%, 35/114l vs NAR, 28%, 33/114; P = 0.159).11 In addition, the platelet count was lower for the patients in the NAR group than for those in the AR group, even after PSM, which indicates that baseline liver function may be worse for patients in the NAR group. On the other hand, the institutional difference in the treatment policy for recurrence in the current study showed that impaired RFS after NAR can be compensated by aggressive curative-intent interventions for recurrence.
Another limitation was that de novo HCC derived from the injured underlying liver was discriminated from recurrence of residual HCC, which confounds the interpretation of the survival outcomes.40 However, the superiority of AR to NAR as an initial procedure for reducing the recurrence, indicated by its improvement to RFS, is a consistent result across PMS studies.10,11
In conclusion, AR for solitary HCC decreases tumor recurrence after initial hepatectomy. However, aggressive curative-intent interventions for tumor recurrence can compensate for the impaired RFS, even in patients undergoing NAR.
References
Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–48.
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatol Baltimore. 2011;53:1020–2.
Makuuchi M, Kokudo N, Arii S, et al. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008;38:37–51.
Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–30.
Nakashima T, Kojiro M. Pathologic characteristics of hepatocellular carcinoma. Semin Liver Dis. 1986;6:259–66.
Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1985;161:346–50.
Ishii M, Mizuguchi T, Kawamoto M, et al. Propensity score analysis demonstrated the prognostic advantage of anatomical liver resection in hepatocellular carcinoma. World J Gastroenterol. 2014;20:3335–42.
Cucchetti A, Qiao GL, Cescon M, et al. Anatomic versus nonanatomic resection in cirrhotic patients with early hepatocellular carcinoma. Surgery. 2014;155:512–21.
Kaibori M, Kon M, Kitawaki T, et al. Comparison of anatomic and non-anatomic hepatic resection for hepatocellular carcinoma. J Hepato-Biliary-Pancreatic Sci. 2017;24:616–26.
Shindoh J, Makuuchi M, Matsuyama Y, et al. Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J Hepatol. 2016;64:594–600.
Kaibori M, Yoshii K, Hasegawa K, et al. Impact of systematic segmentectomy for small hepatocellular carcinoma. J Hepato-Biliary-Pancreatic Sci. 2020;27:331–41.
Okamura Y, Ito T, Sugiura T, Mori K, Uesaka K. Anatomic versus nonanatomic hepatectomy for a solitary hepatocellular carcinoma: a case-controlled study with propensity score matching. J Gastrointest Surg. 2014;18:1994–2002.
Marubashi S, Gotoh K, Akita H, et al. Anatomical versus non-anatomical resection for hepatocellular carcinoma. Br J Surg. 2015;102:776–84.
Shindoh J, Kawamura Y, Kobayashi Y, et al. Time-to-interventional failure as a new surrogate measure for survival outcomes after resection of hepatocellular carcinoma. J Gastrointest Surg. 2020;24(1):50–7.
Oguro S, Yoshimoto J, Imamura H, Ishizaki Y, Kawasaki S. Clinical significance of macroscopic no-margin hepatectomy for hepatocellular carcinoma. HPB. 2018;20:872–80.
Kishi Y, Saiura A, Yamamoto J, et al. Significance of anatomic resection for early and advanced hepatocellular carcinoma. Langenbeck’s Arch Surg. 2012;397:85–92.
Makuuchi M, Kosuge T, Takayama T, et al. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298–304.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Mise Y, Vauthey JN, Zimmitti G, et al. Ninety-day postoperative mortality is a legitimate measure of hepatopancreatobiliary surgical quality. Ann Surg. 2015;262:1071–8.
Xing H, Sun LY, Yan WT, et al. Repeat hepatectomy for patients with early and late recurrence of hepatocellular carcinoma: a multicenter propensity score-matching analysis. Surgery. 2021;169(4):911–20.
Erridge S, Pucher PH, Markar SR, et al. Meta-analysis of determinants of survival following treatment of recurrent hepatocellular carcinoma. Br J Surg. 2017;104:1433–42.
Zhou Y, Sui C, Li B, et al. Repeat hepatectomy for recurrent hepatocellular carcinoma: a local experience and a systematic review. World J Surg Oncol. 2010;8:55.
Chan DL, Morris DL, Chua TC. Clinical efficacy and predictors of outcomes of repeat hepatectomy for recurrent hepatocellular carcinoma - a systematic review. Surg Oncol. 2013;22:e23-30.
Feng Y, Wu H, Huang DQ, et al. Radiofrequency ablation versus repeat resection for recurrent hepatocellular carcinoma (≤5 cm) after initial curative resection. Eur Radiol. 2020;30:6357–68.
Mise Y, Aloia TA, Brudvik KW, Schwarz L, Vauthey JN, Conrad C. Parenchymal-sparing hepatectomy in colorectal liver metastasis improves salvageability and survival. Ann Surg. 2016;263:146–52.
Mise Y, Hasegawa K, Shindoh J, et al. The feasibility of third or more repeat hepatectomy for recurrent hepatocellular carcinoma. Ann Surg. 2015;262:347–57.
Toro A, Pulvirenti E, Palermo F, Di Carlo I. Health-related quality of life in patients with hepatocellular carcinoma after hepatic resection, transcatheter arterial chemoembolization, radiofrequency ablation or no treatment. Surg Oncol. 2012;21:e23-30.
Huang G, Chen X, Lau WY, et al. Quality of life after surgical resection compared with radiofrequency ablation for small hepatocellular carcinomas. Br J Surg. 2014;101:1006–15.
Chie WC, Yu F, Li M, et al. Quality of life changes in patients undergoing treatment for hepatocellular carcinoma. Qual Life Res. 2015;24:2499–506.
Mise Y, Satou S, Ishizawa T, et al. Impact of surgery on quality of life in patients with hepatocellular carcinoma. World J Surg. 2014;38:958–67.
Yamashita Y, Hamatsu T, Rikimaru T, et al. Bile leakage after hepatic resection. Ann Surg. 2001;233:45–50.
Nagano Y, Togo S, Tanaka K, et al. Risk factors and management of bile leakage after hepatic resection. World J Surg. 2003;27:695–8.
Capussotti L, Ferrero A, Viganò L, Sgotto E, Muratore A, Polastri R (2006) Bile leakage and liver resection: where is the risk? Arch Surg. 141:690–4 (discussion 695).
Morimura R, Saiura A, Koga R, et al. Impact of previous hepatectomy on short-term outcomes of repeat hepatectomy for liver tumors with a special concern of operative time. Hepatogastroenterology. 2012;59:809–13.
Sadamori H, Yagi T, Matsuda H, Shinoura S, Umeda Y, Fujiwara T. Intractable bile leakage after hepatectomy for hepatocellular carcinoma in 359 recent cases. Dig Surg. 2012;29:149–56.
Sadamori H, Yagi T, Shinoura S, et al. Risk factors for major morbidity after liver resection for hepatocellular carcinoma. Br J Surg. 2013;100:122–9.
Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radiofrequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–51.
Choi D, Lim HK, Rhim H, et al. Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factors. Ann Surg Oncol. 2007;14:2319–29.
Choi D, Lim HK, Kim MJ, et al. Liver abscess after percutaneous radiofrequency ablation for hepatocellular carcinomas: frequency and risk factors. AJR Am J Roentgenol. 2005;184:1860–7.
Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Minagawa, M., Mise, Y., Omichi, K. et al. Anatomic Resection for Hepatocellular Carcinoma: Prognostic Impact Assessed from Recurrence Treatment. Ann Surg Oncol 29, 913–921 (2022). https://doi.org/10.1245/s10434-021-10380-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-10380-9