Abstract
Background
In the ACOSOG (American College of Surgeons Oncology Group) Z0011 trial and the AMAROS (After Mapping of the Axilla: Radiotherapy or Surgery?) trial, matted nodes with gross extracapsular extension (ECE), a risk factor for locoregional recurrence, were an indication for axillary lymph node dissection (ALND), but the effect of microscopic ECE (mECE) in the sentinel lymph nodes (SLNs) on recurrence was not examined.
Methods
Between 2010 and 2017, 811 patients with cT1-2N0 breast cancer and SLN metastasis were prospectively managed according to Z0011 criteria, with ALND for those with more than two positive SLNs or gross ECE. Management of mECE was not specified. In this study, we compare outcomes of patients with one to two positive SLNs with and without mECE, treated with SLN biopsy alone (n = 685).
Results
Median patient age was 58 years, and median tumor size was 1.7 cm. mECE was identified in 210 (31%) patients. Patients with mECE were older, had larger tumors, and were more likely to be hormone receptor positive and HER2 negative, have two positive SLNs, and receive nodal radiation. At a median follow-up of 41 months, no isolated axillary failures were observed. There were 11 nodal recurrences; two supraclavicular ± axillary, four synchronous with breast, and five with distant failure. The five-year rate of any nodal recurrence was 1.6% and did not differ by mECE (2.3% vs. 1.3%; p = 0.84). No differences were observed in local (p = 0.08) or distant (p = 0.31) recurrence rates by mECE status.
Conclusions
In Z0011-eligible patients, nodal recurrence rates in patients with mECE are low after treatment with SLN biopsy alone, even in the absence of routine nodal radiation. The presence of mECE should not be considered a routine indication for ALND.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The ACOSOG (American College of Surgeons Oncology Group) Z0011 trial and the AMAROS (After Mapping of the Axilla: Radiotherapy or Surgery?) trial both demonstrated no difference in locoregional recurrence, disease-free survival, or overall survival between sentinel lymph node biopsy (SLNB) alone (ACOSOG Z0011) or SLNB plus axillary radiotherapy (AMAROS) compared with axillary lymph node dissection (ALND) in women with cT1-2N0 invasive breast cancer and metastases in one or two sentinel lymph nodes (SLNs) treated with breast-conserving surgery (BCS) and multimodality therapy.1,2,3 As a result, omission of ALND in clinically node-negative patients undergoing BCS with limited disease in the SLNs has become standard practice. These results were confirmed in a prospective cohort of Z0011-eligible patients from our institution with regional control rates of 98% at five years in patients treated with SLNB alone.4
In SLN positive patients, microscopic extracapsular extension (mECE), defined as the growth of tumor cells outside of the lymph node capsule into peri-nodal adipose tissue, is a predictor of poor prognosis5,6,7 as well as non-sentinel node tumor burden.8,9 In a study of 1109 patients with pT1-2, cN0 breast cancer with one to two positive SLNs, four or more additional positive nodes at axillary dissection were identified in 21% of patients with mECE in the SLN, compared with 3% without mECE.8
In ACOSOG Z0011 and AMAROS, matted nodes with gross ECE were an indication for ALND, but the management of mECE was not specified and its effect on recurrence was not examined.1,3 It is uncertain whether the presence of mECE in the SLN in Z0011-eligible patients treated with SLNB alone is associated with a higher rate of nodal recurrence compared with patients without mECE. In this study, we evaluate the association of mECE in the SLN and nodal recurrence in a cohort of cT1-2N0 breast cancer patients with SLN metastasis prospectively managed according to Z0011; to our knowledge, this study is the first to address this question.
Methods
Beginning in August 2010, women with cT1-2N0 invasive breast cancer undergoing BCS were prospectively managed at Memorial Sloan Kettering Cancer Center according to ACOSOG Z0011 criteria, with ALND for more than two SLN metastases or matted nodes with gross ECE. Patients with one to two positive SLNs identified by routine hematoxylin and eosin staining were treated with SLNB alone. The presence or absence of mECE in the SLN was prospectively reported on pathology reports, and, when present, the extent was further stratified as ≤ 2 mm versus > 2 mm, measured as the widest diameter of the invasive front of mECE (either perpendicular or parallel to the lymph node capsule).10 In Z0011-eligible patients with mECE in the SLN, there was no defined policy for completion ALND regardless of the extent of mECE, and the decision to perform ALND was based on surgeon judgment. Whole-breast radiation therapy (RT), with or without nodal RT, was recommended for all patients post-BCS.
Following approval from our Institutional Review Board, clinical and pathologic data, including the presence and extent of mECE (≤ 2 mm vs. > 2 mm), were collected for all eligible patients treated according to Z0011 criteria. Patients were excluded if they were treated with mastectomy or neoadjuvant systemic therapy, or had only isolated tumor cells in the SLN.
A nodal failure was defined as tumor recurrence in the ipsilateral regional nodal basins (axillary, supraclavicular, or internal mammary), occurring alone or concurrent with a breast or distant failure. A local failure was defined as an in-breast tumor recurrence in the absence of a nodal or distant recurrence, and a distant failure included any distant metastases occurring without a nodal recurrence. Time to recurrence was calculated from the date of surgery.
Statistical Analysis
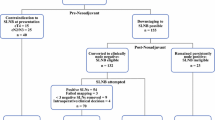
Clinical, demographic, and surgical characteristics were compared using the two-sample t test for continuous variables, and the chi-square test for categorical variables. Cumulative incidence of recurrence locations (nodal, breast, or distant) in patients with one to two positive SLNs with and without mECE treated with SLNB alone (n = 685) was estimated using competing risks.11 Outcomes in the 118 patients treated with ALND are provided for comparison. Patients with more than two SLN metastases treated with SLNB alone (n = 8) were excluded from the analysis (Fig. 1). All analyses were conducted in SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA) and R version 3.5.0 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Clinicopathologic Characteristics of Patients Treated with Sentinel Lymph Node Biopsy Alone
From August 2010 to March 2017, 811 patients with cT1-2N0 invasive breast cancer and SLN metastasis had BCS and were managed according to Z0011 criteria. Overall, 685 patients had one to two positive SLNs and were treated with SLNB alone. In the SLN-only subset, median patient age was 58 years (range 30–92 years) and median pathologic tumor size was 1.7 cm (range 0.1–5.2 cm). Most patients had ductal histology (87%) and were hormone receptor (HR) positive and HER2 negative (84%). Notably, 97% of patients received systemic therapy and 90% received whole-breast RT.
mECE was identified in 210 (31%) patients, with 93 patients having > 2 mm mECE. Patients with mECE were older (median age 61 vs. 57 years; p = 0.0002), had larger tumors (1.8 cm vs. 1.6 cm; p = 0.008), and were more likely to be HR positive and HER2 negative (90% vs. 81%; p = 0.006) and have two (versus one) positive SLNs (23% vs. 16%; p = 0.03) (Table 1) compared to patients without mECE. Patients with mECE were also more likely to receive endocrine therapy (p = 0.03), reflecting the higher proportion of HR positive/HER2 negative patients in this subset.
Nodal RT was more common among patients with mECE than those without mECE (39% vs. 17%; p < 0.0001). Among mECE patients (n = 210), those who received nodal RT (n = 81) had larger tumors, were more likely to have two (versus one) positive SLNs, and were more likely to have > 2 mm of mECE compared with mECE patients who did not receive nodal RT (Table 2).
Recurrence in Patients Treated with Sentinel Lymph Node Biopsy Alone
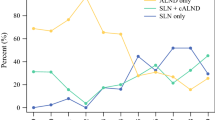
At a median follow-up of 41 months, no isolated axillary nodal failures were observed. There were 11 nodal recurrences. Two were limited to the supraclavicular and supraclavicular + axillary nodal basins, four were synchronous with an in-breast tumor recurrence, and five were synchronous with distant failure. The five-year cumulative incidence of any nodal failure, local failure, and distant failure was 1.6%, 1.3%, and 3.6%, respectively (Fig. 2). Nodal failure in patients with one to two positive SLNs treated with SLNB alone did not differ by mECE (p = 0.84; five-year rate 2.3% [mECE] vs. 1.3% [no mECE]). In the 117 patients with ≤ 2 mm mECE, there were no nodal recurrences; however, in the 93 patients with mECE > 2 mm, there were three nodal recurrences. None of these patients received nodal RT. The small number of nodal failures precluded statistical comparison of five-year nodal failure rates for ≤ 2 mm versus > 2 mm mECE. No differences in local failures (p = 0.08; five-year rate 0% [mECE] vs. 1.9% [no mECE]) and distant failures (p = 0.31; five-year rate 1.2% [mECE] vs. 4.6% [no mECE]) by mECE status were observed.
Axillary Lymph Node Dissection Cohort: Characteristics and Recurrences
Of the 118 patients who had an ALND, 82 (69%) had three or more positive SLNs as the primary reason for the ALND. The remaining 36 patients had one to two positive SLNs, of whom 31 had mECE, and the decision for ALND was based on surgeon judgment. The majority (81%) of ALND patients received nodal RT. The clinical characteristics of the ALND patients stratified by mECE are listed in Table 3.
Among the ALND patients, there was one synchronous nodal and distant failure, and 11 isolated distant failures. The five-year incidence of any nodal failure in the ALND group was 2.2%.
Discussion
In the ACOSOG Z0011 and AMAROS trials,1,2,3 27% and 33% of patients undergoing ALND had additional nodal disease, respectively, suggesting that the patients treated with SLNB alone likely had a similar nodal burden. Yet, in patients treated with SLNB alone, the 10-year rates of nodal failure were low in both studies (1.5% [Z0011] and 1.8% [AMAROS]), and did not differ significantly from the rates of nodal failure in patients treated with ALND.2,12 Although these studies showed that omission of ALND is safe in patients with one to two positive sentinel nodes, the effect of mECE on nodal recurrence was not addressed in the prospective trials, and the poor prognosis associated with macroscopic ECE has raised concern that mECE should also be considered an indication for ALND. Given the shift toward surgical de-escalation in the axilla, with the goal of avoiding the morbidity of ALND, understanding the clinical significance of mECE in the SLN becomes increasingly relevant.
mECE was a common pathologic finding in our study, identified in the SLN in 31% of Z0011-eligible patients treated with SLNB alone. These findings are consistent with the 28–33% rate of mECE reported in other studies of patients with cT1-2 breast cancer and SLN metastasis.13,14 Notably, mECE was more frequent in patients undergoing ALND, identified in 85% who had metastases in more than two SLNs. There was no difference in nodal failure rates between patients with and without mECE in the absence of ALND, and the overall rate of any nodal recurrence at five years was only 1.6%, suggesting that any additional disease burden in the axilla associated with mECE was adequately treated with systemic therapy and RT. Prior studies in early-stage breast cancer patients treated with ALND have similarly shown that although mECE was associated with increased axillary nodal burden, subsequent axillary recurrences were uncommon, even without directed axillary RT.5,7,13,14,15 Choi et al.13 demonstrated no difference in nodal recurrence based on the presence of mECE in 208 T1-2N0 breast cancer patients with one to two positive SLNs treated with ALND (0% [mECE] vs. 3.4% [no mECE]; p = non-significant). Because of the low rates of axillary failure observed among patients with mECE treated with ALND, the use of axillary RT has been questioned in this subset of patients.7,14,15,16 In patients with mECE undergoing more limited axillary surgery with SLNB alone, the utility of nodal RT remains uncertain.
In patients treated with SLNB alone in our study, nodal RT was more common among those with mECE (39%) compared to those without mECE (17%), which may have contributed to the excellent regional control we observed. Patients with mECE who received nodal RT more commonly had other risk factors for locoregional recurrence, including larger tumors, > 2 mm mECE, and two versus one positive SLNs, which likely influenced the decision for nodal RT. The patients with mECE who did not receive nodal RT (61% of the mECE cohort) were not necessarily ‘low risk’, as evidenced by the presence of lymphovascular invasion in nearly 50% of patients and > 2 mm of mECE in 34% of patients. Despite this, nodal recurrence rates were low. Although this retrospective study cannot directly address the need for nodal RT based on mECE, the observed low rates of nodal failure observed among our mECE patients, the majority of whom were treated with standard whole-breast tangents, suggest that nodal RT solely based on the presence of mECE is likely not required.
A bigger challenge is understanding whether the extent of mECE should influence axillary management. Both the presence and extent of mECE are associated with nodal burden at ALND, with Gooch and colleagues8 identifying four or more positive nodes in 33% of patients with > 2 mm mECE, compared to 9% of patients with < 2 mm mECE and 3% of patients with no mECE (p < 0.0001). In our study cohort, 93 of the 210 patients with mECE treated with SLNB alone had > 2 mm of mECE, and yet the five-year nodal failure rate for all mECE patients was only 2.3%. Given the low event rate, we were unable to stratify nodal failures by extent of mECE, but the observation that all three nodal recurrences were observed in patients with > 2 mm of mECE sounds a note of caution in this group. Overall, the low nodal recurrence rate suggests that ALND is likely not necessary based on the extent of mECE alone. The extent of mECE may, however, factor into the decision for nodal RT, as a little more than half of the patients in our study with mECE > 2 mm received nodal RT. Notably, a significant proportion of SLNB patients with mECE > 2 mm were treated with standard tangents (45%) in our study, reinforcing that the decision for nodal RT is complex and should be individualized.
Outcomes of patients in the cohort undergoing ALND due to a heavier axillary tumor burden or the presence of mECE were examined to compare rates of nodal recurrence with those seen in the SLN-only patients. In this higher-risk group, patients treated with ALND had similar rates of nodal failure (five-year rate: 2.2%) to those treated with SLNB, emphasizing that bigger surgery does not eliminate the problem of locoregional recurrence in patients with high-risk biology.
To our knowledge, this study is the first to evaluate nodal recurrence in patients with mECE treated with SLNB without axillary dissection. Strengths of our study include its large, well-defined cohort of patients from a prospectively maintained database with standardized reporting of mECE. Limitations include the fact that this was a non-randomized study where treatment of mECE, including the decision for axillary dissection and nodal RT, was at the discretion of the treating physician. Despite this, the observed event rate was low. Given that the majority of SLNB patients with mECE were treated with standard whole-breast radiation tangents, the use of nodal RT alone cannot account for the excellent outcomes in these patients. Similar to the ACOSOG Z0011 and AMAROS study populations,3 our study cohort comprised largely of HR positive breast cancers, a disease subtype that is known to have a long clinical course and late recurrences. Therefore, the low observed rates of nodal failure may be a result of the short median follow-up. However, even among patients receiving systemic therapy, axillary failures are traditionally early events, as evidenced by the 10-year follow-up of ACOSOG Z0011, which demonstrated only one additional nodal failure in the SLN-only arm between years 5 and 10.17 Therefore, it is unlikely that longer follow-up will result in a significant number of additional nodal failures in our study population.
Conclusions
In this large series of consecutively treated patients prospectively managed according to Z0011 criteria, nodal recurrences in patients with one to two positive SLNs treated with SLNB alone were infrequent, observed in < 2% of patients at 5 years. Patients with mECE had similarly low rates of nodal recurrence compared with those without mECE, even in the absence of routine nodal RT. The presence of mECE in an SLN positive patient should not be considered a routine indication for ALND, but is one of many factors that should be considered in determining the optimal locoregional management strategy.
References
Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 2014;15(12):1303–10.
Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA 2017;318(10):918–26.
Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305(6):569–75.
Morrow M, Van Zee KJ, Patil S, et al. Axillary dissection and nodal irradiation can be avoided for most node-positive z0011-eligible breast cancers: a prospective validation study of 793 patients. Ann Surg 2017;266(3):457–62.
Bucci JA, Kennedy CW, Burn J, et al. Implications of extranodal spread in node positive breast cancer: a review of survival and local recurrence. Breast 2001;10(3):213–9.
Nottegar A, Veronese N, Senthil M, et al. Extra-nodal extension of sentinel lymph node metastasis is a marker of poor prognosis in breast cancer patients: a systematic review and an exploratory meta-analysis. Eur J Surg Oncol 2016;42(7):919–25.
Pierce LJ, Oberman HA, Strawderman MH, Lichter AS. Microscopic extracapsular extension in the axilla: is this an indication for axillary radiotherapy? Int J Radiat Oncol Biol Phys 1995;33(2):253–9.
Gooch J, King TA, Eaton A, et al. The extent of extracapsular extension may influence the need for axillary lymph node dissection in patients with T1-T2 breast cancer. Ann Surg Oncol 2014;21(9):2897–903.
van la Parra RF, Peer PG, Ernst MF, Bosscha K. Meta-analysis of predictive factors for non-sentinel lymph node metastases in breast cancer patients with a positive SLN. Eur J Surg Oncol 2011;37(4):290–9.
Aziz S, Wik E, Knutsvik G, et al. Extra-nodal extension is a significant prognostic factor in lymph node positive breast cancer. PLoS ONE 2017;12(2):e0171853.
Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1141–54.
Rutgers EJ, Donker M, Poncet C, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer patients: 10 year follow up results of the EORTC AMAROS trial (EORTC 10981/22023) [abstract no. GS4-01]. 41st Annual San Antonio Breast Cancer Symposium, 4–8 Dec 2018; San Antonio, TX.
Choi AH, Blount S, Perez MN, et al. Size of extranodal extension on sentinel lymph node dissection in the American College of Surgeons Oncology Group Z0011 trial era. JAMA Surg 2015;150(12):1141–8.
Hetelekidis S, Schnitt SJ, Silver B, et al. The significance of extracapsular extension of axillary lymph node metastases in early-stage breast cancer. Int J Radiat Oncol Biol Phys 2000;46(1):31–4.
Leonard C, Corkill M, Tompkin J, et al. Are axillary recurrence and overall survival affected by axillary extranodal tumor extension in breast cancer? Implications for radiation therapy. J Clin Oncol 1995;13(1):47–53.
Fisher BJ, Perera FE, Cooke AL, et al. Extracapsular axillary node extension in patients receiving adjuvant systemic therapy: an indication for radiotherapy? Int J Radiat Oncol Biol Phys 1997;38(3):551–9.
Giuliano AE, Ballman K, McCall L, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 randomized trial. Ann Surg 2016;264(3):413–20.
Funding
The preparation of this study was funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Dr. Andrea V. Barrio has received speaking honoraria from Roche; Dr. Monica Morrow has received honoraria from Genomic Health and Roche; Dr. Kimberly J. Van Zee served on the advisory board of Genomic Health in 2012; and Dr. Mahmoud El-Tamer has received honoraria from Blue Earth Diagnostic. Stephanie Downs-Canner, Marcia Edelweiss, Hiram S. Cody III, Mary L. Gemignani, Melissa L. Pilewskie, George Plitas, Laurie Kirstein, Deborah Capko, and Sujata Patil have no conflict of interest disclosures to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barrio, A.V., Downs-Canner, S., Edelweiss, M. et al. Microscopic Extracapsular Extension in Sentinel Lymph Nodes Does Not Mandate Axillary Dissection in Z0011-Eligible Patients. Ann Surg Oncol 27, 1617–1624 (2020). https://doi.org/10.1245/s10434-019-08104-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-08104-1