Abstract
Background
The tumor-node-metastasis classification system has proposed that lung cancers presenting as multifocal ground-glass nodules (multi-GGN) on computed tomography scan should be staged as multiple primaries instead of intrapulmonary metastases. However, the problem still exists for those synchronous multiple lung adenocarcinomas (SMLA) involving solid lesions. This study aimed to explore the distinct features of SMLA to better define the diagnosis and staging of this disease.
Methods
Between 2008 and 2016, consecutive patients with complete resection of SMLA were prospectively enrolled in the study. The patients were divided into three groups based on CT images as follows: multi-GGN, one solid nodule plus one or more GGNs (solid-GGN), and multiple solid lesions with or without GGN (multi-solid). Clinicopathologic features and survival outcomes were compared between these groups. Multivariate Cox proportional hazards analyses using bootstrap internal validation were performed to identify independent predictors for recurrence-free survival (RFS) and overall survival (OS).
Results
Of the 695 patients who met the inclusion criteria, 486 (69.9%) presented with multi-GGN tumor, 124 (17.9%) with solid-GGN tumor, and 85 (12.2%) with multi-solid tumor. The three groups had distinguished clinicopathologic features of gender, smoking history, nodal metastases, tumor size, subtype, and location (all P < 0.001). Multivariate analyses demonstrated that multi-solid tumor was an independent predictor for both decreased RFS [hazard ratio (HR) 2.941; 95% confidence interval (CI) 1.07–8.08; P = 0.036] and poor OS (HR 6.13; 95% CI 1.15–32.63; P = 0.034), but neither RFS (P = 0.384) nor OS (P = 0.811) differed between solid-GGN and multi-GGN tumors.
Conclusions
Both multi-GGN and solid-GGN tumors should be staged as multiple primaries, whereas multi-solid tumor was indicated to be advanced disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Recent advances in computed tomography (CT) and widespread screening for lung cancer have led to an increased detection of synchronous multiple lung adenocarcinomas (SMLAs).1 When multiple tumors are present, it is important to distinguish multiple primary lung cancers (MPLCs) from a single cancer with intrapulmonary metastasis because multiple tumors with metastatic designation might benefit from systemic therapy alone, whereas a surgical approach to MPLC may result in prolonged survival.
We previously developed a histologic-mutational strategy for identification of MPLCs.2 However, problems still remain in that all the reported methods rely mainly on pathologic examination of resected specimens to settle this issue.3,4,5,6,7,8,9 Knowing how to identify the differentiation before surgery in order to develop an appropriate treatment plan based on the initial perception of the disease is challenging.
The new edition of the tumor-node-metastasis (TNM) classification has proposed that multiple tumors with prominent ground-glass (multi-GGN) features shown on CT scan should not be staged as intrapulmonary metastasis.10 Survival analysis also has shown that invasive adenocarcinoma presenting as a solid nodule on CT with multifocal ground-glass lesions (solid-GGN) does not behave as advanced disease.11 We hypothesized that these GGN-associated features may serve as a preoperative indicator implying MPLC. Thus, we analyzed 695 consecutive patients with SMLA and explored the clinicopathologic features and survival outcomes. These investigations allowed us to compare the preoperative imaging data and the postoperative pathologic findings with the aim to determine the optimal strategy for identification of multiple primary lung adenocarcinomas.
Methods
Patients Inclusion Criteria
From January 2008 to December 2016, data of consecutive patients with pulmonary tumors were prospectively collected. After intensive preoperative workups (enhanced thin-sliced thoracic CT, abdominal ultrasonography, brain magnetic resonance imaging [MRI], and radionuclide bone scan for all patients and positron emission tomography [PET]/CT for some), to exclude mediastinal metastasis and systemic disease, patients underwent surgery with curative intent at Fudan University Shanghai Cancer Center.
The inclusion criteria for this study specified synchronous multiple pulmonary tumors resected completely with curative intent, final pathology confirmed through immunohistochemistry (IHC) methods, and accessible imaging data of preoperative thoracic CT. The survival period was recorded based on follow-up evaluation from the day of the last operation. For the first 2 years, clinical follow-up procedures included physical examination, chest CT, and abdominal ultrasonography every 3 months, then every 6 months during the years 3–5 and every year in the subsequent years. Brain MRI or CT scan and radionuclide bone scan were performed every year. The Institutional Review Board of Fudan University Shanghai Cancer Center approved this study. All the patients provided written informed consent.
Radiologic Review
All the CT scan images were reviewed and interpreted by two thoracic radiologists (G. L., Q.L.), who were blinded to the pathology. The study defined GGN lesions using the same method described in our previous publications, including both pure and part-solid GGNs.12,13,14 The patients were divided into three groups based on radiologic patterns as follows: multiple lesions with ground-glass components including both pure GGNs and mixed GGNs (multi-GGN), multiple tumors including one solid nodule plus one or more GGNs (solid-GGN), and multiple solid tumors (multi-solid) (Fig. 1). The diagnostic agreement of the two radiologists was measured by the Spearman correlation coefficient.
Classification based on computed tomography (CT) features. The patients were divided into three groups based on the following radiologic patterns: multiple lesions with ground-glass components including both pure ground-glass nodules (GGN) and mixed GGNs (multi-GGN), multiple tumors including one solid nodule plus one or more GGNs (solid-GGN), and multiple solid tumors with or without GGN (multi-solid)
Surgical Approach and Pathologic Review
The surgical indications for the SMLA patients were as follows: all solid and sub-solid nodules suspected to be malignant, easily accessible ipsilateral pure ground-glass nodule (GGN), and contralateral GGN with increasing size or solid component during the follow-up period. The surgical strategy was determined based on the size, location, and CT features of tumors, as well as the performance status and pulmonary function of the patient. The extent of pulmonary resection (lobectomy or sublobar resection including wedge and segmentectomy) and nodal resection was selected depending on the frozen section diagnosis during the operation.15 Adjuvant chemotherapy was suggested for those who had pathologic nodal metastasis.16
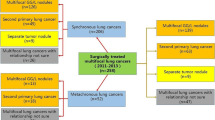
All the resected tumors were histologically reviewed based on the classification of lung adenocarcinoma by the 2011 International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS).17 According to this classification of lung adenocarcinoma and the survival reports,17,18,19,20,21,22 the different histologic subtypes were categorized into three groups: a low-risk group consisting of adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and lepidic-predominant adenocarcinoma; a medium-risk group consisting of acinar, papillary, and invasive-mucinous adenocarcinoma; and a high-risk group consisting of tumors containing 5% or more micropapillary or solid component. The diagnostic strategy for MPLC was determined according to the radiologic and pathologic features of the paired tumors2,10 (Fig. 2).
Diagnostic strategy. The radiologic and histologic indications for multiple primaries were ground-glass nodule (GGN)-associated features on computed tomography (CT) images (multi-GGN or solid-GGN), with either of paired tumors showing adenocarcinoma in situ (AIS) or minimally invasive adenocarcinoma (MIA), major subtypes or variants that differ, or both tumors harboring a noninvasive lepidic background (growth of neoplastic cells along preexisting alveolar structures)
Statistical Analysis
The patients were characterized by demographic and clinical variables. Differences in patient characteristics among the groups were evaluated using Chi square tests for categorical variables and one-way analysis of variance (ANOVA) tests for continuous variables. The patients who had a follow-up period longer than 1 year since the last surgery were selected for prognostic analysis because the survival data of the patients in recent years was not sufficiently mature.
Overall survival (OS) was defined as the time from the last surgery until death from any cause, with the patients who did not die during the study period censored at the date of the last available follow-up evaluation. Recurrence-free survival (RFS) was defined as the time from surgery until recurrence/metastasis or death from any cause. Both OS and RFS were estimated using the Kaplan–Meier method and compared across groups using uni- and multivariate Cox proportional hazards models for the full cohort to identify independent prognostic factors. Internal validation using the bootstrap method was applied to confirm the Cox model outcomes. All statistical analyses were two-sided and performed using SPSS (version 22.0; IBM Corporation, Armonk, NY, USA). A P value of 0.05 or lower indicated statistical significance,.
Results
Patient Demographics
From January 2008 to December 2016, 695 patients who met the inclusion criteria were included in the study. Of these patients, 486 (69.9%) presented as multi-GGN, 124 (17.9%) as solid-GGN, and 85 (12.2%) as multi-solid. The patient demographic characteristics are summarized in Table 1. With the appearance and increase in the number of solid nodules, the proportion of females (78.6% vs. 60.5% vs 37.6%; P < 0.001) and nonsmokers (85.4% vs. 69.4% vs. 50.6%; P < 0.001) decreased, whereas nodal metastases increased (1% vs. 29% vs. 61.2%; P < 0.001). Notably, nearly half of the multi-solid patients were found to have pathologic N2 metastasis (49.4%) (Table 1).
Tumor Characteristics
From the 695 patients, 1695 tumors were resected. Of the 695 patients, 494 (71.1%) had two tumors, 123 (17.7%) harbored three nodules, and 62 (11.2%) carried four to nine tumors. The number of resected tumors among the three groups did not differ statistically (P = 0.363). However, the location and size of the tumors varied. Whereas SMLA presented as multi-GGN, and solid-GGN seemed to be located more often in different pulmonary lobes, multi-solid tumors seemed to be found more often in one single lobe (P < 0.001). Tumors were found to be larger with an increasing number of the solid nodules (P < 0.001). According to the IASLC/ATS/ERS classification of lung adenocarcinoma, most tumors in the multi-GGN group (98.7%) belonged to low- or medium-risk cancer subtypes, whereas 100% of the tumors in the multi-solid group were medium- or high-risk tumors (P < 0.001) (Table 1).
Determination of MPLCs
According to the diagnostic strategy in Fig. 2, for the 610 patients (87.8%) in the multi-GGN and solid-GGN groups, multiple primaries were diagnosed based on CT images by our radiologist blinded to the patients’ information. We used the Spearman correlation coefficient to measure the diagnostic agreement between the two radiologists, which showed significant correlation (correlation coefficient, 0.925; P < 0.001). However, they were not able to reach definite diagnoses solely on the basis of CT images for the 85 patients in multi-solid group. These paired tumors underwent comprehensive pathologic assessment, with 19 cases (22.4%) found to be harboring different subtypes suggestive of independent malignancies. Multiple primaries were diagnosed in 629 patients (90.5%), and the findings for 66 patients (9.5%), using the diagnostic strategy in Fig. 2, were highly suggestive of intrapulmonary metastases. The CT findings for MPLC in this cohort showed a sensitivity of 97%, a specificity of 100%, a negative value of 77.6%, a positive predictive value of 100%, and an accuracy of 97.3%.
Surgical Procedures and Survival Outcomes
For the 186 (26.8%) patients with multiple tumors in the same lobe, lobectomy was the most commonly used procedure (73.2%), especially for the patients with solid tumors (97.6%). However, more than half of the patients with same-lobe GGNs received sublobar resections (54.4%). The 382 (55%) patients with tumors in the unilateral different lobes underwent lobectomy combined with one sublobar resection (50.8%), multiple sublobar resections (40%), or bilobectomy (9.2%). All 127 bilateral-tumor patients (18.2%) underwent two-stage operations. Most of these patients received lobectomy combined with one sublobar resection (59.8%). Notably, 409 (84.2%) of the 486 multi-GGN patients had undergone at least one sublobar resection. All tumors were R0 resected with negative margins.
Of the 448 patients who met the follow-up criteria for at least 12 months since their last lung surgery, 6 (1.3%) were lost to contact and excluded from the survival analysis. Thus, 442 patients finally were enrolled for the survival analysis. The median follow-up time was 21 months (range 12–89 months). No patient died during the perioperative period.
Recurrence for 65 patients included 22 cases of pulmonary dissemination, 10 mediastinal recurrences, 2 pleura/chest wall cases, 4 supra-clavicular mass cases, and 7 cases of brain metastases, as well as 10 bone, 2 adrenal gland, 1 eye, and 2 liver cases. For five cases of multiple metastases, the study could not determine which was the initial malignancy.
The whole cohort had an estimated 5-year RFS rate of 72.6% (95% CI 53.8–91.4%) for CT-defined multiple primaries and 26.6% (95% CI 9.7–43.4%) for multi-solid cases, which CT was unable to define. (P < 0.001; Fig. 2a). During the follow-up period, 30 patients died, including 3 (1.1%) in multi-GGN group, 4 (4.3%) in solid-GGN group, and 22 (32.8%) in multi-solid group.
In the Kaplan–Meier survival analysis for the whole cohort, the estimated 5-year RFS rate was 88.5% (95% CI 78.3–98.7%) for the multi-GGN group, 51.8% (95% CI 20.0–83.6%) for the solid-GGN group, and 22.9% (95% CI 5.7–40.1%) for the multi-solid group. The corresponding estimated 5-year OS rates were respectively 96% (95% CI 90.5–100%), 92.3% (95% CI 84.5–100%), and 39% (95% CI 13.7–64.3%).
For a better evaluation of surgical resection for SMLA, we selected patients who had pathologic N0 disease and at least one invasive adenocarcinoma (excluding AIS/MIA). For these selected patients, the estimated 5-year RFS rate was 79.5% (95% CI 61.3–97.7%) for the multi-GGN group, 63.8% (95% CI 27–100%) for the solid-GGN group, and 41.7% (95% CI 16.0–67.4%) for the multi-solid group. The corresponding estimated 5-year OS rates were respectively 95% (95% CI 85.4–100%), 91.6% (95% CI 81.8–100%), and 71.2% (95% CI 48.5–93.3%).
In the multivariate survival analysis for RFS after adjustment for sex, age, tumor size and location, surgical approach, subtype, nodal status, and lymphovascular invasion, multi-solid tumor proved to be an independent predictor of worse RFS (HR, 2.941; 95% CI 1.07–8.08; P = 0.036). In addition, dominant subtype (HR, 5.174; 95% CI 1.29–20.7; P = 0.02) and pathologic N stage (HR, 2.99; 95% CI 1.604–5.574; P = 0.001) were independent factors that affected tumor recurrence. These significant predictors were rechecked by bootstrap internal validation (Table 2).
In the multivariate analysis for OS, the independent predictors of poor OS were male sex (HR, 2.81; 95% CI 1.10–7.19; P = 0.031), nodal metastases (HR, 2.84; 95% CI 1.16–6.95; P = 0.022), and multi-solid tumor (HR, 6.13; 95% CI 1.152–32.63; P < 0.001), but only multi-solid tumor was confirmed to be positive by bootstrap internal validation (P = 0.007) (Table 3).
Discussion
Synchronous lung cancers were reported for 3.7% to 8.0% of lung cancer patients.23 With the development of thin-slice CT technology and the popularity of lung cancer screening, the detection rate for SMLA has been increasing rapidly. In this study, we prospectively enrolled 695 consecutive patients with SMLA who underwent surgery. We found that most of the multiple primary lung adenocarcinomas could be defined through preoperative CT analysis and characterized as multi-GGN or solid-GGN tumor. The multi-solid tumor was indicated as an independent predictor of poor survival, implying advanced disease.
Identification of MPLC from a single cancer with metastasis poses a significant challenge to chest physicians.2 The new edition of the TNM classification10 has specifically represented four patterns of the disease as follows: MPLC, separate tumor nodules (STNs, defined as intrapulmonary metastasis), multifocal lung cancer presenting as multiple ground-glass/lepidic nodules (multi-GGN), and diffuse pneumonia-type adenocarcinoma (PTA). It also has formulated detailed instructions for each pattern of the disease in the supplementary articles.24,25,26
Several questions still challenge clinicians when multiple pulmonary tumors are present. First, it is difficult to differentiate SPLC from STN without comprehensive histopathologic and genetic analysis before surgery, especially when the lesions have a similar histology.25 Second, although patients with lung adenocarcinoma with multiple pulmonary sites of involvement have been reported by a series of literatures,10 controversy remains regarding appropriate treatment strategies and postsurgical outcomes because information on the risks of recurrence and factors influencing survival is sparse, especially for patients who would benefit from a surgical resection.
In the current study, SMLA showing one solid nodule plus one or more GGNs on CT scan (solid-GGN) accounted for 20.3% of the whole cohort. We deemed this type of lung adenocarcinoma to be MPLC. On one hand, the imaging and histologic features were distinct between the solid and the ground-glass nodules. On the other hand, the surgical outcome for these patients was fairly good, which supported the recommendation that solid-GGN adenocarcinomas be treated as separate tumors. Notably, the prognosis for these patients was dominantly determined by the stage of the solid tumor. Thus, we updated our previous strategy for MPLC presented as Fig. 2. Using these methods, most of the SMLA patients (90.5% in this cohort) can have a definite diagnosis before or during surgery.
In this study, SMLA with the multi-solid pattern was proved to be associated with a high rate of pathologic nodal metastasis even if the preoperative imaging showed no evidence of metastases. This implied that it is more likely to be intrapulmonary metastatic disease. However, we must admit that with the current methods, the differential diagnosis of multiple solid tumors with the same histology is really challenging, even by molecular approaches such as the gene test. Assessment of biomarkers (driver gene mutations) is only suggestive because a substantial rate of discordance exists among different samples of the same tumor, whereas concordance exists among clearly separate tumors.25 Thus, mutational profiling should not be considered definitive and must be considered together with other information.10
Considering the tumor size and nodal metastases of multiple solid lesions in this study, we concluded that in most instances, multiple lesions were highly suggestive of a relation with each other and should be treated as advanced disease. Further work on this problem by our study team is ongoing. From a therapeutic perspective, we can see that after the integrated treatment consisting of surgery, adjuvant chemotherapy, and targeted therapy after recurrence, these patients achieved survival outcomes comparable with those from the IASLC global database,27 implying that these patients still may benefit from comprehensive treatment based on surgery. Importantly, standard lobectomy without limited resections is preferred for each solid nodule to prevent tumor spread through air spaces.28 However, randomized-controlled trials are required to determine which treatment strategy would bring the greatest benefits to the patients with refractory disease.
A series of previous studies have reported favorable OS and RFS for both Asian29 and Caucasian11 patients with multi-GGN tumors. In the current study, the multi-GGN pattern was presented by the majority of the SMLAs (67.6%). This type of tumor often contained low-risk subtypes of adenocarcinoma, such as the lepidic component, and rarely had nodal, airway, or vascular dissemination. Thus, only if the frozen section showed lepidic-predominant tumors were sublobar resection and selective lymphadenectomy expected to yield a good prognosis for these patients.15,30 This surgical strategy would be specially suitable for patients with multiple lung lesions and poor pulmonary reserve.31 As a result, the new TNM classification, which proposes that multi-GGN adenocarcinoma be classified by the T category of the lesion with the highest T and a single N and M for all of the pulmonary lesions, has definite value for the prognosis of these patients. According to the data of this study, the same staging rule and treatment strategy could be applied for the solid-GGN patients as well.
In this study, tumors were classified by their predominant histologic subtype in the multivariate model for survival analysis. This could result in some bias because a tumor can have multiple components. However, we believe this bias would be small and may not affect the outcomes. On the one hand, we defined the predominant subtype based on the criteria of 2011 IASLC/ATS/ERS classification, which emphasizes describing the most invasive component as the predominant subtype. On the other hand, many publications, from both our study team and others, have demonstrated that the predominant histologic subtypes, according to the 2011 IASLC/ATS/ERS classification, have strong prognostic value.12,13,14,15,17,21,32 Additionally, this approach made the model operational and practical compared with assigning each tumor to multiple histologic subtypes in the survival analysis.
Several limitations of our study need to be addressed. First, although this study may have had the largest number of enrolled patients with SMLAs, it was inevitable that selection bias would be present due to the nature of a single-institution study. Second, the study population was limited to East Asians, thereby raising concerns about the generalizability of our results because disease spectra may differ among different ethnicities. Third, the short follow-up period and small sample for some of the subgroups hampered the analysis of overall survival and limited the power of the survival analysis. Therefore, a larger cohort with a longer follow-up period is needed (Fig. 3).
Kaplan-Meier curves including recurrence-free survival (RFS) and overall survival (OS) for the whole cohort. a The estimated 5-year RFS rates were 88.5% (95% CI 78.3–98.7%) for multi-GGN group, 51.8% (95% CI 20.0–83.6%) for solid-GGN group, and 22.9% (95% CI 5.7–40.1%) for multi-solid group. b The corresponding estimated 5-year OS rates were respectively 96% (95% CI 90.5–100%), 92.3% (95% CI 84.5–100%), and 39% (95% CI 13.7–64.3%)
In summary, this study presented a comprehensive analysis of clinicopathologic characteristics, surgical treatment, and prognosis of SMLA in large cohort of patients. The results showed that among the three groups classified by the CT features, both the multi-GGN and solid-GGN tumors should be staged as multiple primaries, whereas the multi-solid tumors indicate advanced disease. Based on both these data and our previous work,2 we proposed a diagnostic strategy for SMLA (Fig. 2) that could practically and effectively stratify the stages of patients before, during, and after surgery. Future studies to investigate biologic mechanisms of the multiple tumors are warranted, and clinical trials that enroll more patients and have a longer follow-up period are needed to evaluate and improve the staging and management of the disease.
References
Ishikawa Y, Nakayama H, Ito H, et al. Surgical treatment for synchronous primary lung adenocarcinomas. Ann Thorac Surg. 2014;98:1983–8.
Zhang Y, Hu H, Wang R, et al. Synchronous non-small cell lung cancers: diagnostic yield can be improved by histologic and genetic methods. Ann Surg Oncol. 2014;21:4369–74.
Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70:606–12.
Chung JH, Choe G, Jheon S, et al. Epidermal growth factor receptor mutation and pathologic-radiologic correlation between multiple lung nodules with ground-glass opacity differentiates multicentric origin from intrapulmonary spread. J Thorac Oncol. 2009;4:1490–5.
Girard N, Deshpande C, Lau C, et al. Comprehensive histologic assessment helps to differentiate multiple lung primary non-small cell carcinomas from metastases. Am J Surg Pathol. 2009;33:1752–64.
Girard N, Deshpande C, Azzoli CG, et al. Use of epidermal growth factor receptor/Kirsten rat sarcoma 2 viral oncogene homolog mutation testing to define clonal relationships among multiple lung adenocarcinomas: comparison with clinical guidelines. Chest. 2010;137:46–52.
Warth A, Macher-Goeppinger S, Muley T, et al. Clonality of multifocal non-small cell lung cancer: implications for staging and therapy. Eur Respir J. 2012;39:1437–42.
Murphy SJ, Aubry MC, Harris FR, et al. Identification of independent primary tumors and intrapulmonary metastases using DNA rearrangements in non-small cell lung cancer. J Clin Oncol. 2014;32(36):4050–8.
Sun W, Liu Y, Liu XY, Lin DM, Lv N. Significance of nonmucinous lepidic component with mild nuclear atypia in the discrimination of multiple primary lung cancers from intrapulmonary metastases. Int J Clin Exper Pathol. 2014;7:7583–96.
Detterbeck FC, Nicholson AG, Franklin WA, et al. The IASLC Lung Cancer Staging Project: summary of proposals for revisions of the classification of lung cancers with multiple pulmonary sites of involvement in the forthcoming eighth edition of the TNM classification. J Thorac Oncol. 2016;11:639–50.
Gu B, Burt BM, Merritt RE, et al. A dominant adenocarcinoma with multifocal ground glass lesions does not behave as advanced disease. Ann Thorac Surg. 2013;96:411–8.
Ye T, Deng L, Wang S, et al. Lung adenocarcinomas manifesting as radiological part-solid nodules define a special clinical subtype. J Thorac Oncol. 2019;14:617–27.
Fu F, Zhang Y, Wen Z, et al. Distinct prognostic factors in patients with stage I non-small cell lung cancer with radiologic part solid or solid lesions. J Thorac Oncol. 2019;14(12):2133–42.
Ye T, Deng L, Xiang J, et al. Predictors of pathologic tumor invasion and prognosis for ground-glass opacity featured lung adenocarcinoma. Ann Thorac Surg. 2018;106:1682–90.
Liu S, Wang R, Zhang Y, et al. Precise diagnosis of intraoperative frozen section is an effective method to guide resection strategy for peripheral small-sized lung adenocarcinoma. J Clin Oncol. 2016;34:307–13.
Ettinger DS, Wood DE, Akerley W, et al. Non-small cell lung cancer, version 1.2015. JNCCN J Natl Comprehensive Cancer Network. 2014;12:1738–61.
Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol. 2011;6:244–85.
Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol. 2012;30:1438–46.
Hu H, Sun Z, Li Y, et al. The histologic classifications of lung adenocarcinomas are discriminable by unique lineage backgrounds. J Thorac Oncol. 2016;11(12):2161–72.
Zhang Y, Wang R, Cai D, et al. A comprehensive investigation of molecular features and prognosis of lung adenocarcinoma with micropapillary component. J Thorac Oncol. 2014;9:1772–8.
Zhang Y, Li J, Wang R, et al. The prognostic and predictive value of solid subtype in invasive lung adenocarcinoma. Sci Rep. 2014;4:7163.
Tsao MS, Marguet S, Le Teuff G, et al. Subtype classification of lung adenocarcinoma predicts benefit from adjuvant chemotherapy in patients undergoing complete resection. J Clin Oncol. 2015;33:3439–46.
Gazdar AF, Minna JD. Multifocal lung cancers: clonality vs field cancerization and does it matter? J Natl Cancer Institute. 2009;101:541–3.
Detterbeck FC, Marom EM, Arenberg DA, et al. The IASLC Lung Cancer Staging Project: background data and proposals for the application of TNM staging rules to lung cancer presenting as multiple nodules with ground-glass or lepidic features or a pneumonic type of involvement in the forthcoming eighth edition of the TNM classification. J Thorac Oncol. 2016;11:666–80.
Detterbeck FC, Franklin WA, Nicholson AG, et al. The IASLC Lung Cancer Staging Project: background data and proposed criteria to distinguish separate primary lung cancers from metastatic foci in patients with two lung tumors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:651–65.
Detterbeck FC, Bolejack V, Arenberg DA, et al. The IASLC Lung Cancer Staging Project: background data and proposals for the classification of lung cancer with separate tumor nodules in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:681–92.
Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The 8th-edition lung cancer stage classification. Chest. 2017;151(1):193–203.
Kadota K, Nitadori J, Sima CS, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol. 2015;10:806–14.
Kim HK, Choi YS, Kim K, et al. Management of ground-glass opacity lesions detected in patients with otherwise operable non-small cell lung cancer. J Thorac Oncol. 2009;4:1242–6.
Donington JS. An additional step toward personalization of surgical care for early-stage non-small cell lung cancer. J Clin Oncol. 2016;34:295–6.
Baisi A, De Simone M, Raveglia F, Cioffi U. What really affects synchronous pulmonary adenocarcinoma management? Ann Thorac Surg. 2015;100:1506–7.
Zhao Y, Wang R, Shen X, et al. Minor components of micropapillary and solid subtypes in lung adenocarcinoma are predictors of lymph node metastasis and poor prognosis. Ann Surg Oncol. 2016;23:2099–105.
Acknowledgments
This work was supported by grants from The National Natural Science Foundation of China (81572253), the Shanghai Shenkang Hospital Development Center City Hospital Emerging Cutting-Edge Technology Joint Research Project (SHDC12017102), and the Shanghai Municipal Health Commission Key Discipline Project (2017ZZ02025 and 2017ZZ01019).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure
The author declares that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Li, G., Li, Y. et al. Imaging Features Suggestive of Multiple Primary Lung Adenocarcinomas. Ann Surg Oncol 27, 2061–2070 (2020). https://doi.org/10.1245/s10434-019-08109-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-08109-w