Abstract
Objective
The aim of this retrospective study was to compare the outcomes of patients resected for intrahepatic cholangiocarcinoma (ICC) with upfront surgery or after downstaging treatment.
Methods
All consecutive patients with ICC between January 1997 and November 2017 were included in a single-center database and retrospectively reviewed. Patients were divided into two groups: upfront resection or resection after downstaging using either chemotherapy alone or selective internal radiation therapy (SIRT) combined with chemotherapy. Survival rates of patients who underwent upfront surgery for ICC were compared with those of patients who underwent surgery after downstaging therapy.
Results
A total of 169 patients resected for ICC were included: 137 underwent upfront surgery and 32 received downstaging treatment because their tumor was initially unresectable (13 received chemotherapy, 19 received SIRT). Median OS was not different between the two groups: 32.3 months [95% confidence interval (CI) 23.9–40.7] with primary surgery versus 45.9 months (95% CI 32.3–59.4) with downstaging treatment (p = 0.54, log-rank test). In a multivariable Cox regression model, downstaging treatment was not associated with a better or worse prognosis; however, delivery of SIRT as a downstaging treatment was associated with a significant benefit in multivariable analysis (hazard ratio 0.34, 95% CI 0.14–0.84; p = 0.019).
Conclusions
Overall survival of patients resected after downstaging treatment was not different compared with the OS of patients resected upfront. Patients should therefore again be discussed with the surgeon following medical treatment. SIRT may be an efficient neoadjuvant therapy in patients with resectable ICC, in order to improve surgical results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cholangiocarcinoma is a malignant tumor that arises from the extra- or intrahepatic biliary tree. Intrahepatic cholangiocarcinoma (ICC) represents 40% of all biliary tract cancers.1 Its worldwide incidence has significantly increased over the last few decades 2 and because the early disease is usually asymptomatic, most ICCs are detected when the tumors are large or multifocal with a poorer prognosis.3,4
Complete surgical resection remains the only potential curative treatment, providing an approximately 30% 5-year overall survival (OS) rate.5 Whether postoperative chemotherapy improves survival remains controversial, given a recent negative trial of gemcitabine and oxaliplatin (GEMOX) or gemcitabine versus a borderline positive trial of capecitabine.6,7,–8
Only 30–40% of ICC patients can benefit from upfront surgery.9 When ICC remains localized to the liver and is not initially resectable, the role of downstaging treatments remains unclear. Guidelines from the International Liver Cancer Association stipulate that locoregional treatments are optional for locoregional ICC.10 A recent retrospective analysis suggested that patients who had ICC downstaged with preoperative chemotherapy could have long-term outcomes similar to those treated with upfront surgery.11 In this setting, selective internal radiation therapy (SIRT), also known as Yttrium-90 microsphere radioembolization, has given promising results.12,13,14,15,16,17,–18 In a phase II multicentric trial, downstaging to surgery was shown in 9 of 41 initially unresectable patients with improved survival.19
Whether surgery in patients downstaged after medical therapy may be beneficial to patients with initially unresectable ICC remains unclear. The aim of the present study was to compare the survival of patients who were resected either upfront or after downstaging with chemotherapy or SIRT.
Methods
Selection of Patients
All patients with ICC who underwent liver resection with curative intent at Pontchaillou Hospital, Rennes, France, between January 1997 and November 2017 were selected from a prospectively maintained ICC database and were reviewed retrospectively.
Selected patients had pathologically proven ICC, an Eastern Cooperation Oncology Group performance status of ≤ 2, adequate liver function, and no extrahepatic disease at the time of resection. Patients with mixed hepatocholangiocellular carcinoma, as well as patients who had incomplete macroscopic (R2) resection, were excluded from the analysis. Prior to treatments, all selected cases were reviewed by a Multidisciplinary Board that included expert hepatobiliary surgeons and nuclear medicine physicians. Resectability was defined at presentation according to clinical condition, liver function test results, tumor size and location, vascular involvement, intrahepatic metastasis, lymphadenopathy, and extrahepatic metastasis.
The selected patients had either upfront resection or delayed resection after downstaging due to initially unresectable disease. Downstaging treatments included chemotherapy alone or SIRT plus chemotherapy. Three patients whose ICC was downstaged using external radiotherapy or transarterial chemoembolization were excluded from the study. One patient with a mistaken diagnosis (ICC was confused with breast cancer hepatic metastasis, having initially received breast cancer-directed chemotherapy) was also excluded from the studied population.
Treatment Received
Eligibility criteria for upfront resection were tumors amenable to complete extirpation regardless of margin width and leaving an adequate volume of liver tissue (minimum of two contiguous Couinaud liver segments representing at least 30% of the total liver volume) with adequate perfusion, venous, and biliary drainage. All surgical procedures were performed by one senior surgeon (KB). After exploration of the abdominal cavity to exclude a contraindication to liver resection (extrahepatic disease, distant lymphadenopathy, or unresectable lesions in the future remnant liver), intraoperative ultrasonography was performed to ensure resectability of the tumor. Vascular and biliary reconstructions were performed when necessary. Major liver resection was defined as the resection of three or more liver segments. The evidence of hilum lymph node invasion via frozen section biopsy at the time of laparotomy would undermine the usefulness of an excision. The extrahepatic bile duct was resected when necessary and bilioenteric anastomosis using a Roux-en-Y was performed.
Patients who did not complete criteria for upfront resection were offered primary local or systemic treatment with chemotherapy and/or SIRT. The choice of treatment mostly depended on the availability of SIRT, due to the period of treatment (SIRT was available from 2008 in our center) and the center referring the patient (patients from referral centers could have received chemotherapy in their referral center and be discussed at our center after downstaging). The time between the last chemotherapy cycle and the surgery was at least 1 month. Unresectability was eventually decided when liver remnant volume was insufficient or when the tumor was close to the main vascular structures of the remnant liver. Patients with extrahepatic metastases or distant lymphadenopathy were considered definitively to have unresectable ICC (metastatic ICC).
From 2008, patients who were primarily referred to our center were offered SIRT as frontline downstaging treatment. SIRT was used mostly concomitantly or after chemotherapy. The SIRT therapeutic procedure was performed as previously described.20 Briefly, at the end of the diagnostic angiography, 99mTc-macroaggregated albumin was injected selectively in the right, left, or segmental hepatic arterial branch to assess the percentage of pulmonary shunting and confirm the absence of digestive uptake. SIRT was performed 8–15 days later during a second angiography, using Yttrium-90 glass microspheres. Patients with unilobar disease were submitted to unilobar injection. When possible, in case of bilobar disease, the injection was administered in such a way so as to spare the potential liver remnant (e.g. by injecting the right artery, then, selectively, the segment 4 artery, sparing segments 2 and 3 rather than a whole-liver approach). Activity injected was calculated with the aim of administering a dose of 120 ± 20 Gy to the injected liver volume without exceeding a cumulative dose of 30 Gy to the lungs. However, in the case of segmental or bi-segmental injection, the dose to the segment could be higher than 120 Gy, as previously described.21 To obtain the injected activity, the injected liver volume was calculated from single-photon emission computed tomography/computed tomography (SPECT/CT) data, and not from the angiographic and CT data usually used, as previously described.22 When patients received concomitant gemcitabine and SIRT, the dose of gemcitabine was reduced to 300 mg/m2 for the cycles preceding and after SIRT by analogy to the recommended dose for concomitant chemoradiotherapy in pancreatic cancer.23 Concomitant chemotherapy was administered on the day before or the day after SIRT, but not on the same day.
The first preoperative treatment was chemotherapy alone. Seven different chemotherapy regimens were administered; the modified LV5FU2–cisplatin regimen consisted of cisplatin at 50 mg/m2 on day 1, 5-fluorouracil bolus at 400 mg/m2 on day 1, and 5-fluorouracil continuous infusion at 2400 mg/m2 at 46 h, every 2 weeks; the capecitabine–cisplatin regimen consisted of cisplatin at 80 mg/m2 on day 1 and oral capecitabine at 1000 mg/m2 twice daily on days 2–15, every 3 weeks; the cisplatin regimen consisted of cisplatin 80 mg/m2 every 3 weeks; the GEMOX regimen consisted of gemcitabine 1000 mg/m2 on day 1 and oxaliplatin 100 mg/m2 on either day 1 or 2, every 2 weeks; the gemcitabine plus cisplatin (GEMCIS) regimen consisted of cisplatin 25 mg/m2 on days 1 and 8 and gemcitabine 1000 mg/m2 on days 1 and 8, every 3 weeks; the FOLFIRINOX regimen consisted of oxaliplatin 85 mg/m2 on day 1, irinotecan 180 mg/m2 on day 1, 5-fluorouracil bolus at 400 mg/m2 on day 1, and 5-fluorouracil continuous infusion at 2400 mg/m2 at 46 h, every 2 weeks; and the capecitabine regimen consisted of oral capecitabine at 1000 mg/m2 twice daily on days 2–15, every 3 weeks.
Follow-Up Assessments
All patients underwent a CT scan before the initiation of downstaging treatments and were closely monitored with physical examinations, biology tests, and evaluations of the adverse effects after each step of treatment. Secondary resection was assessed by the same Board who had estimated the initial unresectability. Response was evaluated using the Response Evaluation Criteria for Solid Tumors (RECIST) 1.1 criteria. After resection, patients underwent CT scans every 3–6 months over a period of 5 years. Clinicopathological, intraoperative, and postoperative data were collected.
Statistical Analysis
The primay endpoint of the study was the impact of downstaging treatment on patient OS, while the secondary endpoints were the impact of the different downstaging treatments on OS and recurrence-free survival (RFS).
Baseline characteristics are presented as mean (standard deviation) or median (range) (minimum–maximum) and 95% confidence interval (CI) for continuous variables, as appropriate, and were compared using Student’s t test or Mann–Whitney U test, respectively. Categorical parameters are expressed as the number of patients and associated percentages. Comparisons of patient variables between the upfront and downstaging groups were performed using the Chi square or Fisher’s exact test.
Endpoint criteria (OS and RFS) were calculated from the date of surgery to the date of the event (death or recurrence, respectively), and survival rates were determined using the Kaplan–Meier method. Multivariable analysis for prognostic factors was performed using Cox proportional hazards regression of the preoperative and intraoperative factors considered significant at univariable analysis (entry in the model for p <0.1). Results were expressed as hazard ratios (HRs) with 95% CIs. Statistical analysis was performed using SPSS software version 18 (IBM Corporation, Armonk, NY, USA).
Results
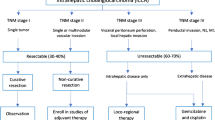
A total of 169 patients who underwent resection for ICC between January 1997 and November 2017 were enrolled in the study. Among these patients, 137 (81%) were defined as having resectable tumors and underwent upfront surgery, and 32 (29%) were resected after downstaging therapy. Downstaging therapy was chemotherapy alone or SIRT with chemotherapy in 13 and 19 cases, respectively. The study flow chart is shown in Fig. 1. Baseline characteristics and clinical data of the studied groups are reported in Table 1. Non-resectability details for the SIRT group are reported in electronic supplementary Table 1.
Downstaging treatment is described in Table 2. After downstaging treatment, tumoral response was evaluated using RECIST 1.1 criteria; investigators identified five stabilities (17%) and 24 responses (83%, all partial responses). In cases of RECIST stable disease, a decrease of < 30% of lesions was seen, allowing for reconsideration of surgery.
There was no biliary tree injuries post-SIRT, such as intrahepatic biliary strictures or bilomas. The median time between the diagnosis of non-primary resectability and secondary surgery after downstaging treatment was 5.8 months; the mean time between the start of downstaging treatment and surgery was 4 months.
Major hepatectomies were performed in 135 cases (79.9%). Details of the surgical procedures are reported in electronic supplementary Table 2. In addition, no biliary-enteric anastomoses were performed that were not initially planned.
Table 3 compares the demographics and pathological characteristics of patients between the treatment allocation groups. Patients who had surgery after chemotherapy were younger (p = 0.034) than patients in the two other groups. The extent of surgery (major hepatectomy required, number of segments resected) was greater in patients resected following downstaging. R1 resection was seen in 12% of patients resected after SIRT, 39% of patients resected after chemotherapy, and 22% of patients with upfront resection, but the difference was not statistically significant (p = 0.174).
Postoperative complications are detailed in electronic supplementary Table 3. There was no difference in overall morbidity, except the rate of pulmonary complications was significantly higher in the chemotherapy-alone group [3/13 (23.1%)] compared with the SIRT [0/18 (0%)] or upfront surgery groups [5/135 (3.7%)] (p = 0.005). The 90-day mortality was 7/137 (5.1%) in the upfront surgery group, 0/13 (0%) in the chemotherapy-alone group, and 2/19 (10.5%) in the SIRT group (p = 0.414).
Median follow-up was 85.5 months (97.6 months for patients in the upfront surgery group, not reached but > 133 months in the chemotherapy group versus 44.0 months in the SIRT group; p < 0.01). During follow-up, 117 patients (69.2%) died. Median OS for the entire cohort was 35.8 months (95% CI 25.8–45.8). There was no significant difference in OS between the two cohorts: 32.3 months (95% CI 23.9–40.7) with primary surgery versus 45.9 months (95% CI 32.3–59.4) with downstaging treatment (p = 0.537) (Fig. 2a). In the chemotherapy group, the median OS was 36 months (95% CI 0.4–71.7) and no difference was observed compared with the upfront resection-alone group (p = 0.43). In the SIRT group, the median OS was not reached but compared favorably with the upfront resection group, although the difference did not reach significance (p = 0.11) (Fig. 2b). Median RFS was 11.3 months (95% CI 7.4–15.3) in the upfront surgery group, 7.2 months (95% CI 0–21.8) in the chemotherapy group, and 18.5 months (95% CI 4.7–32.2) in the group treated with SIRT before surgery (log-rank p = 0.28) (Fig. 3). Recurrence was seen in the liver in 45 (33%) patients treated with upfront surgery, 6 (46%) patients in the chemotherapy group, and 4 patients (21%) in the SIRT group, but the differences were not statistically significant (p = 0.21).
Univariable analysis of variables affecting OS are reported in Table 4. There was no significant difference in OS between patients operated upfront and patients operated following downstaging treatment. Age, extent of hepatectomy, tumor size, number of tumors, and lymph node involvement on histological examination of the specimens were significantly associated with poorer prognosis. In multivariable analysis, when downstaging treatment was entered in the model along with variables associated with OS in univariable analysis (p < 0.1), downstaging treatment was not associated with a better or worse prognosis. However, when focusing on the type of downstaging treatment, delivery of SIRT was associated with a significant benefit, with an HR of 0.34 (95% CI 0.14–0.84) (p = 0.019).
Discussion
Surgical resection of ICC remains the mainstay of potentially curative therapy, with 5-year disease-free survival at 20–25%.5 However, in patients initially unresectable, it is not clear whether surgery offers similar survival when downstaging was achieved following medical treatment. The aim of the present study was to compare the postoperative evolution of ICC patients who were readily operated, with ICC patients who were operated after downstaging. The main result of this study is that patients downstaged and patients initially resectable had similar prognosis. The other important result is that SIRT-based downstaging is suggested to improve postoperative survival compared with chemotherapy-alone downstaging, or even upfront surgery. We thus propose that surgery should be discussed following response to medical treatment in ICC.
A study by Le Roy et al. 11 conducted in 2018 evaluated the outcomes of patients downstaged following chemotherapy. The authors showed that patients with secondarily resectable ICC had the same survival as patients who were resected upfront. In the population of initially unresectable but localized ICC patients, a group of potentially resectable patients should be individualized, following a similar strategy as is applied to patients with initially unresectable colorectal liver metastases.24
There are no recommended downstaging treatments for ICC to date, and different potential strategies may be applied, i.e. chemotherapy, SIRT, or a combination of SIRT and chemotherapy. Other locoregional approaches could also be discussed but were not reported to achieve downstaging in ICC. Cisplatin plus gemcitabine has been shown to be associated with a significant survival advantage over gemcitabine alone.25,26 In a short series of patients with initially unresectable ICC, patients with huge tumors were amenable to complete resection and substantial survival benefit after pretreatment with SIRT alone or SIRT plus chemotherapy.27 We previously published the results of our patients treated with SIRT in regard to the potential for downstaging. In the retrospective analysis of patients across different lines of treatment, we found that 12/64 (19%) patients could be resected following SIRT, when focusing on first-line patients; 11/24 (46%) were downstaged to surgery.14,15 In the prospective, multicentre, phase II MISPHEC study, this proportion was 22% (30% when the tumor was limited to a hemi-liver).19
In our series, there was no difference in terms of postoperative complications in each group. We noticed that in the SIRT group, even if the surgery was more challenging for large tumors regarding adhesions between the liver tumor and the diaphragm, the necrosis induced by SIRT 28 could help the surgeon by delineating tumor margins, avoiding tumoral cell spread during manipulation,27 and reducing blood loss.
Furthermore, SIRT induces liver hypertrophy contralateral to the tumor and tumor necrosis. Potential downstaging of the chemotherapy-only regimen is not well-described in the literature because limited case reports exist.
There was no difference in survival outcomes and RFS despite the initial unbalanced characteristics of the patients reflecting two different populations. In the downstaging group, patients had worse baseline characteristics, especially in the SIRT group (higher number of segments involved, higher frequency of macrovascular invasion), which translated into a more frequent requirement for major hepatectomy. Despite these initially worse characteristics, overall survival was similar to that of patients with upfront surgery. One important conclusion is that surgery should be discussed again for patients with locally advanced disease responding to medical treatment, even if patients were initially considered unresectable by the surgical team and did not immediately conclude palliative treatment in those situations. Two downstaging treatments were evaluated—chemotherapy and SIRT. Multivariable analysis has shown a benefit with SIRT as downstaging treatment in terms of survival compared with patients treated by chemotherapy or upfront surgery. This study included patients treated before the results of the BILCAP trial 7 were released, and partly during conduct of the PRODIGE 12 study in France.6 The PRODIGE 12 study tested GEMOX in an adjuvant setting, similar to most of the chemotherapy used in the downstaging setting in this study. The PRODIGE 12 failed to show the benefit of GEMOX in an adjuvant setting.
The results suggest that there could be a benefit to include SIRT before surgery in a neoadjuvant strategy of ICC, either in unresectable disease, such as the patients presented in this study, or perhaps also for resectable disease. This must be confirmed by prospective studies. The SIRCCA phase III trial (ClinicalTrials.gov identifier NCT02807181) 29 randomized patients with unresectable ICC to either chemotherapy alone or SIRT followed by chemotherapy. Unfortunately, the study closed early due to slow accrual; however, information on whether SIRT could improve the probability of downstaging in this context may be available, even if the study lacked the power to demonstrate OS improvement.
Our study has some limitations. This was a retrospective, single-center study, and some patients were referred for surgery from other institutions after different types of treatment for their initially unresectable tumors; we were unable to compare the probability of downstaging as all patients treated with chemotherapy were not followed in our institution. In addition, this study was subject to selection bias because it was considered patients underwent surgery by the same multidisciplinary team of expert liver surgeons, Furthermore, there are limited data on the subgroup of patients who failed to be successfully downstaged because most patients had been followed-up at other centers. Nonetheless, the aim of this study was not to evaluate the performances of downstaging treatments.
Moreover, the selection of patients who underwent surgery after neoadjuvant therapy could have been biased since patients were resected only if the tumor had downstaged. The time between downstaging treatment and surgery could also have allowed the withdrawal of patients with early disease progression. However, this could be considered an interesting point in the adjuvant therapy approach, avoiding unnecessary surgery in patients with rapid extrahepatic progression.
The retrospective nature of this study exposes other biases, i.e. the small sample size of the chemotherapy-alone and SIRT groups induces difficulty in interpreting the findings.
Conclusions
This study suggests that when ICC cannot be resected upfront, secondary resectability can be obtained after downstaging treatment, without a reduction in survival probability. Moreover, SIRT appears to be associated with the potential for long-term benefit. These results highlight the potential for multimodality treatment for ICC, allowing for potential curative approaches. The role of neoadjuvant treatment in this population needs to be clarified in prospective studies.
References
Al Mahjoub A, Bouvier V, Menahem B, Bazille C, Fohlen A, Alves A, et al. Epidemiology of intrahepatic, perihilar, and distal cholangiocarcinoma in the French population. Eur J Gastroenterol Hepatol. 2019;31(6):678–84.
Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–44.
Doussot A, Gonen M, Wiggers JK, Groot-Koerkamp B, DeMatteo RP, Fuks D, et al. Recurrence patterns and disease-free survival after resection of intrahepatic cholangiocarcinoma: preoperative and postoperative prognostic models. J Am Coll Surg. 2016;223:493–505.e2.
Spolverato G, Kim Y, Alexandrescu S, Popescu I, Marques HP, Aldrighetti L, et al. Is hepatic resection for large or multifocal intrahepatic cholangiocarcinoma justified? Results from a multi-institutional collaboration. Ann Surg Oncol. 2015;22:2218–25.
Sulpice L, Rayar M, Boucher E, Pracht M, Meunier B, Boudjema K. Treatment of recurrent intrahepatic cholangiocarcinoma. Br J Surg. 2012;99:1711–7.
Edeline J, Benabdelghani M, Bertaut A, Watelet J, Hammel P, Joly J-P, et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): a randomized phase III study. J Clin Oncol. 2019;37:659–68.
Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663–673.
Ebata T, Hirano S, Konishi M, Uesaka K, Tsuchiya Y, Ohtsuka M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg. 2018;105:192–202.
Tan JCC, Coburn NG, Baxter NN, Kiss A, Law CHL. Surgical management of intrahepatic cholangiocarcinoma: a population-based study. Ann Surg Oncol. 2008;15:600–8.
Bridgewater J, Galle PR, Khan SA, Llovet JM, Park J-W, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol .2014;60:1268–89.
Le Roy B, Gelli M, Pittau G, Allard M-A, Pereira B, Serji B, et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg. 2018;105:839–47.
Cucchetti A, Cappelli A, Ercolani G, Mosconi C, Cescon M, Golfieri R, et al. Selective internal radiation therapy (SIRT) as conversion therapy for unresectable primary liver malignancies. Liver Cancer. 2016;5:303–11.
Hoffmann R-T, Paprottka PM, Schön A, Bamberg F, Haug A, Dürr E-M, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol. 2012;35:105–16.
Bourien H, Palard X, Rolland Y, Le Du F, Beuzit L, Uguen T, et al. Yttrium-90 glass microspheres radioembolization (RE) for biliary tract cancer: a large single-center experience. Eur J Nucl Med Mol Imaging. 2019;46;669–76.
Edeline J, Du FL, Rayar M, Rolland Y, Beuzit L, Boudjema K, et al. Glass microspheres 90Y selective internal radiation therapy and chemotherapy as first-line treatment of intrahepatic cholangiocarcinoma. Clin Nucl Med. 2015;40:851–5.
Gangi A, Shah J, Hatfield N, Smith J, Sweeney J, Choi J, et al. Intrahepatic cholangiocarcinoma treated with transarterial yttrium-90 glass microsphere radioembolization: Results of a single institution retrospective study. J Vasc Interv Radiol. 2018;29:1101–8.
Al-Adra DP, Gill RS, Axford SJ, Shi X, Kneteman N, Liau S-S. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and pooled analysis. Eur J Surg Oncol .2015;41:120–7.
Filippi L, Schillaci O, Cianni R, Bagni O. Yttrium-90 resin microspheres and their use in the treatment of intrahepatic cholangiocarcinoma. Future Oncol. 2018;14(9):809–18.
Edeline J, Touchefeu Y, Guiu B, Farge O, Tougeron D, Baumgaertner I, et al. Radioembolization plus chemotherapy for first-line treatment of locally advanced intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. Epub 31 Oct 2019. https://doi.org/10.1001/jamaoncol.2019.3702.
Salem R, Lewandowski RJ, Gates VL, Nutting CW, Murthy R, Rose SC, et al. Research reporting standards for radioembolization of hepatic malignancies. J Vasc Interv Radiol. 2011;22:265–78.
Vouche M, Habib A, Ward TJ, Kim E, Kulik L, Ganger D, et al. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology. 2014;60:192–201.
Garin E, Lenoir L, Rolland Y, Laffont S, Pracht M, Mesbah H, et al. Effectiveness of quantitative MAA SPECT/CT for the definition of vascularized hepatic volume and dosimetric approach: phantom validation and clinical preliminary results in patients with complex hepatic vascularization treated with yttrium-90-labeled microspheres. Nucl Med Commun. 2011;32:1245–55.
Zhu C-P, Shi J, Chen Y-X, Xie W-F, Lin Y. Gemcitabine in the chemoradiotherapy for locally advanced pancreatic cancer: a meta-analysis. Radiother Oncol. 2011;99:108–13.
Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240(4):644–57; discussion 657–658.
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;8;362:1273–81.
Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D, et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:28–37.
Rayar M, Sulpice L, Edeline J, Garin E, Levi Sandri GB, Meunier B, et al. Intra-arterial yttrium-90 radioembolization combined with systemic chemotherapy is a promising method for downstaging unresectable huge intrahepatic cholangiocarcinoma to surgical treatment. Ann Surg Oncol. 2015;22:3102–8.
Beuzit L, Edeline J, Brun V, Ronot M, Guillygomarc’h A, Boudjema K, et al. Comparison of Choi criteria and response evaluation criteria in solid tumors (RECIST) for intrahepatic cholangiocarcinoma treated with glass-microspheres Yttrium-90 selective internal radiation therapy (SIRT). Eur J Radiol. 2016;85:1445–52.
SIRT followed by CIS-GEM chemotherapy versus CIS-GEM chemotherapy alone as 1st line treatment of patients with unresectable intrahepatic cholangiocarcinoma (SIRCCA). ClinicalTrials.gov. Available AT: https://clinicaltrials.gov/ct2/show/NCT02807181.
Acknowledgments
We are grateful to Peter Tucker for language editing assistance.
Author information
Authors and Affiliations
Contributions
DR and ADM contributed equally to the data collection and data analysis, and wrote the manuscript. KB and JE designed and supervised the project. All authors discussed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Disclosure
Diane Riby, Alessandro D. Mazzotta, Damien Bergeat, Lucas Verdure, Laurent Sulpice, Heloise Bourien, Astrid Lièvre, Yan Rolland, Etienne Garin, Karim Boudjema, and Julien Edeline declare there are no conflicts of interest regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Riby, D., Mazzotta, A.D., Bergeat, D. et al. Downstaging with Radioembolization or Chemotherapy for Initially Unresectable Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 27, 3729–3737 (2020). https://doi.org/10.1245/s10434-020-08486-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08486-7