Abstract
Background
Perioperative use of nonsteroidal anti-inflammatory drugs (NSAIDs) is known to reduce inflammatory response in relation to surgery. Inflammation may promote recurrence of cancer, thus inhibition by use of NSAIDs could reduce recurrence after surgery.
Objective
The aim of this study was to examine the association between perioperative use of NSAIDs and cancer recurrence, as well as disease-free survival (DFS) and mortality after colorectal cancer surgery.
Methods
This was a cohort study based on data from a prospective clinical database, electronic medical records, and nationwide registers, and included patients from six major colorectal centers in Denmark. The primary outcome was cancer recurrence, while secondary outcomes included 5-year mortality and DFS.
Results
Overall, 2308 patients undergoing colorectal cancer surgery between 1 January 2006 and 31 December 2009 were included. A total of 909 patients received at least 2 days of treatment with NSAIDs, of whom 702 (77.2%) received ibuprofen and 204 (22.4%) received diclofenac. Cox regression analysis adjusting for NSAIDs resulted in decreased recurrence risk (adjusted hazard ratio [HRadjusted] 0.84, 95% confidence interval [CI] 0.72–0.99; p = 0.042). Competing risk analysis confirmed the finding, with an HRadjusted of 0.76 (95% CI 0.60–0.97; p = 0.026). There was no significant effect on mortality or DFS. Sensitivity analysis of the effect of ibuprofen reported an HRadjusted of 0.83 (95% CI 0.70–1.00; p = 0.047). In restricted analyses of localized disease only (Union for International Cancer Control [UICC] I–II) and elective surgery only, no effect was found (localized: HRadjusted 0.81, 95% CI 0.62–1.06, p = 0.12; elective: HRadjusted 0.85, 95% CI 0.72–1.01, p = 0.063).
Conclusions
Perioperative use of NSAIDs was associated with a reduced risk of cancer recurrence after resection for colorectal cancer. No effect on 5-year mortality or DFS was found.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In curative-intent treatment of colorectal cancer, surgery remains the cornerstone. Surgery in itself induces an inflammatory response with the purpose of optimizing the healing process after surgical trauma.1 This neural, inflammatory, angiogenetic response results in optimal wound healing.2 At the same time, cancer cells thrive in this inflammatory environment, where local and systemic responses to tissue injury promote malignant growth.3 Cancer cells that are not resected therefore have improved growth conditions, and postsurgical micrometastases have increased potential of leading to recurrence of cancer.3 This has been demonstrated in several types of cancer, where surgery has been shown to increase the rate of recurrence.4,5,6 Indeed, a prolonged inflammatory state leads to a greater risk of recurrence.7,8 In addition, the magnitude of the surgical stress response could also influence the risk of recurrence.3
Nonsteroidal anti-inflammatory drugs (NSAIDs), often used as part of a multimodal analgesic regimen after surgery, have been shown to decrease the tumor-associated inflammation in animal models and potentially prevent metastasis.9,10,11 In humans, a decreased inflammatory response at surgery is found with perioperative use of NSAIDs. Furthermore, studies have shown a lower recurrence rate after surgery for breast cancer12,13 and hepatocellular carcinoma.14
In an historic cohort of prospectively collected data from 2006 to 2009, postoperative use of diclofenac, a cyclooxygenase (COX)-2 selective NSAID, led to an increased risk of anastomotic leakage after primary colorectal resection for cancer.15 This implies decreased local tissue healing after surgery, and thus decreased inflammatory response. The same effect was not found with respect to a nonselective NSAID such as ibuprofen. Paradoxically, anastomotic leakage itself has been shown to increase the risk of cancer recurrence due to inflammatory response to leakage.16
The aim of this study was to investigate whether the use of NSAIDs in the immediate postoperative period after elective colorectal resection influenced the risk of cancer recurrence.
Methods
Study Cohort
This historic cohort study was reported according to the RECORD guidelines, an extension of the existing STROBE guidelines,17 and was based on data from the Danish Colorectal Cancer Group’s (DCCG) national prospective database. Patients from the six major colorectal cancer surgery centers in eastern Denmark were included. Patients underwent a curative-intent operation for colorectal cancer between 1 January 2006 and 31 December 2009 with either colonic or rectal resection, and received a primary anastomosis.
The DCCG database includes, among others, information on demographic factors, such as comorbidities and perioperative and postoperative treatment. Overall, 98.6% of patients with a diagnosis of colorectal cancer are included as part of the database population.18 Information including age at diagnosis, American Society of Anesthesiologists (ASA) score (1–5), Charlson Comorbidity Index (CCI),19 WHO performance score (1–5), alcohol consumption (categorized as the number of weekly drinks of alcohol [1 drink = 12 g ethanol]: 0 drinks, 1–14 drinks, 15–21 drinks, > 21 drinks), tobacco use (current smoker, former smoker, or never smoker), blood transfusion in the perioperative period, body mass index (BMI; < 20 kg/m2, 20–24.9 kg/m2, 25–29.9 kg/m2, > 30 kg/m2), tumor classification (TNM and Union for International Cancer Control [UICC]), operative procedure(s), type of surgery (emergency or elective surgery), and date of surgery was obtained from the DCCG database. A unique personal identification number (CPR number) is assigned to all Danish citizens and information on mortality is documented in the Danish Civil Registration System. The database is complete and virtually no registered individuals are lost to follow-up.20 The CPR number is used as an inter-source linkage throughout all registries. Hospital contacts are registered in the Danish National Patient Register (NPR),21 where data regarding hospital admissions, such as date of admission and discharge, diagnoses, and procedures, as well as outpatient visits, are registered.
Regarding biological specimens, standard data are coded using the Danish version of the Systemized Nomenclature of Medicine (SNOMED) codes by all Danish pathology departments. The Danish Pathology Register (DPR)22 use SNOMED codes, and pathologically diagnosed recurrences are documented in the Register.
Assessment of Nonsteroidal Anti-inflammatory Drug Use
Electronic medical records have been used in Danish hospitals since 2003. All treatments administered at a hospital are prospectively documented. Medical staff administer treatment and simultaneously complete electronic registration, rendering data collection thorough and close to complete. Postoperative NSAID consumption, including type, regimen, date of prescription and dose, was registered for each patient by three independent reviewers. Postoperative consumption of NSAIDs was defined as a minimum of 2 days of treatment with a clinically relevant dose of an NSAID through the first 7 days after surgery, while relevant daily dose was defined as at least 50 mg for diclofenac and at least 800 mg for ibuprofen.23 The presence or absence of NSAID treatment in the perioperative period was confirmed to be based primarily on perioperative standard analgesic treatment, and not based on indication.23
Study Design
Patients from the historical cohort were included in the study and grouped/stratified according to the presence or absence of NSAID treatment as defined above. The primary outcome was recurrence of cancer within 5 years after surgery, while overall survival (OS) and disease-free survival (DFS) were secondary outcomes.
Recurrence during follow-up was estimated using a validated algorithm described in detail elsewhere.24 This algorithm is based on:
-
NPR-registered metastasis codes 180 days after the first colorectal cancer surgery, and, at the same time, no new primary cancer diagnosis from the date of colorectal cancer surgery to the date of the metastasis code.
-
Cytostatic therapy, registered in the NPR, at least 180 days after primary colorectal cancer surgery and at least 60 days after the last NPR-registered cytostatic therapy code, without a new occurrence of primary tumor from the date of colorectal cancer surgery to the date of the cytostatic therapy code.
-
DPR-registered SNOMED combinations indicating recurrence documented at least 180 days after the primary colorectal cancer surgery, with no new primary cancer diagnosis.
-
Specific codes for local colorectal cancer recurrence in the NPR (used since the beginning of 2012), any time after primary colorectal surgery: DC189X and DC209X.
Exclusion criteria were death within 180 days after surgery, or metastases at surgery or within 180 days after surgery, since investigation of recurrence is irrelevant in such cases.24 Furthermore, other cancer diagnoses (except nonmelanoma skin cancer) before the registered date of colorectal cancer diagnosis were also considered exclusion criteria as the NPR-registered metastasis codes do not specify the origin of metastasis.
Statistical Methods
Cox regression models were used to estimate all-cause survival, recurrence-free survival (RFS), and DFS. Patients underwent censoring if a new primary tumor occurred or if death from any cause occurred within 180 days of surgery.
RFS was defined as the time from 180 days after surgery to cancer recurrence. Death was not defined as an event for this outcome. OS was defined as the time from 180 days after surgery to death from any cause within 5 years of surgery. Finally, DFS, combined the two abovementioned definitions with the occurrence of a new primary cancer as it was defined as the first of three possible events within 5 years after surgery: (1) recurrence of cancer; (2) death by any cause; and (3) development of a new primary cancer.
Primary analyses were adjusted for age and sex, whereas the multivariate models were adjusted for age at diagnosis, sex, BMI, preoperative oncological treatment, CCI, perioperative blood transfusions, surgical priority, anatomical cancer localization (colon or rectum), UICC stage, and anastomotic leakage. Results are presented as hazard ratios (HRs) with 95% confidence intervals (CIs). A p value < 0.05 was considered statistically significant. Competing risk analyses were performed using the Fine and Grey method25 in regard to RFS.
Sensitivity Analyses
Three separate sensitivity analyses were conducted. In one, a sensitivity analysis regarding the type of NSAID was conducted, adjusting in the same manner as the primary analysis. In the subsequent two analyses, the primary analysis was repeated using restrictions: (1) to create an homogenous group, patients undergoing acute surgery were excluded; and (2) to identify those with localized disease, only patients with UICC stage I or II disease were included.
SAS® Proprietary Software 9.4 (SAS Institute Inc., Cary, NC USA) was used for statistical analysis. This study was approved by the Scientific Council of the DCCG, and by the Danish Data Protection Agency (REG-081-2018) prior to initiation.
Results
Overall Identification of Population
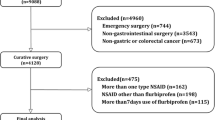
A total of 2756 patients fulfilled the inclusion criteria and were eligible for inclusion in this study. Of these, 448 patients had missing data regarding the date of operation (n = 40) or were excluded due to other reasons (Fig. 1). Thus, 2308 patients were included in the study, of whom 909 received at least 2 days of treatment with any type of NSAID. Of the treated patients, 702 (77.2%) received ibuprofen and 204 (22.4%) received diclofenac; 3 (0.4%) patients received a different type of NSAID. No patients received two or more types of NSAIDs.
Table 1 presents demographic variables and patient characteristics. The mean age of the entire population was 68.5 years (standard deviation ± 10.8), and sex was equally distributed (1189 [52%] men). Overall, 630 (27%) patients were diagnosed with recurrence of cancer, and 610 (26%) patients died within 5 years after surgery. A total of 1751 (76%) patients had colonic tumors, whereas the remaining had rectal tumors. Among patients who did not receive NSAIDs, 63 (5%) experienced an anastomotic leak, along with 73 (8%) of the treated patients (p < 0.001).
Table 2 presents demographic variables and patient characteristics regarding the type of NSAID use.
Recurrence-Free Survival
Recurrence of cancer occurred in 390 (28%) controls, compared with 240 (26%) NSAID-treated patients (p = 0.44). In the primary analysis, no association with RFS was found (HR 0.94, 95% CI 0.80–1.01; p = 0.41); however, in the adjusted analysis, an association was found where treatment with NSAIDs resulted in a decreased risk of recurrence (adjusted HR [HRadjusted] 0.84, 95% CI 0.72–0.99; p = 0.042) (Table 3). This association was confirmed in the competing risk model, with an HRadjusted of 0.76 (95% CI 0.60–0.97; p = 0.026).
Disease-Free Survival and Overall Survival
In the primary analysis, use of an NSAID was not associated with benefits in either DFS (HR 1.04, 95% CI 0.93–1.17; p = 0.49) or 5-year mortality (HR 1.10, 95% CI 0.94–1.30; p = 0.24). In the subsequent multivariate analyses, no association between NSAID use and DFS (HRadjusted 0.97, 95% CI 0.86–1.10; p = 0.65) or 5-year mortality was found (HRadjusted 0.96, 95% CI 0.81–1.13, p = 0.60) (Table 3). The open approach was a risk factor for overall mortality (HRadjusted 1.27, 95% CI 1.06–1.52; p = 0.009).
Sensitivity Analyses
All sensitivity analyses found similar tendencies regarding the association between NSAID use and RFS, as well as 5-year OS and DFS. Analysis regarding the type of NSAID, as seen in Table 4, presented an association between the use of ibuprofen and RFS (HRadjusted 0.83, 95% CI 0.67–1.00; p = 0.047).
The sensitivity analyses with restrictions are presented in Table 5. For the population consisting of elective surgical patients only, an HRadjusted of 0.85 (95% CI 0.72–1.01; p = 0.063) was found with respect to DFS. For the population with limited disease only, i.e. patients presenting with UICC stage I and II disease, no significant association was found with respect to RFS (HRadjusted 0.81, 95% CI 0.62–1.06; p = 0.12).
Discussion
In this study, based on prospective data from nationwide clinical databases and electronic medical records including information on postoperative treatment, we used multivariate analyses to demonstrate a decreased risk of cancer recurrence with NSAID treatment after surgical resection for colorectal cancer. A competing risk analysis confirmed this finding. No effect was found on DFS or OS. Likewise, patients receiving postoperative ibuprofen had a significant reduction in risk of recurrence. In sensitivity analyses of UICC stage I–II, elective surgery, and treatment with diclofenac, similar tendencies were found as NSAIDs reduced the risk of recurrence but failed to reach statistical significance, perhaps due to the limited sample size in these subgroups. This is the first study to provide novel evidence that treatment with NSAIDs in immediate relation to surgery for colorectal cancer may influence the risk of recurrence.
NSAIDs are known to inhibit tumor-associated inflammation and reduce angiogenesis and lymphangiogenesis, thereby inhibiting the recurrence of cancer, as well as having several chemopreventive effects.3 This is in part explained by inhibition of COX-2, which has been shown to be a promising antineoplastic target.11,26 The COX-2 inflammatory pathway relies on prostaglandins, which are known as tumor-promoting agents.27 The COX-2 pathway has been shown to be overexpressed in > 80% of colorectal cancers,28 and overexpression affects the effect of aspirin treatment, which also inhibits COX-2.29,30 Furthermore, alternative targets have been suggested as an explanation for the anticancer activities of NSAIDs, such as inhibition of the cancer-specific surface protein tNOX31 and increased expression of human leukocyte antigen (HLA) class I and HLA-DR antigen of cancer cells.32,33 In our study, we observed a pooled effect of NSAIDs, as well as ibuprofen alone. Diclofenac34 is known to primarily inhibit the inducible COX-2, whereas nonselective NSAIDs such as ibuprofen also inhibit the constitutively expressed COX-1.35 An effect of ibuprofen alone is not commonly described or examined in the literature, but several factors beyond COX inhibition could play a role.36
NSAIDs are often used in postoperative analgesic regimens. The period immediately after surgery is known to be particularly valuable in improving long-term cancer outcomes.3 In our study, an effect is observed with the use of NSAIDs for a minimum of 2 days within the first 7 postoperative days. In our population, an increased rate of postoperative anastomotic leakage was previously found when treated with diclofenac, but not ibuprofen.23 This adds to the point that NSAIDs play a role at the surgical lesion during postsurgical inflammation, influencing the risk of leakage.37 Paradoxically, anastomosis leakage is known to increase the risk of recurrence,38 but this seemed to be counteracted by the anti-inflammatory benefits of NSAIDs. Given that the association of anastomotic leakage with ibuprofen is either non-existent or weak, and that in our sensitivity analysis we could demonstrate an association with lower recurrence, ibuprofen might be the drug to investigate in more detail with respect to an effect on recurrence.
The use of NSAIDs in relation to colorectal cancer has been investigated in several studies. A 2011 study investigated colorectal cancer-specific survival and patient-reported use of NSAIDs prior to a diagnosis of cancer, and found improved survival in patients reporting use of NSAIDs.39 Another study investigated the effect of aspirin preoperatively and found a higher rate of downstaging when receiving neoadjuvant therapy in users of aspirin.40
Several investigators have reported an association between postoperative treatment with NSAIDs and outcome. A nationwide cohort study including 15,544 patients found a reduced risk of early recurrence within 2 years after resection for hepatocellular carcinoma. The use of NSAIDs was assessed by the pharmacy register and included both COX-2 selective and nonselective NSAIDs, and aspirin, until 4 months after surgery. No evaluation of type, dose, or timing of NSAIDs was made.14
A 2018 study prospectively investigated the influence of ketorolac, a COX-2 selective NSAID, in relation to primary surgery for breast cancer. The authors demonstrated that a single intraoperative dose of ketorolac was associated with a reduced risk of recurrence. But this was not seen in patients receiving intraoperative diclofenac.13 Finally, in a study investigating cancer recurrence after mastectomy, a retrospective analysis of 327 cases favored ketorolac over other analgesics with respect to recurrence of cancer.12
The primary strength of this study relies on the study design, as well as the accuracy and validity of the data. The DCCG database is consecutively controlled, and validation showed that 98.6% of all patients diagnosed with colorectal cancer are registered.18,41 Furthermore, our results regarding cancer recurrence rely on a previously validated algorithm used to identify recurrence in both colonic and rectal cancer.24,42 The assessment of NSAID use was not based on analgesic regimens, but instead performed as individual patient-by-patient registrations by separate observers, including only doses registered as taken. In our multivariate analysis, a purposeful selection of variables was made, based on the potential clinical influence on the primary outcome, taking the validity of each variable into account.
The findings of this study should be interpreted with caution as a causal association between NSAIDs and the risk of recurrence of colorectal cancer cannot be deduced on the basis of an observational study. Potential unmeasured confounders may cause a difference in outcomes; for instance, our data lack the granularity to investigate specific segments of colonic and rectal resections as the tissue trauma varies, resulting in variation in the surgical stress response.
In our study, no data regarding NSAID use beyond postoperative day 7 are included, and patients treated with only 1 day of NSAID use were interpreted as non-users; however, both these limitations would only lead to an underestimation of our findings. Furthermore, our study was constrained to a limited sample size, leading to an increased risk of type II errors of primary and secondary analyses. Our results failed to show an effect on mortality and DFS, which in part could be explained by these limitations. Furthermore, in recent years, the treatment of recurrence of cancer has improved, and it is possible that the effect of NSAIDs is too small to break through on the two secondary outcomes within the time frame of our study.
In our sensitivity analyses, the additional reduction in study population impacts the power of the analyses, and results in insignificant results, while similar tendencies are still observed with respect to NSAID use and recurrence of cancer. Comparable results are observed with respect to both our restrictions, as well as both specific NSAIDs in the sensitivity analyses. Future studies should increase the size of the study population to repeat the sensitivity analyses, as well as investigate whether an effect on mortality or DFS can also be demonstrated. A future prospective study should also investigate biomarkers related to inflammation in order to add translational evidence to the epidemiological evidence provided in this article.
Conclusion
This study showed that postoperative use of NSAIDs was associated with a reduced risk of recurrence after resection for colorectal cancer. Our results justify the requirement for further studies on the potential of NSAIDs in the colorectal cancer treatment routine.
References
Arias JI, Aller MA, Arias J. Surgical inflammation: a pathophysiological rainbow. J Transl Med. 2009;7:19.
Weiss U. Inflammation. Nature. 2008;454(7203):427.
Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol. 2018;15(4):205–18.
Oosterling SJ, van der Bij GJ, van Egmond M, van der Sijp JRM. Surgical trauma and peritoneal recurrence of colorectal carcinoma. Eur J Surg Oncol. 2005;31(1):29–37.
Kelsey CR, Fornili M, Ambrogi F, Higgins K, Boyd JA, Biganzoli E, et al. Metastasis dynamics for non-small-cell lung cancer: effect of patient and tumor-related factors. Clin Lung Cancer. 2013;14(4):425–32.
Lee J-W, Shahzad MMK, Lin YG, Armaiz-Pena G, Mangala LS, Han H-D, et al. Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res. 2009;15(8):2695–702.
Dillekås H, Demicheli R, Ardoino I, Jensen SAH, Biganzoli E, Straume O. The recurrence pattern following delayed breast reconstruction after mastectomy for breast cancer suggests a systemic effect of surgery on occult dormant micrometastases. Breast Cancer Res Treat. 2016;158(1):169–78.
Isern AE, Manjer J, Malina J, Loman N, Martensson T, Bofin A, et al. Risk of recurrence following delayed large flap reconstruction after mastectomy for breast cancer. Br J Surg. 2011;98(5):659–66.
Le CP, Nowell CJ, Kim-Fuchs C, Botteri E, Hiller JG, Ismail H, et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun. 2016;7:10634.
Glasner A, Avraham R, Rosenne E, Benish M, Zmora O, Shemer S, et al. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a β-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184(5):2449–57.
Yakar I, Melamed R, Shakhar G, Shakhar K, Rosenne E, Abudarham N, et al. Prostaglandin E 2 suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann Surg Oncol. 2003;10(4):469–79.
Forget P, Vandenhende J, Berliere M, MacHiels JP, Nussbaum B, Legrand C, et al. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth Analg. 2010;110:1630–5.
Desmedt C, Demicheli R, Fornili M, Bachir I, Duca M, Viglietti G, et al. Potential benefit of intra-operative administration of ketorolac on breast cancer recurrence according to the patient’s body mass index. J Natl Cancer Inst. 2018;110:1115–22.
Yeh C-C, Lin J-T, Jeng L-B, Ho HJ, Yang H-R, Wu M-S, et al. Nonsteroidal anti-inflammatory drugs are associated with reduced risk of early hepatocellular carcinoma recurrence after curative liver resection: a nationwide cohort study. Ann Surg. 2015;261(3):521–6.
Klein M, Andersen LPH, Harvald T, Rosenberg J, Gögenur I. Increased risk of anastomotic leakage with diclofenac treatment after laparoscopic colorectal surgery. Dig Surg. 2009;26(1):27–30.
Lu ZR, Rajendran N, Lynch AC, Heriot AG, Warrier SK. Anastomotic leaks after restorative resections for rectal cancer compromise cancer outcomes and survival. Dis Colon Rectum. 2016;59(3):236–44.
Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLoS Med. 2015;12(10):e1001885. https://doi.org/10.1371/journal.pmed.1001885.
Kronborg O. Danish Colorectal Cancer Group (DCCG). Dan Med Bull. 2016;55(2):129.
Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51.
Pedersen CB, Gøtzsche H, Møller JØ, Mortensen PB. The Danish civil registration system. A cohort of eight million persons. Dan Med Bull. 2006;53:441–9.
Lynge E, Sandegaard J, Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39:30–3.
Erichsen R, Lash TL, Hamilton-Dutoit SJ, Bjerregaard B, Vyberg M, Pedersen L. Existing data sources for clinical epidemiology: the Danish national pathology registry and data bank. Clin Epidemiol. 2010;2(1):51–6.
Klein M, Gogenur I, Rosenberg J. Postoperative use of non-steroidal anti-inflammatory drugs in patients with anastomotic leakage requiring reoperation after colorectal resection: cohort study based on prospective data. BMJ. 2012;345:e6166.
Lash TL, Riis AH, Ostenfeld EB, Erichsen R, Vyberg M, Thorlacius-Ussing O. A validated algorithm to ascertain colorectal cancer recurrence using registry resources in Denmark. Int J Cancer. 2015;136(9):2210–5.
Fine JP, Gray RJ. A Proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509
Dannenberg AJ, Subbaramaiah K. Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell. 2003;4:431–6.
Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–22.
Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, Dubois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107(4):1183–8.
Chan AT. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302(6):649–59.
Fuchs C, Meyerhardt JA, Heseltine DL, Niedzwiecki D, Hollis D, Chan AT, et al. Influence of regular aspirin use on survival for patients with stage III colon cancer: findings from Intergroup trial CALGB 89803. J Clin Oncol. 2005;23(16 Suppl):3530.
Morré DJ, Morre DM. tNOX, an alternative target to COX-2 to explain the anticancer activities of non-steroidal anti-inflammatory drugs (NSAIDS). Mol Cell Biochem. 2006;283(1–2):159–67.
Reimers MS, Engels CC, Putter H, Morreau H, Liefers GJ, van de Velde CJH, et al. Prognostic value of HLA class I, HLA-E, HLA-G and Tregs in rectal cancer: a retrospective cohort study. BMC Cancer. 2014;14(1):486.
Arvind P, Papavassiliou ED, Tsioulias GJ, Qiao L, Lovelace CIP, Duceman B, et al. Prostaglandin E2Down-regulates the expression of HLA-DR antigen in human colon adenocarcinoma cell lines. Biochemistry. 1995;34(16):5604–9.
Pichot Pla C, Ruiz López R. Diclofenac. DOLOR. 2004;19(4):227–36.
Orlando BJ, Lucido MJ, Malkowski MG. The structure of ibuprofen bound to cyclooxygenase-2. J Struct Biol. 2015;189(1):62–6.
Matos P, Jordan P. Beyond COX-inhibition: “side-effects” of ibuprofen on neoplastic development and progression. Curr Pharm Des. 2015;21(21):2978–82.
Modasi A, Pace D, Godwin M, Smith C, Curtis B. NSAID administration post colorectal surgery increases anastomotic leak rate: systematic review/meta-analysis. Surg Endosc. 2019;33(3):879–885.
Branagan G, Finnis D. Prognosis after anastomotic leakage in colorectal surgery. Dis Colon Rectum. 2005;48(5):1021–6.
Coghill AE, Newcomb PA, Campbell PT, Burnett-Hartman AN, Adams SV, Poole EM, et al. Prediagnostic non-steroidal anti-inflammatory drug use and survival after diagnosis of colorectal cancer. Gut. 2011;60(4):491–8.
Restivo A, Cocco IMF, Casula G, Scintu F, Cabras F, Scartozzi M, et al. Aspirin as a neoadjuvant agent during preoperative chemoradiation for rectal cancer. Br J Cancer. 2015;113(8):1133–9.
Nickelsen TN, Harling H, Kronborg O, Bulow S, Jorgensen T. The completeness and quality of the Danish Colorectal Cancer clinical database on colorectal cancer [in Danish]. Ugeskr Laeger. 2004;166(36):3092–5
Colov EP, Fransgaard T, Klein M, Gögenur I. Validation of a register-based algorithm for recurrence in rectal cancer. Dan Med J. 2018;65(10):pii:5507.
Funding
No sources of funding were used to assist in the preparation of this study.
Author information
Authors and Affiliations
Contributions
Anders Schack participated in study design, data collection, data analysis, data interpretation, and drafting and critical revision of the manuscript. Tina Fransgaard participated in study design, data collection, data analysis, data interpretation, and critical revision of the manuscript. Mads Klein participated in data collection, data interpretation, and critical revision of the manuscript. Ismail Gögenur participated in study design, data interpretation, and critical revision of the manuscript.
Corresponding author
Ethics declarations
Disclosure
Anders Schack, Tina Fransgaard, Mads Falk Klein, and Ismail Gögenur have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schack, A., Fransgaard, T., Klein, M.F. et al. Perioperative Use of Nonsteroidal Anti-inflammatory Drugs Decreases the Risk of Recurrence of Cancer After Colorectal Resection: A Cohort Study Based on Prospective Data. Ann Surg Oncol 26, 3826–3837 (2019). https://doi.org/10.1245/s10434-019-07600-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07600-8