Abstract
Background
The systemic inflammatory response caused by host-tumor interactions is currently recognized as a hallmark feature of cancer. No study has confirmed which systemic inflammatory factors can accurately predict the progression and long-term prognosis of gastric cancer (GC).
Methods
Through the analysis of receiver operating characteristic curve (ROC), in the discovery cohort, a variety of indicators composed of usual inflammatory factors were compared. Fibrinogen-albumin ratio (FAR), which can accurately predict the long-term survival of GC patients was selected and was further verified in the test cohort and the external validation cohort.
Results
The ROC curve analysis showed that the area under curve (AUC) value of FAR on the overall survival (OS) of GC patients was higher than that of other combined markers (P < 0.01). Patients in the high FAR group showed more advanced pathological stages, larger tumor diameters, and more poorly differentiated pathological type than those in the low FAR group (P < 0.05). Logistic regression analysis elucidated that, FAR was an independent risk factor for LN metastasis and tumor invasion of GC. High FAR was an independent risk factor for poor prognosis of GC patients. The relationship between FAR and pathological stage of GC and long-term prognosis of patients was verified in the test cohort and the external validation cohort with the same FAR cutoff value. The results are consistent with those of the discovery cohort.
Conclusions
As a new developed inflammation-related marker, FAR can independently and effectively predict the tumor burden and long-term prognosis of patients with advanced GC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Although the incidence of GC has declined, it remains one of the most common cancers in the world, ranked as the second-leading cause of cancer-related death.1,2,3,4 Accumulating studies have revealed that the inflammatory response is a persistent and abnormal systemic response to malignancy and plays an important role in tumor progression and long-term survival in cancer patients.5,6,7,8 Since Virchow first reported the relationship between inflammation and cancer in 1863, increasing research has focused on the potential prognostic value of systemic inflammatory markers, such as C-reactive protein (CRP), fibrinogen, albumin, and neutrophil and platelet and lymphocyte counts, as well as their combination into ratios or cumulative scores (e.g., CRP to albumin ratio (CAR), neutrophil to lymphocyte ratio (NLR), and platelet to lymphocyte ratio (PLR)) in cancer.9,10,11 These markers of systemic inflammation, which are usually assessed in terms of ratios or cumulative scores of peripheral circulating white blood cells or acute phase proteins, represent systemic responses in two different organs: the lymphoid/medullary tissue and the liver.12,13,14 At present, no studies have confirmed which systemic inflammatory factors or combinations of indicators can accurately predict the tumor progression and/or long-term prognosis of GC patients. Moreover, there is no cutoff for each inflammatory marker that can consistently predict therapeutic efficacy in GC, which is a prerequisite for clinical use and promotion. In this study, large-volume data were retrospectively analyzed using ROC curves. Traditional combinations of inflammatory factors were compared to assess the potential of such indexes in predicting long-term prognosis in patients with GC. Ultimately, a new combination of indicators FAR was developed and confirmed to accurately predict the long-term prognosis of GC patients. We explored the clinical significance of the FAR in patients with GC and verified the prognostic value of the FAR for GC patients using a consistent cutoff value in both data from an independent prospective trial at our center and external center.
Methods
Study Population
We selected two hospitals, Fujian Medical University Union Hospital and Qinghai University Affiliated Hospital, that have facilities for electronic storage of clinical data, including medical records, images, or laboratory data. All consecutive patients with GC who underwent gastrectomy during the study period at these two hospitals were considered for the study. All patients at these institutions who met the inclusion criteria below were enrolled (Fig. 1): (a) diagnosis of histologically confirmed adenocarcinoma of the stomach, (b) no evidence of tumors invading adjacent organs, paraaortic lymph node enlargement or distant metastasis demonstrated by abdominal computed tomography and/or abdominal ultrasound and posteroanterior chest radiography, and (c) receipt of D1/D1+/D2 lymphadenectomy with curative R0 resection. The exclusion criteria were as follows: (a) stage T4b tumors, (b) metastatic disease, (c) gastric stump carcinoma, and (d) incomplete or inaccurate medical records. We retrospectively reviewed data collected from 2401 patients who underwent radical gastrectomy at Fujian Medical University Union Hospital from June 2007 to December 2013. Of these, 88 patients lacked information on inflammatory markers, such as Fb and Alb, and were excluded. As such, 2313 patients who underwent radical gastrectomy were ultimately selected as the discovery group in this study. Between January 1, 2015 and April 1, 2016, a total of 438 patients admitted to Fujian Medical University Union Hospital were recruited for the trial, and 419 patients were included in the final analysis (ClinicalTrials.gov number NCT02327481). A prospective, phase 3, randomized, controlled trial was conducted to determine whether the use of 3D laparoscopic gastrectomy would shorten the operation time compared with the use of the traditional 2D procedure. After excluding 10 patients with neuroendocrine carcinoma, 6 patients treated with palliative surgery, and 2 patients without evidence of GC, 401 patients were enrolled as a testing cohort in the present study. Between January 1, 2010 and April 1, 2014, the clinicopathological data of 448 patients from Qinghai University Affiliated Hospital were retrospectively analyzed. Overall, 197 patients with incomplete clinicopathological and follow-up data were excluded. A total of 251 patients undergoing radical gastrectomy for gastric cancer were finally selected as the external validation population in this study. The study was approved by the ethics committees of Fujian Medical University Union Hospital and Qinghai University Affiliated Hospital. All patients underwent routine preoperative imaging examinations, including chest radiography, computed tomography (CT) scanning, ultrasonography (US) of the abdomen, positron emission tomography–computed tomography (PET–CT), and endoscopic ultrasonography, as needed to evaluate the clinical stage. The eighth edition of the UICC classification system was used to assess the clinical and pathological stages.
Laboratory Measurements of Inflammation-related Factors
Blood samples from each patient were obtained before breakfast within 1 week before the surgical resection of their primary tumor. Based on thrombin clotting times, fibrinogen was assayed according to the Clauss method using Datafai Fibrinogen (Sysmex Corporation) and a CA7000 Analyzer (Sysmex Corporation). Serum albumin was measured using the bromocresol green (BCG) dye method. Based on the comprehensive analysis of previous studies on inflammatory indicators related to the prognosis of GC, this study included five traditional key inflammatory indicators (upregulated indicators during tumor progression: neutrophil, platelet, and fibrinogen; downregulated indicators during tumor progression: lymphocyte and albumin), and different combinations of these markers were used to create ten combined inflammatory parameters. The FAR was calculated as the plasma fibrinogen concentration (A; g/L) divided by the albumin concentration (B; g/L), i.e., FAR = A/B.
Follow-up Care
GC patient postoperative follow-up was performed in outpatient and inpatient manners every 3 months within 2 years, every 6 months within 3–5 years, and every year after 5 years. The vast majority of patients routinely underwent physical examinations, laboratory tests (including CA19-9, CA72-4, CEA, and CA125 levels), chest radiographs, abdominopelvic US or CT scans, and endoscopic examination every year.
Statistical Analysis
Statistical analysis was performed using MedCalc version 19.0.7 (Broekstraat 52, 9030; Mariakerke, Belgium), SPSS 22.0 (SPSS Inc., Chicago, IL) and Python software (version 3.7.0). The data are presented as the mean ± standard deviation for continuous variables and as a number for categorical variables. The distributions of each continuous and categorical variable were compared using Student’s t test, the χ2 test, or categorical Fisher’s exact test as appropriate for each variable. The random forest method is a machine learning algorithm that uses input samples of class markers to build a class prediction model and sorts the input variables according to the degree of association with classification. After training the random forest model, the importance of each feature can be obtained by directly calling the feature importance attribute. X-tile software is used to determine the best cutoff point for continuous variables and convert them into categorical variables. Comparison of ROC curve data was used to calculate the standard error of the AUC, and the differences between AUC values were determined. Survival estimates were calculated using Kaplan–Meier analysis, and groups were compared with the log-rank test. Overall survival (OS) was measured from the date the patient underwent surgery until the date of death from any cause or the last known follow-up date for patients who survived. Cox proportional hazards models were used to estimate hazard ratios (HRs) for death or recurrence. All p values were two-sided, and values less than 0.05 were considered statistically significant.
Results
Demographic and Clinicopathological Characteristics
In this study, there were a total of 2313 patients with GC in the discovery cohort, including 1728 males and 585 females. The numbers of patients whose tumors were located in the lower, middle, or upper layers or within multiple layers of the stomach were 1112 (48.1%), 377 (16.3%), 576 (24.9%), and 248 (10.7%), respectively. Overall, 615 patients (26.6%) had stage I disease, 473 patients (20.4%) had stage II disease, and 1,225 patients (53.0%) had stage III disease in the discovery cohort. A total of 724 patients received postoperative adjuvant chemotherapy (Supplementary Table 1). Overall, 401 GC patients, including 271 men and 130 women, were enrolled in the test cohort of our center. The numbers of patients with tumors in the lower, middle, and upper sites or within multiple sites of the stomach were 183 (45.6%), 69 (17.2%), 112 (27.9%), and 37 (9.3%), respectively. A total of 135 (33.7%) patients had stage I disease, 84 patients (20.9%) had stage II disease, and 182 patients (45.4%) had stage III disease in the testing cohort (Supplementary Table 2). The external validation cohort included a total of 251 patients with GC, including 182 men and 69 women. Fifty-one patients (20.3%) had stage I, 71 patients (28.3%) had stage II, and 129 patients (51.4%) had stage III disease in the external validation cohort (Supplementary Table 3). The median follow-up times for the discovery cohort, the test cohort, and the external validation cohort were 56, 42, and 71 months, respectively.
Accuracy in Predicting the Long-Term Prognosis of GC Patients
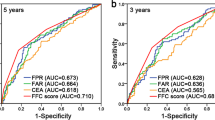
Five key inflammatory indicators (neutrophil, platelet, fibrinogen, lymphocyte, and albumin) were included in the study, and combination indicators were established according to the above methods (Fig. 2). Analysis of the ROC curves showed that several inflammatory indicators could well predict OS in patients with GC, but the newly developed indicator FAR showed the highest accuracy for predicting the OS of GC patients (Supplementary Fig. 1). According to ROC analysis, the FAR combination was better at predicting patients’ OS and had a better AUC value than the other indexes, with a statistically significant difference (P < 0.01; Fig. 3). Features were extracted from all preoperative inflammatory indicators and combined indicators, a random forest model was established, and the importance of features in predicting the overall survival rate of gastric cancer patients was ranked. The results elucidated that the importance of FAR was higher than that of other indicators. The Pearson correlation test suggested that there was no significant correlation between FAR and other combination indexes except fibrinogen-lymphocyte ratio (FLR) and Platelet X Fibrinogen (PXF) (Supplementary Fig. 2). Based on these findings, we further evaluated the utility of the preoperative FAR, its prognostic impact in GC patients, and its value as a biomarker.
Schematic chart for the combination of systemic inflammatory factors in the discovery cohort. Two inflammatory factors down-regulated during disease development: lymphocyte, albumin. Three inflammatory factors upregulated during disease development: neutrophil, platelet, fibrinogen. Ten combined inflammatory factors to find the highest accuracy to predict oncological outcomes in GC patients
Comparison of ROC curves analysis to evaluate the predictive value of each combination marker for OS in GC patients in the discovery cohort. Comparison of ROC curves analysis showed that AUC value of FAR was significantly higher than almost all of combination markers for poor OS in GC patients. All statistical tests were two-sided. *P < 0.05
High FAR Related to Aggressive GC in the Discovery Cohort
X-tile software was used to determine the optimal cutoff value for the FAR (0.10), and patients with FAR ≤ 0.10 were categorized into the low FAR group. The patients with FAR > 0.10 were categorized into the high FAR group (Supplementary Fig. 3). In the discovery cohort, the correlation between the FAR and the clinicopathological data of patients was further analyzed. Patients in the high FAR group showed more advanced pathological stages, larger tumor diameters, and more poorly differentiated pathological tumors than those in the low FAR group, with statistically significant differences (all P < 0.05) (Supplementary Table 4). The proportion of patients in the high FAR group increased with progressing N, T, and TNM stage ((Supplementary Fig. 4). Similarly, the kernel density estimation curve confirmed that the FAR was a continuous variable that increased with increasing postoperative pathological stage in GC (Supplementary Fig. 5). Logistic regression analysis showed that in addition to tumor diameter, tumor differentiation, and tumor location, FAR was an independent risk factor for lymph node metastasis and tumor invasion of GC (Table 1).
High FAR Associated with a Poor OS in the Discovery Group
The Kaplan-Meier survival curve showed that in the discovery group, the OS of patients with a low FAR was significantly higher than that of patients with a high FAR, and the difference was statistically significant (P < 0.001; Fig. 4a). Stratified analysis according to pTNM stage showed that there was no difference in OS between patients with a low FAR and patients with a high FAR in stage I (P = 0.358), whereas the OS of patients with a low FAR was significantly higher than that of patients with a high FAR in stage II-III (P < 0.001; Supplementary Fig. 6). Based on the Cox univariate proportional hazards analysis, age ≥65 years, body mass index (BMI) <25 kg/m2, high Charlson score, high ASA score, presence of lymphovascular invasion, complications, tumor diameter ≥45 mm, tumor location in the middle-upper stomach, advanced pT stage, presence of LN metastasis, undifferentiated grade, and high FAR were associated with poor OS. Upon the multivariate analysis, in addition to age ≥65 years, BMI <25 kg/m2, tumor diameter ≥45 mm, advanced pT stage, and presence of LN metastasis, high FAR was an independent prognostic factor for OS in GC patients within the discovery cohort (Table 2). The relationship between the FAR and OS was not modified by other usual inflammatory factors (Supplementary Table 5).
High FAR Associated with Patient Clinicopathological Characteristics in the Test Cohort and the External Validation Cohort
To verify the clinical significance of the FAR in predicting the long-term survival of GC patients and its potential as a biomarker, patients from an independent prospective database of our center were used as a test cohort. Patients from a retrospective database of the Affiliated Hospital of Qinghai University were used as the external validation group. The cutoff threshold for the FAR was consistent with that used in the discovery group. First, the correlations between clinicopathological factors of gastric cancer patients and preoperative FAR were evaluated in the test group. The results showed that the high FAR group showed more advanced pathological stages, larger tumor diameters, more poorly differentiated pathological types, and more tumor recurrence than the low FAR group. The results also were confirmed in the external validation group (Supplementary Tables 6, 7). In the test group, the proportion of patients in the high FAR group increased with increasing N stage, T stage, and TNM stage (Supplementary Fig. 7). According to the multivariate logistic regression analysis, in addition to tumor diameter ≥45 mm, undifferentiated tumor type, and upper-middle site, high FAR was a risk factor for local invasion of gastric cancer (pT2-4) and lymph node metastasis (pN+) (all P < 0.05; Supplementary Table 8). In the external validation group, with increasing T stage and TNM stage, the proportion of patients in the high FAR also group increased. These findings were consistent with the findings in the discovery cohort.
Prognostic Value of the FAR in Validation Cohorts
The Kaplan-Meier survival curve showed that the OS of patients with low FAR was significantly higher than that of patients with high FAR in the test cohort and the external validation cohort (P < 0.001 and P = 0.017, respectively) (Fig. 4b, c). Based on a Cox univariate proportional hazards analysis, presence of lymphovascular invasion, adjuvant chemotherapy, tumor diameter ≥45 mm, advanced pT stage, presence of LN metastasis, undifferentiated grade, and high FAR were associated with poor OS. In the multivariate analysis, in addition to tumor diameter ≥45 mm, adjuvant chemotherapy, age ≥65 years, advanced pT stage, and presence of LN metastasis, high FAR status was an independent prognostic factor for OS in GC patients within the testing cohort, while high FAR status was not an independent prognostic factor for RFS in GC patients (Supplementary Table 9). In the multivariate analysis of the external validation cohort, in addition to presence of lymph node metastasis, high FAR status was an independent prognostic factor for OS in GC patients (Supplementary Table 10).
Discussion
Although gastrectomy with adequate LN dissection can improve survival in patients with GC, the OS of patients remains poor.15 Several studies have confirmed that preoperative hematological indicators can effectively predict the long-term prognosis of GC patients, but no studies have assessed which indicators are more effective in predicting the long-term survival of GC patients.16,17,18,19,20,21 In this study, we systematically and comprehensively investigated the effects of multiple systemic inflammatory factor combinations on the long-term prognosis of GC patients. The ROC analysis found that the combination of preoperative fibrinogen and albumin levels, named FAR, was a more reliable indicator predicting poor prognosis of GC patients than other combinations of inflammatory markers. Investigators have demonstrated that the preoperative FAR is an independent prognostic factor in esophageal squamous cell carcinoma patients.22,23,24,25 Studies by Yao Liang et al. have noted that the preoperative FAR is associated with tumor progression and can be considered an independent factor for OS in resected soft tissue sarcoma patients.26 Fibrinogen is an acute phase systemic inflammatory glycoprotein synthesized by liver epithelial cells that can enhance the adhesion between cells, connect malignant cells and vascular endothelium, and thus can promote tumor progression and metastasis.27,28 Hyperfibrinogenemia is thought to be associated with excessive production of inflammatory cytokines in malignant cells, suggesting that inflammatory responses and aggressive tumor behavior may be reflected in fibrinogen levels.29,30 In 1975, Brajerski reported that increased fibrinogen levels could be observed in 67% of GC patients and that preoperative fibrinogen levels were associated with tumor progression and metastasis.31 Because advanced gastric cancer often is associated with the inflammatory response, high fibrinogen levels in patients with LN metastasis may be secondary to the increased systemic inflammatory response caused by tumor progression.32 Masaaki Yamamoto et al. concluded that the preoperative plasma fibrinogen level had the highest predictive value for recurrence compared with other known prognostic markers and was useful for predicting prognosis after gastric cancer surgery.33 A retrospective study from Slovakia elucidated that fibrinogen levels were associated with LN involvement and overall survival in GC patients.32 Albumin, as a chronic phase protein, is an indicator of the nutritional status of the host and a marker of systemic inflammation. Hypoalbuminemia is associated with poor prognosis for a variety of cancers, including gastric, lung, and colon cancers.34 Malnutrition weakens the immune system, increases the chance of infection, and further accelerates the progression of malignant tumors.35
In this study, five key blood indicators (neutrophil, platelet, fibrinogen, lymphocyte, and albumin) and their combinations were analyzed. Compared with other preoperative hematological indicators and combination indicators, the FAR had the highest predictive accuracy for OS. We evaluated the clinical significance of our newly developed FAR using a large cohort of GC patients and demonstrated that higher FAR levels were significantly correlated with a number of important clinicopathological parameters shown to be predictive of worse outcomes. In this study, FAR is a composite index composed of fibrinogen and albumin, including tumor factors and patient factors. Therefore, patient factors, such as age, gender, BMI, and comorbidities, are related to FAR. However, stratification analysis based on age, BMI, and comorbidities confirmed that the OS of patients with high FAR was significantly worse than that of patients with low FAR in each subgroup. The trend of survival curve was consistent with that of the whole group. The FAR was treated as a continuous variable in the discovery cohort, with patients grouped into high and low FAR groups according to a defined cutoff value. This cutoff value was used for subsequent analyses of the test and external validation cohorts, and the FAR was found to be associated with important clinicopathologic data and predictive of overall survival. In the discovery cohort of this study, the logistic regression analysis showed that a high FAR was an independent risk factor for local invasion and lymph node metastasis of gastric cancer, which were verified in the test cohort from our center. In this study, FAR is a composite index composed of fibrinogen and albumin, including tumor factors and patient factors. Therefore, patient factors, such as age, gender, BMI, and comorbidities are related to FAR. However, stratification analysis based on age, BMI, and comorbidities confirmed that the OS of patients with high FAR was significantly worse than that of patients with low FAR in each subgroup, and the trend of survival curve was consistent with that of the whole group (Supplementary Fig. 8). TNM stage is used to assess the risk of postoperative recurrence of gastric cancer and long-term prognosis, but patients of different TNM stages can have the same predicted prognosis, indicating that heterogeneity exists. This highlights that more reliable biomarkers for predicting the long-term survival of patients with GC and to identify people at high risk are urgently needed, as patients at high risk need aggressive chemotherapy and close follow-up. A high preoperative FAR could predict prognosis in terms of OS independent of pathological stage in GC patients undergoing surgery. All of these findings regarding preoperative FAR utility were successfully validated in an independent testing and external validation cohort. Patients with an elevated FAR may require additional neoadjuvant therapy or more intense adjuvant chemotherapy to reduce the risk of recurrence. The value of FAR may be applied to select the appropriate regimens and/or cycles of neoadjuvant chemotherapy. The FAR has the advantage of being inexpensive, repeatable, and standardized, thus offering reduced costs and increased convenience for prognostication.
There are some potential limitations in this study. First, in this study, we only focused on five inflammatory factors and their combinations and then selected the best indicator, the FAR, for predicting overall survival in patients with GC. The test and external validation cohorts used a consistent cutoff value to successfully validate the clinical value of the preoperative FAR, but the accuracy and sensitivity of the FAR as a biomarker for screening in actual clinical environments may not be sufficient. Second, the testing cohort from prospective clinical trials has excluded patients who underwent neoadjuvant chemotherapy. In addition, as subjects of discovery cohort in this study are patients from 2007 to 2013, among whom few received neoadjuvant chemotherapy, this study, pitifully, does not gain the chance to study FAR in patients receiving neoadjuvant chemotherapy. Third, due to limitations of the retrospective data in the modeling group, CRP and IL-6, two important inflammatory indicators, were not included in this study for comparative analysis. Therefore, further studies should include a broader range of hematological indicators or other scores to screen for more reliable markers. Although we have successfully validated the study results through test and external cohorts, the sample sizes of the two validation cohorts are still relatively small. Baseline data, such as general clinicopathological characteristics of patients, differ among these populations. Therefore, larger, multicenter, prospective trials, especially in populations including different races and nationalities, are required to confirm the clinical effectiveness of the FAR and assess its abilities to predict long-term survival in patients with GC, identify the long-term prognosis of high-risk groups, and support individualized treatment strategies.
Conclusions
Our systemic and comprehensive analysis identified the ratio of fibrinogen to albumin, namely, the FAR, as an easily calculated, new systemic inflammatory score. It was a promising biomarker for predicting long-term prognosis in GC patients. Quantification of the preoperative FAR may help to design more effective perioperative management strategies, such as neoadjuvant chemotherapy or doublet adjuvant chemotherapy strategies, and postoperative oncological follow-up strategies in GC patients, supporting an ultimate goal of individualized treatment.
References
Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917.
Jemal A, Bray F, Center MM, et al. Global cancer statistics, 2012. Ca Cancer J Clin. 2011;61:69–90.
Dikken JL, Jansen EPM, Cats A, et al. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol. 2010;28:2430–6.
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7.
Hanahan D et al. Hallmarks of cancer: the next generation. Cell. 2011.
Diakos CI, Charles KA, Mcmillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493-503.
Colotta F, Allavena P et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009.
Urabe M, Yamashita H, Seto Y. Pretreatment neutrophil to lymphocyte ratio independently predicts disease-specific survival in patients with resectable gastroesophageal junction and gastric cancer. Ann Surg. 2016;1.
Hsu JT, Wang CC, Le PH, et al. Lymphocyte-to-monocyte ratios predict gastric cancer surgical outcomes. J Surg Res. 2016;284-90.
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45.
Dolan RD, Mcsorley ST, Park JH, et al. The prognostic value of systemic inflammation in patients undergoing surgery for colon cancer: comparison of composite ratios and cumulative scores. Biochim Biophys Acta Molec Cell Res. 2018;25:34.
Dolan RD, Lim J, Mcsorley ST et al. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: Systematic review and meta-analysis. Scientific Rep. 2017;7.
Ross, Dolan, Stephen, et al. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017.
Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–62.
Jian-Xian, Lin, Jun-Peng, et al. Prognostic value and association of sarcopenia and systemic inflammation for patients with gastric cancer following radical gastrectomy. Oncologist. 2019.
You X, Zhou Q, Song J, et al. Preoperative albumin-to-fibrinogen ratio predicts severe postoperative complications in elderly gastric cancer subjects after radical laparoscopic gastrectomy. BMC Cancer. 2019; 19.
Kim DK, Oh SY, Kwon HC, et al. Clinical significances of preoperative serum interleukin-6 and C-reactive protein level in operable gastric cancer. BMC Cancer. 2009;9:155.
Ikeda M, Furukawa H, Imamura H, et al. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol. 2002;9:287–91.
Zhou X, Du Y, Huang Z, et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One. 2014;9:e101119.
Shimada H, Takiguchi N, Kainuma O, et al. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13:170–6.
Matsuda S, Takeuchi H, Kawakubo H, et al. Cumulative prognostic scores based on plasma fibrinogen and serum albumin levels in esophageal cancer patients treated with transthoracic esophagectomy: comparison with the Glasgow Prognostic Score. Ann Surg Oncol. 2015;22:302–10.
Takeuchi H, Ikeuchi S, Kitagawa Y, et al. Pretreatment plasma fibrinogen level correlates with tumor progression and metastasis in patients with squamous cell carcinoma of the esophagus. J Gastroenterol Hepatol. 2010;22:2222–7.
Matsuda S, Takeuchi H, Fukuda K, et al. Clinical significance of plasma fibrinogen level as a predictive marker for postoperative recurrence of esophageal squamous cell carcinoma in patients receiving neoadjuvant treatment. Dis Esophagus. 654-61.
Zihui, Tan, Man, et al. A novel blood tool of cancer prognosis in esophageal squamous cell carcinoma: the fibrinogen/albumin ratio. J Cancer. 2017.
Yao, Liang, Wei, et al. Prognostic value of the fibrinogen/albumin ratio (FAR) in patients with operable soft tissue sarcoma. Bmc Cancer 2018.
KIM, Injune, KIM, et al. Hepatic expression, synthesis and secretion of a novel fibrinogen angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochem J. 2000.
Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. 2000;96:3772–8.
Polterauer S, Seebacher V, Hefler-Frischmuth K, et al. Fibrinogen plasma levels are an independent prognostic parameter in patients with cervical cancer. Am J Obstet Gynecol. 2009;200:e641–7.
Seebacher V, Polterauer S, Grimm C, et al. The prognostic value of plasma fibrinogen levels in patients with endometrial cancer: a multi-centre trial. Br J Cancer.
Brajerski W, Sikorska K, Bisztyga A, Ilenda M. Blood fibrinogen level in peptic ulcer and gastric carcinoma. Polish Med Sci Hist Bull. 1975;15:557.
Palaj J, Kečkéš Š, Marek V, et al. Fibrinogen levels are associated with lymph node involvement and overall survival in gastric cancer patients. Anticancer Res. 2018;38:1097.
Yamamoto M, Kurokawa Y, Miyazaki Y, et al. Usefulness of preoperative plasma fibrinogen versus other prognostic markers for predicting gastric cancer recurrence. World J Surg. 2016;40:1904–9.
Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69.
Lis CG, Gupta D, Lammersfeld CA, et al. Role of nutritional status in predicting quality of life outcomes in cancer: a systematic review of the epidemiological literature. Nutr J. 2012;11.
Author information
Authors and Affiliations
Contributions
Conception/Design: CMH, CHZ, GTL, QYC. Provision of study material or patients: GTL, YBM, QYC, QZ, CHZ, PL, JWX, JBW, JXL, CMH. Collection and/or assembly of data: QYC, QZ, GTL, PL, JWX, JBW, JXL. Data analysis and interpretation: GTL, QYC. Manuscript writing: GTL, QYC, YBM, CHZ, CMH. Final approval of manuscript: GTL, YBM, QYC, QZ, CHZ, PL, JWX, JBW, JXL, CMH.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supported by Scientific and technological innovation joint capital projects of Fujian Province (2017Y9011, 2017Y9004, 2018Y9041); Construction Project of Fujian Province Minimally Invasive Medical Center (No. [2017]171); The second batch of special support funds for Fujian Province innovation and entrepreneurship talents (2016B013); The general project of sailing fund of Fujian Medical University (2019QH1052).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lin, GT., Ma, YB., Chen, QY. et al. Fibrinogen-Albumin Ratio as a New Promising Preoperative Biochemical Marker for Predicting Oncological Outcomes in Gastric Cancer: A Multi-institutional Study. Ann Surg Oncol 28, 7063–7073 (2021). https://doi.org/10.1245/s10434-021-10027-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-10027-9