Abstract
Background
Immunotherapy has become a standard treatment option for non-small cell lung cancer (NSCLC), with the tumor microenvironment attracting significant attention. CD8 + and forkhead box protein P3 + (FoxP3 +) tumor-infiltrating lymphocytes (TILs) influence the tumor microenvironment, but the clinical significance of CD8 + and FoxP3 + TILs in stage IA lung adenocarcinoma (LAD) is poorly understood.
Methods
We analyzed 203 patients with stage IA primary LAD who had undergone surgery at Kyushu University from January 2003 to December 2012. We evaluated CD8 + and FoxP3 + TILs by immunohistochemistry. We set the cutoff values at 50 cells/0.04 mm2 for CD8 + TILs and 20 cells/0.04 mm2 for FoxP3 + TILs, respectively. We divided the patients into four groups: CD8-Low/FoxP3-Low; CD8-High/FoxP3-Low; CD8-Low/FoxP3-High; and CD8-High/FoxP3-High. We compared clinical outcomes among them. Programmed cell death ligand-1 (PD-L1) expression by tumor cells was also evaluated as previously reported.
Results
Respectively, 104 (51.2%), 46 (22.7%), 22 (10.8%), and 31 (15.3%) patients were classified as CD8-Low/FoxP3-Low, CD8-High/FoxP3-Low, CD8-Low/FoxP3-High, and CD8-High/FoxP3-High. Both disease-free survival (DFS) and overall survival (OS) were significantly worse in the CD8-Low/FoxP3-High group than the other groups (5-year DFS: 66.3% vs. 90.5%; P = 0.0007, 5-year OS: 90.9% vs. 97.0%; P = 0.0077). In the multivariate analysis, CD8-Low/FoxP3-High and PD-L1 expression were independent prognostic factors of DFS, and lymphatic invasion, surgical procedure, and PD-L1 expression were independent prognostic factors of OS.
Conclusions
CD8-Low/FoxP3-High was an independent prognostic factor of DFS (hazard ratio: 3.22; 95% confidence interval: 1.321–7.179; P = 0.0121) in stage IA LAD. Immunosuppressive conditions were associated with poor prognosis in stage IA LAD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recently, a treatment paradigm shift for patients with lung cancer has occurred with the development of immune checkpoint inhibitors, such as nivolumab, pembrolizumab, atezolizumab, and durvalumab.1,2,3,4,5 Also, the immune mechanisms within the tumor microenvironment have attracted much attention. Tumor-infiltrating lymphocytes (TILs) play a central role in the tumor microenvironment, and there have been several studies analyzing the significance of TILs in lung cancer.6,7,8,9,10,11,12 TILs showed an association with survival, recurrence, and malignancy of lung cancer.6,7,8,9,10 Furthermore, TILs predict therapeutic responses to immunotherapy in non-small cell lung carcinoma (NSCLC).11, 12
Cluster of differentiation 8 + (CD8 +) lymphocytes, known as cytotoxic T lymphocytes, are activated via major histocompatibility complex class I antigen, and lyse target cells, such as tumor cells or virus infected cells, through the release of perforin and granzymes.13 Forkhead box protein P3 (FoxP3) is a transcription factor specific to regulatory T lymphocytes. FoxP3 + lymphocytes exert their immunosuppressive effects through various mechanisms: consumption of interleukin 2, cytotoxic T lymphocyte antigen 4 signal, and production of immune inhibitory cytokines.14 In NSCLC, past meta-analyses have shown that a high density of CD8 + TILs indicated good prognosis for overall survival (OS), disease-free survival (DFS), and recurrence-free survival (RFS), and high levels of FoxP3 + TILs had unfavorable prognostic effects for OS and RFS.6, 7 However, in lung adenocarcinoma (LAD), there are several studies that show that a high density of CD8 + TILs was associated with poor prognosis for death and recurrence.8,9,10 Based on these findings, we considered that the histologic type of lung cancer and patient characteristics had a strong effect on the significance of TILs. Therefore, in this study, to eliminate the bias of the histologic type of lung cancer and patient characteristics, we selected patients with stage IA LAD and elucidated the significance of CD8 + and FoxP3 + TILs in stage IA LAD, exclusively.
Methods
Study Patients
A total of 459 patients with LAD who had undergone surgery between January 2003 and December 2012 at the Department of Surgery and Science, Graduate School of Medical Sciences, Kyushu University were enrolled in the study. Of them, 229 patients were pathologically diagnosed with stage IA adenocarcinoma according to the 7th edition of the TNM Classification.15 Finally, 203 formalin-fixed paraffin-embedded specimens were available for immunohistochemical staining. After surgery, routine follow-up, including physical examination, blood tests, and chest radiographs, were performed at 3-month intervals for the first 3 years and 6-monthly thereafter. Clinicopathological characteristics, DFS, and OS were retrospectively analyzed. Clinicopathological characteristics assessed included age, sex, smoking history, vascular invasion (v), lymphatic invasion (ly), histological subtypes, surgical procedure, and EGFR mutation. This study was approved by our institutional review board (Kyushu University, IRB No. 29-402).
Immunohistochemical Staining
Immunohistochemical staining was performed in 203 surgically resected stage IA LADs. Sections were cut at 4-μm thickness from formalin-fixed and paraffin-fixed tissue blocks, then dewaxed with xylene, and rehydrated through a graded concentration series of ethanol. After inhibition of endogenous peroxidase activity with 3% hydrogen peroxide in methanol for 30 min, the sections were pretreated with Target Retrieval Solution (Dako) in a decloaking chamber at 121 °C for 15 min and then incubated with primary antibodies at 4 °C overnight. The primary antibodies were mouse monoclonal anti-human CD8 antibody (clone #C8/144B, dilution 1:100, Dako) and mouse monoclonal anti-human FoxP3 antibody (clone #236A/E7, dilution 1:100, eBioscience). The immune complex was detected with a DAKO EnVision Detection System (Dako). The sections were finally reacted in 3,30-diaminobenzidine, counterstained with hematoxylin, and mounted. Sections of tonsil were used as positive controls for CD8 and FoxP3. Stained slides were scanned using the NanoZoomer (Hamamatsu Photonics KK). In this study, all hematoxylin–eosin images and immunohistochemical images were reviewed by at least two investigators, including a pathologist, and TILs were distinguished from other cancer cells by their morphology.
The density of CD8 + and Foxp3 + TILs was evaluated by counting the number of CD8 + and Foxp3 + TILs per 0.04 mm2 over 5 fields, then averaging the cell counts. Samples were evaluated by at least two investigators, including a pathologist. The cutoff values of the number of CD8 + and FoxP3 TILs were 50 (cells/0.04 mm2) and 20 (cells/0.04 mm2), defined by ROC curve analysis (Supplementary Fig. 1).
Programmed cell death ligand-1 (PD-L1) expression was detected by immunohistochemical staining with rabbit monoclonal anti-human PD-L1 antibody (clone #SP142, dilution 1:100, Spring Bioscience), as described previously.16 In this study, more than 1% tumor membrane staining was considered to denote PD-L1 positivity.16
Statistical Analysis
Fisher’s exact test was used to analyze patients’ characteristics. DFS was defined as the period between surgery and the date of the last follow-up, recurrence or death, and OS as the period between surgery and the date of last follow-up or death. Survival curves were estimated by using the Kaplan–Meier method with the log-rank test. Cox proportional hazards regression analysis was performed to estimate the hazard ratios for the positive risk factors with the backward elimination method. All results were considered as statistically significant at P < 0.05. JMP pro 13.0 software (SAS Institute) was used for all statistical analyses.
Results
Clinicopathological Characteristics in Patients with Stage IA LAD
The study cohort comprised 203 patients with stage IA LAD who had undergone surgical resection (Table 1). The mean age was 68 (range 34–85) years, and 115 patients (56.7%) were female. On histological examination of resected tumors, 22 patients (10.8%) had tumors of noninvasive (adenocarcinoma in situ or minimally invasive adenocarcinoma), and 181 were invasive adenocarcinomas (89.2%). The surgical procedures included sublober resection performed on 74 patients (36.5%). Seventy (53.8%) patients had EGFR mutation, and 48 (23.6%) patients showed PD-L1 positivity.
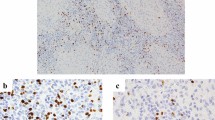
The mean numbers of CD8 + and FoxP3 + TILs were 39.2 (2.6–114.2; cells/0.04 mm2) and 12.4 (0–9.4; cells/0.04 mm2), respectively. Seventy-eight (38.4%) and 52 (25.6%) patients were classified as having high infiltrations of CD8 + (CD8-High) and FoxP3 + (FoxP3-High) TILs, respectively. Representative images with CD8 and FoxP3 staining with CD8-Low, CD8-High, FoxP3-Low, and FoxP3-High are shown in Figs. 1a–d, respectively.
Representative images of immunohistochemical staining of CD8 and FoxP3 in surgically resected specimens from patients with stage IA LAD. Typical CD8 staining of LAD with CD8-Low (a) and CD8-High (b), and typical FoxP3 staining of LAD with FoxP3-Low (c) and FoxP3-High (d). Scale bars: 100 mm. LAD lung adenocarcinoma; CD8 cluster of differentiation 8; FoxP3 Forkhead box protein P3
We examined the relationship between clinicopathological characteristics and CD8 + and FoxP3 + TILs (Supplementary Table 1). Patients with CD8-High were significantly associated with vascular invasion positivity, lobectomy and increased infiltration of FoxP3 + cells. Conversely, patients with FoxP3-High were significantly associated with vascular invasion positivity, invasive subtypes, PD-L1 positivity and CD8-High status.
Prognosis Analysis of Patients with Stage IA LAD According to CD8 + and FoxP3 + TILs
Prognostic analysis in relation to CD8 + and FoxP3 + TILs was performed using the Kaplan–Meier method. There was no significant difference between CD8-High and CD8-Low groups in both DFS (5-year DFS: 93.0% vs. 84.4%; P =0.2833; Fig. 2a) and OS (5-year OS: 97.4% vs. 95.6%; P =0.9583; Fig. 2b). In FoxP3 + TILs, the DFS was not significantly different in patients with FoxP3-High and FoxP3-Low groups (5-year DFS: 81.7% vs. 89.9%; P =0.0775; Fig. 2c); however, the OS was significantly worse in the FoxP3-High group than the FoxP3-Low group (5-year OS: 92.2% vs. 97.8%; P =0.0192; Fig. 2d).
Kaplan-Meier curves showing survival of patients with stage IA LAD according to CD8 + and FoxP3 + TILs. (a) Disease-free survival and (b) overall survival of CD8-Low and CD8-High groups. (c) Disease-free survival and (d) overall survival of FoxP3-Low and FoxP3-High groups. LAD lung adenocarcinoma; CD8 cluster of differentiation 8; FoxP3 Forkhead box protein P3; TILs tumor-infiltrating lymphocytes
Combined Evaluation of CD8 + and FoxP3 + TILs in Stage IA LAD and Survival Analysis
We further conducted combinatory analysis of CD8 + and FoxP3 + TILs. Patients were categorized into the following four groups: CD8-Low/FoxP3-Low, CD8-High/FoxP3-Low, CD8-Low/FoxP3-High, and CD8-High/FoxP3-High. The DFS and OS among the four groups had significant differences (P =0.0096 and P = 0.0463, respectively). In particular, the CD8-Low/FoxP3-High group had significantly worse prognosis in DFS and OS than the other groups (5-year DFS: 66.3% vs. 90.5%; P =0.0007; Fig. 3A, 5-year OS: 90.9% vs. 97.0%; P =0.0077; Fig. 3B). In our multivariate analysis, CD8-Low/FoxP3-High remained an independent predictor of DFS (hazard ratio: 3.22; 95% confidence interval: 1.321–7.179; P =0.0121; Table 2). We verified the association between the CD8-Low/FoxP3-High group and clinicopathological characteristics, and there were no characteristics associated with CD8-Low/FoxP3-High (Supplementary Table 2). There was an association between CD8-Low/FoxP3-High and high PD-L1 expression; however, it was not significant (P = 0.0607).
Kaplan-Meier curves showing survival of patients with stage IA LAD among the following four groups; CD8-Low/FoxP3-Low, CD8-High/FoxP3-Low, CD8-Low/FoxP3-High, and CD8-High/FoxP3-High. (a) Disease-free survival and (b) overall survival of CD8-Low/FoxP3-Low, CD8-High/FoxP3-Low, CD8-Low/FoxP3-High, and CD8-High/FoxP3-High groups. LAD lung adenocarcinoma; CD8 cluster of differentiation 8; FoxP3 Forkhead box protein P3
Discussion
In this study, we described the significance of CD8 + and FoxP3 + TILs in stage IA LAD. The density of CD8 + TILs did not have a significant effect on prognosis. In contrast, the high levels of FoxP3 TILs were associated with worse prognosis of OS but were not independent predictive factors for poor survival. However, the combined evaluation of CD8 + and FoxP3 + TILs elucidated that CD8-Low/FoxP3-High was significantly associated with poor prognosis in both OS and DFS and was an independent predictive factor for DFS. These results demonstrated that the combined evaluation of CD8 + and Foxp3 + TILs provides accurate prognosis for stage IA LAD.
Several reports mentioned the significance of CD8 + TILs in lung cancer. Past meta-analyses indicated that a high density of CD8 + TILs was associated with good prognosis in NSCLC.6,7 However, especially in LAD, some papers described the high infiltration of CD8 + TILs as an unfavorable prognostic factor.8,9,10 Thus, we thought that the significance of CD8 + TILs in lung cancer was not yet fully clarified. Shimizu et al. demonstrated that the high levels of CD8 + TILs were significantly related with PD-L1 expression positivity.17 Additionally, in the evaluation of prognosis of LAD by combining CD8 + TILs with PD-L1 expression, patients with CD8-Low and high PD-L1 expression had poor prognosis; conversely, patients with CD8-High and low PD-L1 expression had good prognosis.17 Furthermore, Kim et al. also described that high levels of CD8 + TILs and low PD-L1 expression together were associated with favorable prognosis in NSCLC.18 Koh et al.19 reported that in LAD, the high density of CD8 + TILs was a good prognostic factor; however, the high ratio of PD-1 + TILs to CD8 + TILs was correlated with a poor prognosis. Furthermore, Kinoshita et al. elucidated that high levels of CD8 + TILs were associated with poor prognosis in LAD, particularly in non-smokers, and further analysis showed that immunoregulatory CD8 + lymphocytes co-expressed FoxP3 and immunodysfunctional CD8 + lymphocytes co-expressed GATA-binding protein 3 were increased in the LADs of nonsmokers.10 Based on these past studies, the significance of CD8 + TILs might fluctuate by histology, patient characteristics, and immune environment surrounding CD8 + TILs.
The methods for evaluating FoxP3 + TILs were diverse, such as ratio of FoxP3/CD8, FoxP3/CD4 and FoxP3/CD3.11,20,21 However, almost all past studies reported that high infiltration of FoxP3 + TILs was associated with poor prognosis in NSCLC.6,7,20,21 Furthermore, FoxP3 + TILs are important to the field of immunotherapy where an association between FoxP3 + TILs and PD-L1 expression was demonstrated.22 A low ratio of FoxP3/CD8 was reported as a therapeutic predictor of PD-1 inhibitor.11
As described above, CD8 + and FoxP3 + TILs were closely connected with the prognosis of lung cancer and the therapeutic effect of immunotherapy. However, while the significance of FoxP3 + TILs was consistent, the significance of CD8 + TILs was still controversial. One of the reasons was that past studies on TILs included patients with advanced as well as early stage lung cancer. We considered that the cancer stage was associated with changes in the immune microenvironment of lung cancer. Another reason was that the evaluation method of TILs varied widely. Our study analyzed whole tumor sections and assessed TILs evenly in the tumor tissue. In contrast, several studies were analyzed by tissue microarray or evaluated separately as cancer stoma and nests.8,18,21,23,24 Furthermore, the functions of CD8 + TILs were controlled by many factors, such as FoxP3 + TILs or PD-1/PD-L1 signal, and it was difficult to elucidate the significance of CD8 + TILs only by assessment of the number. Therefore, the analysis of only CD8 + TILs might be insufficient for assessing the significance of CD8 + TILs in lung cancer. Thus, we analyzed the combination of cytotoxic CD8 + and immunosuppressive FoxP3 + TILs in stage IA LAD.
Our study showed that the prognosis of the CD8-Low/FoxP3-High group was worse than other groups; however, there was no significant difference among the CD8-Low/FoxP3-Low, CD8-High/FoxP3-Low, and CD8-High/FoxP3-High groups. Our study cohort included only stage IA LAD and the prognosis was relatively good. Therefore, only the CD8-Low/FoxP3-High group with worse immune status might show significant differences with other groups. We propose that a larger study is needed to elucidate the difference between CD8-Low/FoxP3-Low, CD8-High/FoxP3-Low, and CD8-High/FoxP3-High groups.
We analyzed PD-L1 expression in tumor cells in addition to CD8 + and FoxP3 + TILs. In a multivariate analysis, PD-L1 expression was an independent prognostic predictor for both DFS and OS. While not significant, CD8-Low/FoxP3-High group tended to have higher PD-L1 expression. This trend is possibly one of the reasons why the CD8-Low/FoxP3-High group had a poor prognosis.
The standard therapy for stage IA LAD is surgical resection and patients with pathological stage IA disease tend to have a long survival time after complete surgical resection. However, the survival rate after recurrence is very poor.25 Therefore, it is important to identify survival-associated factors for stage IA lung cancer. If we could predict the stage IA lung cancer patients with poor prognosis, adjuvant chemotherapy would be one of the treatment options for the patients after surgical resection.
One limitation of our study is that we could not evaluate all factors that had effects on the function of CD8 + TILs. Furthermore, the design of this study was retrospective, and the study cohort was relatively small due to the selection of patients with stage IA LAD. In addition, the PD-L1 analysis was performed using a specific antibody against PD-L1 (SP142). According to the report by the Blueprint Working Group, the detection rate for the SP142 clone was low compared with other antibodies, such as 28-8, 22C3, and SP263.26 Thus, we should investigate PD-L1 expression using other antibodies in future studies.
Conclusions
We showed the importance of analyzing the combination of CD8 + and Foxp3 + TILs in stage IA LAD. We consider that combinational analysis of TILs was required to further elucidate the significance of TILs in lung cancer.
References
Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39.
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35.
Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28.
Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65.
Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–29.
Geng Y, Shao Y, He W, et al. Prognostic role of tumor-infiltrating lymphocytes in lung cancer: a meta-analysis. Cell Physiol Biochem. 2015;37:1560–71.
Zeng DQ, Yu YF, Ou QY, et al. Prognostic and predictive value of TILs for clinical therapeutic research in patients with NSCLC. Oncotarget. 2016;7:13765–81.
Wakabayashi O, Yamazaki K, Oizumi S, et al. CD4 + T cells in cancer stroma, not CD8 + T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. 2003;94:1003–9.
Lin C, Chen X, Li M, et al. Programmed death-Ligand 1 expression predicts tyrosine kinase inhibitor response and better prognosis in a cohort of patients with epidermal growth factor receptor mutation-positive lung adenocarcinoma. Clin Lung Cancer. 2015;16:e25–35.
Kinoshita T, Kudo-Saito C, Muramatsu R, et al. Determination of poor prognostic immune features of tumour microenvironment in non-smoking patients with lung adenocarcinoma. Eur J Cancer. 2017;86:15–27.
Kim H, Kwon HJ, Han YB, et al. Increased CD3 + T cells with a low FOXP3 +/CD8 + T cell ratio can predict anti-PD-1 therapeutic response in non-small cell lung cancer patients. Mod Pathol. 2019;32:367–75.
Uryvaev A, Passhak M, Hershkovits D, Sabo E, Bar-Sela G. The role of tumor-infiltrating lymphocytes (TILs) as a predictive biomarker of response to anti-PD1 therapy in patients with metastatic non-small cell lung cancer or metastatic melanoma. Med Oncol. 2018;35:25.
Barnes TA, Amir E. HYPE or HOPE: the prognostic value of infiltrating immune cells in cancer. Br J Cancer. 2017;117:451–60.
Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression—implications for anticancer therapy. Nat Rev Clin Oncol 2019;16:356–71.
Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14.
Takada K, Okamoto T, Shoji F, et al. Clinical significance of PD-L1 protein expression in surgically resected primary lung adenocarcinoma. J Thorac Oncol. 2016;11:1879–90.
Shimizu K, Okita R, Saisho S, Maeda A, Nojima Y, Nakata M. Prognostic value of Cox-2 and PD-L1 expression and its relationship with tumor-infiltrating lymphocytes in resected lung adenocarcinoma. Cancer Manag Res. 2017;9:741–50.
Kim S-H, Go S-I, Song DH, et al. Prognostic impact of CD8 and programmed death-ligand 1 expression in patients with resectable non-small cell lung cancer. Br J Cancer. 2019;120:547–54.
Koh J, Go H, Keam B, et al. Clinicopathologic analysis of programmed cell death-1 and programmed cell death-ligand 1 and 2 expressions in pulmonary adenocarcinoma- comparison with histology and driver oncogenic alteration status. Mod Pathol. 2015;28:1154–66.
Kinoshita T, Muramatsu R, Fujita T, et al. Prognostic value of tumor-infiltrating lymphocytes differs depending on histological type and smoking habit in completely resected non-small-cell lung cancer. Ann Oncol. 2016;27:2117–23.
Suzuki K, Kadota K, Sima CS, et al. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor beta2 (IL-12Rbeta2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol. 2013;31:490–8.
Giatromanolaki A, Banham AH, Harris AL, Koukourakis MI. FOXP3 infiltrating lymphocyte density and PD-L1 expression in operable non-small cell lung carcinoma. Exp Lung Res. 2019;45:76–83.
Schalper KA, Brown J, Carvajal-Hausdorf D, et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst. 2015;107.
Bremnes RM, Busund LT, Kilvaer TL, et al. The role of tumor-infiltrating lymphocytes in development, progression, and prognosis of non-small cell lung cancer. J Thorac Oncol. 2016;11:789–800.
Hung JJ, Jeng WJ, Chou TY, et al. Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann Surg. 2013;258:1079–86.
Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017;12:208–22.
Acknowledgment
The authors thank S. J. Win, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kinoshita, F., Takada, K., Yamada, Y. et al. Combined Evaluation of Tumor-Infiltrating CD8 + and FoxP3 + Lymphocytes Provides Accurate Prognosis in Stage IA Lung Adenocarcinoma. Ann Surg Oncol 27, 2102–2109 (2020). https://doi.org/10.1245/s10434-019-08029-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-08029-9






