Abstract
Background
The 8th edition of the American Joint Committee on Cancer (AJCC) TNM staging system provided a specific ‘ypTNM’ stage grouping for patients with esophageal cancer.
Objective
This study aimed to evaluate the clinical utility of the AJCC 8th edition ypTNM stage grouping for patients with esophageal squamous cell carcinoma (ESCC).
Methods
We enrolled 152 patients with ESCC who underwent surgery after neoadjuvant cisplatin plus 5-fluorouracil (CF) therapy between June 2005 and December 2011. ypStage was evaluated according to the AJCC 7th and 8th editions. Predictive performance for disease-specific survival (DSS) and overall survival (OS) was compared between both editions. The prognostic significance of ypTNM stage grouping was evaluated using univariate and multivariate analyses.
Results
Revision of the AJCC 7th edition to the 8th edition was associated with a change in ypStage in 96 patients (63.2%). The AJCC 8th edition revealed a better predictive performance than the 7th edition in terms of DSS (Akaike’s information criterion [AIC] 499 vs. 513; Bayesian information criterion [BIC] 505 versus 519; concordance index [C-index] 0.725 versus 0.679) and OS (AIC 662 vs. 674; BIC 669 vs. 681; C-index 0.662 vs. 0.622). On univariate and multivariate analyses, ypStage in the 8th edition was an independent prognostic factor for both DSS and OS.
Conclusions
ypTNM stage grouping in the AJCC 8th edition provided a better predictive performance for DSS and OS than that in the 7th edition. ypStage in the 8th edition was the most reliable prognostic factor for ESCC patients who underwent surgery after neoadjuvant CF therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Radical surgery is one of the primary curative treatments for esophageal cancer; 1 however, surgery alone does not result in a sufficient outcome for patients with advanced esophageal cancer who frequently develop local or systemic recurrence.2,3 To improve the prognosis of locally advanced esophageal cancer, multidisciplinary treatment combining surgery with neoadjuvant chemotherapy and/or radiotherapy has been attempted.4,5 In Japan, according to a multi-institutional randomized controlled study, neoadjuvant chemotherapy with cisplatin plus 5-fluorouracil (CF) followed by esophagectomy with lymphadenectomy has been regarded as one of the standard treatments for patients with locally advanced esophageal squamous cell carcinoma (ESCC).6
The American Joint Committee on Cancer (AJCC) TNM staging system is most widely accepted and commonly used worldwide to predict prognosis and determine treatment strategies for cancer.7 In the revision of the AJCC TNM staging system from the 7th edition to the 8th edition, the tumor grading system for esophageal cancer was remarkably changed.8,9,10 Until the 7th edition of the AJCC TNM staging system (AJCC 7th edition), pathological stage was evaluated using the identical stage grouping regardless of the presence or absence of neoadjuvant therapy.11 The 8th edition of the AJCC TNM staging system (AJCC 8th edition) provided a specific ‘ypTNM’ stage grouping for patients with esophageal cancer who underwent neoadjuvant therapy followed by surgery.12 The TNM staging system for esophageal cancer in the AJCC 8th edition was based on a large database of the Worldwide Esophageal Cancer Collaboration (WECC), which included 43,727 patients;8,9,10 however, the advantages of the novel ypTNM stage grouping in the AJCC 8th edition have not been fully confirmed, especially in ESCC treated with neoadjuvant CF therapy.
This study therefore aimed to elucidate the clinical utility of ypTNM stage grouping in the AJCC 8th edition for ESCC patients who underwent neoadjuvant CF therapy followed by surgery.
Materials and Methods
Patient Eligibility and Data Collection
This study was approved by the Human Ethics Review Committee of Niigata University and Niigata Cancer Center Hospital. The eligibility criteria of this study were squamous cell carcinoma of the thoracic esophagus; neoadjuvant CF therapy followed by esophagectomy; and treated at Niigata University Medical and Dental Hospital or Niigata Cancer Center Hospital from 2005 to 2011. Exclusion criteria were death during the operation, and simultaneous surgical resection of concomitant cancers. Clinicopathologic features of the patients were retrospectively extracted from medical records. The conditions of pretreatment comorbidities were evaluated using the Charlson Comorbidity Index (CCI).13 The CCI was calculated by summation of the weighted scores for 19 comorbid conditions, such as liver disease or diabetes.
Treatment Strategy
The neoadjuvant chemotherapy regimen consisted of a protracted infusion of 5-fluorouracil 800 mg/m2/day on days 1–5 combined with a 2-h infusion of cisplatin 80 mg/m2/day on day 1.6 Basically, two courses of chemotherapy were planned with a 3-week interval. Surgery consisting of open esophagectomy with right thoracotomy (OE), transhiatal esophagectomy (THE), or minimally invasive esophagectomy with video-assisted thoracoscopic surgery (MIE) was performed 4–6 weeks after the chemotherapy. MIE was introduced in 2007 and was performed in patients with tumors of a relatively early stage, such as clinical stage I or II. OE and MIE were performed with either two-field (mediastinal and abdominal) or three-field (cervical, mediastinal, and abdominal) lymphadenectomy. Two-field lymphadenectomy was considered if the tumor was located at the lower-third of the esophagus. THE with abdominal and limited mediastinal lymphadenectomy was considered an option for elderly patients or patients with low respiratory function who were not eligible for OE or MIE.
Tumor Stage and Histopathological Examination
Tumor stage was evaluated according to both the AJCC 7th and 8th editions. In the AJCC 7th edition, a ‘ypT0’ classification was not provided. In this study, a primary tumor that showed complete regression after neoadjuvant chemotherapy was classified as ‘ypT0’, based on the AJCC 8th edition. Pathological histologic grade was assigned according to the areas with the highest grade. Pathological response to neoadjuvant chemotherapy was evaluated according to the proportion of viable tumor cells in the primary tumor tissues, and classified as follows: grade 3 (G3), complete disappearance of viable cancer cells; grade 2 (G2), less than one-third of viable cancer cells remained; grade 1b (G1b), one-third to two-thirds of viable cancer cells remained; grade 1a (G1a), more than two-thirds of viable cancer cells remained; or grade 0 (G0), no significant pathological response.14 Although these ypT0N0M0 cases were classified as ypStage I in the AJCC 8th edition, there was no specific staging for these cases in the 7th edition. In this study, we classified ypT0N0M0 cases as ‘Stage 0’ in the AJCC 7th edition, as with ypTisN0M0 cases according to a previous study.15
Follow-Up and Patient Survival
As follow-up after surgery, contrast-enhanced computed tomography was performed at least every 6 months to detect possible tumor recurrence. In the present study, all enrolled patients were not administered postoperative adjuvant chemotherapy, therefore we defined the date of surgery as the start of the follow-up period. Disease-specific survival (DSS) was defined as the time from surgery to death caused by ESCC-implicated disease or to the last follow-up, and overall survival (OS) was defined as the time from surgery to death from any cause or the last follow-up.
Statistical Analysis
Categorical variables were compared using the Fisher’s exact test or Chi square test, as appropriate. The prognostic significance of ypStage and clinicopathological features were evaluated using univariate and multivariate analyses for DSS and OS. Cumulative survival rates were estimated using the Kaplan–Meier method, and the differences were evaluated using the log-rank test for the univariate analysis. The Cox proportional hazards regression model was applied to the multivariate analysis calculating hazard ratios (HRs) with 95% confidence intervals (CIs). The variables with a p value < 0.05 in the univariate analysis were selected as potential co-factors for the multivariate analysis. The strength of association between the potential co-factors assessed by phi or Cramer’s V value was applied to select the co-factors for avoiding multicollinearity. All statistical analyses were performed using the PASW Statistics 23.0 software package (SPSS Japan, Tokyo, Japan). A two-tailed p-value < 0.05 was considered statistically significant. Akaike’s information criterion (AIC), the Bayesian information criterion (BIC), and the concordance index (C-index) of ypStage were calculated using the Cox proportional hazard regression model in the R programming language and environment (version 4.0.1; http://www.r-project.org/). Lower AIC and BIC values revealed more preferable predictive outcomes, whereas a higher C-index demonstrated a more accurate prognostic prediction.16,17 Comparisons of the C-index between the AJCC 7th and 8th editions for the prognostic prediction were conducted using the ‘Hmisc’ package. Internal validation for the accuracy of ypStage was performed using the calibration curve comparing ypStage predicted versus observed Kaplan–Meier estimates of survival probability under the bootstraps with 1000 resamples, using the ‘rms’ package.
Results
Characteristics of the Enrolled Patients
A total of 155 consecutive patients were eligible for inclusion in this study. Among these patients, one patient who died during the operation and two patients who underwent simultaneous surgical resection of concomitant cancers were excluded. Finally, 152 patients were enrolled in this study (Fig. 1). A summary of the patient characteristics is described in Table 1. Among the 152 patients, 25 (16.5%) were female and 127 (83.5%) were male. The median age at diagnosis was 65 years (range 47–79). Two courses of neoadjuvant CF therapy were completed in 135 patients (88.8%). Surgical procedures were OE for 102 patients (67.1%), THE for 9 patients (5.9%), and MIE for 41 patients (27.0%). The stomach was used for the conduit of reconstruction in 143 patients (94.1%), and the retrosternal route was selected in 98 patients (64.5%). Regarding residual tumor status, 135 patients (88.9%) had no residual tumor (R0) after surgery. Metastasis was classified as ypM1 in five patients (3.3%) because of the presence of supraclavicular and para-aortic lymph node metastasis in four patients (2.6%) and one patient (0.7%), respectively. Ninety-eight patients (64.5%) had tumors with lymphatic invasion and 77 patients (50.7%) had tumors with venous invasion. The median follow-up time after surgery was 88 months (range 36–146). Death from any cause at the last follow-up was observed in 72 patients (47.3%), including death from ESCC in 56 patients (36.8%).
Change in the Distribution of ypStage from the American Joint Committee on Cancer (AJCC) 7th Edition to 8th Edition
The distribution of patients classified by ypStage according to each edition of the AJCC TNM staging system is shown in Fig. 2. The revision from the 7th edition to the 8th edition was associated with a change in ypStage in 96 patients (63.2%). Among these patients, 89 (58.6%) had an increase in ypStage from the 7th to 8th edition, as follows: ypStage 0 in 5 patients was classified as I, ypStage IIB in 21 patients was classified as IIIA, ypStage IIIA in 40 patients was classified as IIIB, and ypStage IIIC due to ypT4aN1-2/T4b/N3 in 23 patients was classified as IVA. On the other hand, a decrease in ypStage was observed in only seven patients (4.6%): ypStage IIA (two patients) and IIB (five patients) in the 7th edition were classified as ypStage I in the 8th edition. These changes resulted in a remarkable decrease in the proportion of patients with ypStage II, from 30.3% in the 7th edition to 11.8% in the 8th edition, as well as an increase in the proportion of patients with ypStage IV, from 3.3% in the 7th edition to 18.4% in the 8th edition.
Characteristics in Patients with ypStage Change
The clinicopathologic features of the three groups classified according to the status of ypStage changes are shown in electronic supplementary Table S1. With the application of ypTNM stage grouping in the AJCC 8th edition, the proportion of patients with ypT4 tumors and lymph node metastases was significantly higher in the group with increased ypStage than in the other groups (p < 0.001). Furthermore, lymphatic invasion was more frequently observed in the group with increased ypStage than in the other groups (p = 0.001). A lower pathological response to neoadjuvant chemotherapy was also more frequently observed in the group with increased ypStage, but the difference was not statistically significant. The 5-year DSS rate after surgery was 54.6% in the group with increased ypStage, 71.3% in the group with no change in ypStage, and 100% in the group with decreased ypStage (p = 0.046).
Prognosis Stratified by ypStage in the AJCC 7th and 8th Editions
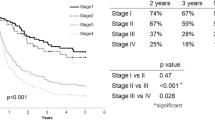
In the 152 patients included in this study, the 5-year DSS rate after surgery was 62.7%. The differences in DSS stratified by ypStage were statistically significant in the AJCC 7th (p < 0.001) and 8th editions (p < 0.001), as shown in Fig. 3 and electronic supplementary Table S2. The 5-year DSS rate for ypStage III was higher in the 8th edition than in the 7th edition (60.6% vs. 47.5%), but the 5-year DSS rate for ypStage IV was lower in the 8th edition than in the 7th edition (24.5% vs. 40.0%). As a result, ypStage in the 8th edition was more able to adequately stratify DSS, especially in advanced disease even after neoadjuvant therapy. Univariate analysis showed that ypStage in the 7th and 8th editions, as well as the additional six factors (surgical procedure, lymphadenectomy, residual tumor, lymphatic invasion, venous invasion, and histologic response), were significantly associated with DSS (Tables 2 and 3). Among the six potential co-factors, lymphatic invasion had a relatively strong association with venous invasion (Phi coefficient = 0.606, p < 0.001) and histologic response (Phi coefficient = 0.376, p < 0.001). Lymphatic invasion is a well-known, strong, unfavorable prognostic indicator for ESCC;18 thus, we excluded venous invasion and histologic response and adjusted the ypStage HRs in the 7th and 8th editions for DSS using the remaining four co-factors in the multivariate analysis. As a result, ypStage in the 7th edition was not a significant prognostic factor for DSS when adjusted for the four co-factors (Table 2). On the other hand, ypStage IV in the 8th edition (HR 19.056, 95% CI 2.332–155.714; p = 0.006) was a significant independent unfavorable prognostic factor for DSS (Table 3). Similar to DSS, ypStage in the 8th edition more adequately stratified OS (Fig. 3, electronic supplementary Table S2) compared with the 7th edition. In the multivariate analysis, ypStage in the 7th edition was not a significant independent prognostic factor for OS (electronic supplementary Table S3), whereas, multivariate analysis demonstrated that ypStage IV in the 8th edition (HR 3.547, 95% CI 1.360–9.250; p = 0.010) was a significant independent unfavorable factor for OS (electronic supplementary Table S4). The associations between the potential co-factors identified in the univariate analyses for DSS and OS are detailed in electronic supplementary Table S5.
Predictive Performance of ypStage in the AJCC 7th and 8th Editions
Regarding the predictive performance for DSS, ypStage in the AJCC 8th edition had a lower AIC (499 vs. 513) and BIC (505 vs. 519), and higher C-index (0.725, 95% CI 0.668–0.782 vs. 0.679, 95% CI 0.623–0.735, p < 0.001) than in the 7th edition (Table 4). Similar to DSS, ypStage in the 8th edition showed higher predictive performance for OS compared with the 7th edition. Calibration plots demonstrated that the predictive probability of 5-year DSS and OS of ypStage in the 8th edition were closer to the actual 5-year survival than that of ypStage in the 7th edition, especially in the high-risk population (electronic supplementary Fig. S1).
Discussion
Neoadjuvant therapies including chemotherapy and chemoradiotherapy followed by surgery are standard multidisciplinary treatments for locally advanced esophageal cancer worldwide.4,5,19 Because of the current state of the art in treatment, an important issue in esophageal cancer has been whether the pathological staging system after neoadjuvant therapy shares the same prognostic implications as that after surgery alone. The pathological data in the WECC database demonstrated that prognosis after neoadjuvant therapy followed by surgery was not equivalent to that after surgery alone in the same pathological category.10 The AJCC 8th edition was an epoch-making classification that defined ypTNM stage grouping after neoadjuvant therapy differently from pTNM stage grouping for surgery alone. In the present study, we found that ypTNM stage grouping according to the AJCC 8th edition was the most reliable prognostic indicator for ESCC patients who underwent neoadjuvant CF therapy followed by surgery.
The WECC database represents worldwide data but this imposes limitations related to heterogeneity in the etiology and pathological characterization of esophageal cancer among countries and continents.12 ypTNM stage grouping in the AJCC 8th edition was mainly based on the WECC database, which included 7773 patients with esophageal cancer who underwent neoadjuvant therapy. Although the histopathologic cell type was squamous cell carcinoma in 2045 of the included patients, only 20.0% (408/2045) of patients originated from Asian countries. Moreover, the WECC included five Chinese institutions but no Japanese institution.10 This new version of the AJCC staging system has been validated in several retrospective cohorts,15,20,21,22 but data are limited regarding its validation in Asian patient groups, particularly in Japanese patients with ESCC. In our study, we confirmed the validity of ypTNM stage grouping in the AJCC 8th edition in a Japanese cohort of ESCC patients. Additionally, chemoradiotherapy was the predominant neoadjuvant treatment option in the WECC database. Only 22.2% (454/2045) of the ESCC patients received neoadjuvant chemotherapy in the database cohort, and treatment regimens or protocols of neoadjuvant chemotherapy were not uniform among participating institutions.10 Thus, our study is novel in that we focused only on ESCC patients who underwent neoadjuvant CF therapy, which is one of the important regimens in current multidisciplinary treatments.
In this study, we found intriguing stage changes from the AJCC 7th to 8th editions. Thirty-five patients with ypStage IIB in the 7th edition, including ypT2-3/N0 and ypT1-2/N1, experienced the most diverse changes, ranging from ypStage I–IIIA in the 8th edition. Twenty-three patients with ypStage IIIC in the 7th edition, including ypT4a/N1-2, ypT4b, and ypN3, were classified as having ypStage IVA in the 8th edition. These changes clearly represent the concepts of ypTNM stage grouping in the AJCC 8th edition, emphasizing the prognostic impact of the ypT classification in patients with ypN0, and considering ypT4 or ypN1-3 even after neoadjuvant therapy as an extremely poor prognostic classification.20 Furthermore, with an increase in stage in the 8th edition, we identified specific characteristics in patients, as follows: more frequent lymphatic invasion, lower pathological response to neoadjuvant chemotherapy, and more unfavorable prognosis. Thus, ypTNM stage grouping in the AJCC 8th edition could definitely identify tumors with potential malignant behaviors after neoadjuvant therapy as advanced-stage tumors with an unfavorable prognosis.
As shown in the Kaplan–Meier analysis in Fig. 3, the overlapping survival curves of ypStage III and IV in the 7th edition were clearly distinguished in the 8th edition for both DSS and OS. Patients with a high risk of death from ESCC are more adequately classified into the advanced-stage according to ypTNM stage grouping in the 8th edition, than in the 7th edition. On the other hand, discriminative values for OS between ypStage I and II in the 8th edition were less compared with those in the 7th edition (Fig. 3d), which may be the consequence of the relatively high incidence of death from other causes in patients with ypStage I and II in the AJCC 8th edition. Indeed, among all the deaths in ypStage I and II, the rate of death from other causes in the 8th edition was higher compared with that in the 7th edition (69.2% vs. 54.5%, data not shown). Regarding the overall predictive performance for DSS and OS, ypTNM stage grouping in the 8th edition had a lower AIC and BIC and higher C-index than in the 7th edition. These results indicate that ypTNM stage grouping in the 8th edition provides superior prognostic stratification than in the 7th edition, especially in patients with advanced-stage ESCC who underwent neoadjuvant chemotherapy followed by surgery.
We evaluated the clinical utility of ypTNM stage grouping as a prognostic factor for ESCC patients undergoing neoadjuvant therapy. According to the multivariate analysis in the AJCC 8th edition, only ypStage was identified as an independent prognostic factor for both DSS and OS (Table 3 and electronic supplementary Table S4). Previous studies using the WECC database, and classifying patients according to ypTNM stage grouping in the 8th edition, showed that the prognostic effects of tumor location and histologic grade (non-anatomic categories) were less evident after neoadjuvant therapy than after surgery alone.10 Thus, ypTNM stage grouping was only based on ypT, ypN, and ypM (anatomic categories).12,23 These consistent results of no association between non-anatomic categories and both DSS and OS were identified in this present study of ESCC patients who underwent neoadjuvant CF therapy.
This study has two main limitations. First, this was a retrospective design with a small sample size; however, we enrolled 152 of 155 (98.1%) consecutive patients who underwent neoadjuvant CF therapy followed by surgery in two institutions from 2005 to 2011. Such a high inclusion rate and short inclusion period might contribute to reduced selection bias. Second, ypStage was simplified into merged stages of four tiers in the survival analyses because of the small number of patients in each ypStage group. Further large-scale, multi-institutional studies are necessary to more accurately confirm the prognostic performance of ypTNM stage grouping in the AJCC 8th edition for ESCC. Nonetheless, our findings could provide useful information for clinical practice and future clinical trials to improve the prognosis of ESCC patients.
Conclusion
This study revealed that ypTNM stage grouping in the AJCC 8th edition provided more accurate predictive values for DSS and OS than in the 7th edition, and classified patients with infiltration of other remaining organs or lymph node metastases even after neoadjuvant therapy as the worse prognostic group. Furthermore, we found ypStage in the 8th edition to be the most reliable prognostic factor for ESCC patients who underwent neoadjuvant CF therapy followed by surgery.
References
Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol. 2007;8(6):545–553.
Dresner SM, Griffin SM. Pattern of recurrence following radical oesophagectomy with two-field lymphadenectomy. Br J Surg. 2000;87(10):1426–1433.
Nakagawa S, Kanda T, Kosugi S, Ohashi M, Suzuki T, Hatakeyama K. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg. 2004;198(2):205–211.
van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084.
Mayanagi S, Irino T, Kawakubo H, Kitagawa Y. Neoadjuvant treatment strategy for locally advanced thoracic esophageal cancer. Ann Gastroenterol Surg. 2019;3(3):269–275.
Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19(1):68–74.
Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99.
Rice TW, Apperson-Hansen C, DiPaola LM, et al. Worldwide Esophageal Cancer Collaboration: clinical staging data. Dis Esophagus. 2016;29(7):707–714.
Rice TW, Chen LQ, Hofstetter WL, et al. Worldwide Esophageal Cancer Collaboration: pathologic staging data. Dis Esophagus. 2016;29(7):724–733.
Rice TW, Lerut TE, Orringer MB, et al. Worldwide Esophageal Cancer Collaboration: neoadjuvant pathologic staging data. Dis Esophagus. 2016;29(7):715–723.
Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC cancer staging manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17(7):1721–1724.
Rice TW, Ishwaran H, Kelsen DP, Hofstetter WL, Apperson-Hansen C, Blackstone EH. Recommendations for neoadjuvant pathologic staging (ypTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus. 2016;29(8):906–912.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–383.
Sato H, Tsubosa Y, Kawano T. Correlation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World J Surg. 2012;36(3):617–622.
Kroll D, Noser L, Erdem S, et al. Application of the 8th edition of the AJCC yTNM staging system shows improved prognostication in a single center cohort of esophageal carcinomas. Surg Oncol. 2018;27(1):100–105.
Fang WL, Huang KH, Chen MH, et al. Comparative study of the 7th and 8th AJCC editions for gastric cancer patients after curative surgery. PloS ONE. 2017;12(11):e0187626.
Liu K, Feng F, Chen XZ, et al. Comparison between gastric and esophageal classification system among adenocarcinomas of esophagogastric junction according to AJCC 8th edition: a retrospective observational study from two high-volume institutions in China. Gastric Cancer. 2019;22(3):506–517.
Tachimori Y, Ozawa S, Numasaki H, et al. Comprehensive registry of esophageal cancer in Japan, 2010. Esophagus. 2017;14(3):189–214.
Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098.
Zhang D, Zheng Y, Wang Z, et al. Comparison of the 7th and proposed 8th editions of the AJCC/UICC TNM staging system for esophageal squamous cell carcinoma underwent radical surgery. Eur J Surg Oncol. 2017;43(10):1949–1955.
D’Journo XB. Clinical implication of the innovations of the 8(th) edition of the TNM classification for esophageal and esophago-gastric cancer. J Thorac Dis. 2018;10 Suppl 22:S2671–S2681.
Park SY, Kim DJ, Suh JW, Byun GE. Comparison of the 11(th) Japanese classification and the AJCC 7(th) and 8(th) staging systems in esophageal squamous cell carcinoma patients. J Thorac Dis. 2018;10(8):5039–5046.
Kamarajah SK, Burns WR, Frankel TL, Cho CS, Nathan H. Validation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with pancreatic adenocarcinoma: a Surveillance, Epidemiology and End Results (SEER) analysis. Ann Surg Oncol. 2017;24(7):2023–2030.
Acknowledgment
This project was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI, Grant Number JP19K09212 for HI, and Grant Number 17K00813 for KS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclocure
Natsuru Sudo, Hiroshi Ichikawa, Yusuke Muneoka, Takaaki Hanyu, Yosuke Kano, Takashi Ishikawa, Yuki Hirose, Kohei Miura, Yoshifumi Shimada, Masayuki Nagahashi, Jun Sakata, Takashi Kobayashi, Takeo Bamba, Satoru Nakagawa, Shin-ichi Kosugi, and Toshifumi Wakai have no disclosures to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2020_9181_MOESM1_ESM.pptx
Supplementary Fig. S1 The calibration curve comparing ypStage-predicted versus observed Kaplan–Meier estimates of survival probability under the bootstraps with 1000 resamples. Disease-specific survival in the (a) 7th and (b) 8th editions, and overall survival in the (c) 7th and (d) 8th editions. (PPTX 71 kb)
Rights and permissions
About this article
Cite this article
Sudo, N., Ichikawa, H., Muneoka, Y. et al. Clinical Utility of ypTNM Stage Grouping in the 8th Edition of the American Joint Committee on Cancer TNM Staging System for Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 28, 650–660 (2021). https://doi.org/10.1245/s10434-020-09181-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-09181-3