Abstract
Background
Immune checkpoint and BRAF-targeted inhibitors have demonstrated significant survival benefits for advanced melanoma patients within the context of clinical trials. We sought to determine their impact on overall survival (OS) at a population level in order to better understand the current landscape for patients diagnosed with clinical stage III melanoma.
Methods
A retrospective study was performed using the National Cancer Database. Patients diagnosed with clinical stage III melanoma were categorized by diagnosis year into two cohorts preceding the advent of novel therapies (P1: 2004–2005, P2: 2008–2009) and a contemporary group (P3: 2012–2013). OS was estimated using standard time-to-event statistical methods.
Results
Of 3720 patients, 525 (14%) were diagnosed in P1, 1375 (37%) in P2, and 1820 (49%) in P3. Median age at diagnosis increased over time (58, 59, and 61 years in P1, P2, and P3, respectively, P = 0.004). OS increased between P2 (median 49.3 months) and P3 (median 58.2 months, Bonferroni-corrected log-rank P < 0.001) but did not differ between P1 (median 50.5 months) and P2 (Bonferroni-corrected log-rank P > 0.99). These differences persisted on multivariable analysis. OS improved for patients diagnosed in P3 compared with P1 [hazard ratio (HR) 0.76, P < 0.001] but not P2 compared with P1 (HR 0.96, P = 0.52).
Conclusions
OS has significantly improved nationally for patients newly diagnosed with clinical stage III melanoma in the era of novel melanoma therapies. OS outcomes will likely continue to evolve as these agents are increasingly utilized in the adjuvant setting. These data may help to better inform affected patients with respect to prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Melanoma incidence has increased steadily over the past few decades, and there were over 91,000 new diagnoses in 2018.1 Survival outcomes for melanoma are highly dependent on American Joint Committee on Cancer (AJCC) stage, as determined by Breslow thickness, ulceration, and presence of nodal and distant metastases.2,3,4,5 Stage III melanoma is defined by presence of lymph node (LN) or in-transit/satellite metastases.2,3
Since 2011, the treatment options for advanced melanoma have rapidly evolved with the advent of immune checkpoint and BRAF/MEK pathway inhibitors.6,7,8,9,10,11 More recently, these novel therapies have been approved for the adjuvant setting for resectable stage III melanoma.10,12,13,14 While these therapies have demonstrated significant survival benefits within the context of well-designed clinical trials in selected patient populations, few studies have evaluated their impact at a population level. In a recent National Cancer Database (NCDB) study by Sinnamon et al., diagnosis in the novel therapeutic era was associated with improved overall survival (OS) for patients with stage IV melanoma.15 The purpose of the current study is to estimate OS at a national population level in patients diagnosed with clinical stage III melanoma in the current landscape of melanoma therapies compared with historical cohorts. We hypothesized that OS has significantly improved, and historical survival data for clinical stage III melanoma are outdated.
Methods
Data Source
A retrospective study was performed using the melanoma participant use file of the NCDB. The NCDB is a collaborative effort between the American College of Surgeons’ Commission on Cancer and the American Cancer Society.16 Reporting to the NCDB has increased over time, and the database currently captures hospital registry data from more than 1500 Commission on Cancer-accredited facilities and represents more than 70% of newly diagnosed cancer cases nationally.17 All data are deidentified and compliant with the Health Insurance Portability and Accountability Act. The Institutional Review Board of the University of Pennsylvania deemed this study exempt from review.
Patient Selection
Patients 18 years of age or older diagnosed with clinical stage III melanoma, as defined by the AJCC 6th and 7th editions, were identified (Supplementary Figure 1). Only patients with clinically evident stage III disease were included in the study; patients with microscopic melanoma lymph node (LN) metastases identified by sentinel LN biopsy were not included. We chose to evaluate patients with clinical stage III melanoma as they may have higher risk of recurrence and be more likely to benefit from systemic therapy. Clinical stage III melanoma was defined as clinical N staging of N1, N1b, N2, N2b, N2c, and N3 with pathologic confirmation of nodal disease. Patients were categorized by diagnosis year into two historical cohorts preceding the advent of novel systemic therapies (P1: 2004–2005, P2: 2008–2009) and one contemporary group (P3: 2012–2013). Those with discrepancies in coding (e.g., documented has having distant metastases) or having unknown vital status or follow-up time were excluded.
Variables
The primary outcome variable was OS, defined as the interval (in months) between diagnosis and death. Patients who were alive were censored at time of last follow-up. Diagnosis period, rather than receipt of adjuvant immunotherapy, was used as the primary independent variable in order to additionally capture the potential effects of BRAF-targeted therapies and treatments for recurrent and metastatic disease. Approval for adjuvant indication was first obtained for ipilimumab in 2015.14 Therefore, patients diagnosed in 2012–2013 were unlikely to have received these treatments in the adjuvant setting, but would rather have benefited from treatment of recurrent disease. Moreover, the NCDB does not differentiate immune checkpoint inhibitors from interferon-α. Therefore, diagnosis period was thought to better reflect the current landscape of melanoma care.
Additional variables analyzed were patient demographics (age at diagnosis, sex, race, ethnicity, Charlson–Deyo score, primary payer, and residence), hospital characteristics (facility type and region), tumor characteristics (primary site, T stage, and N stage), and first-course treatments (primary-site surgery, regional lymphadenectomy, and radiation therapy, immunotherapy, and chemotherapy). Extent of regional lymphadenectomy was stratified as < 5 LNs, 5–9 LNs, and ≥ 10 LNs resected. Anatomic LN basin (e.g., axilla, cervical, superficial/deep inguinal) involved was not provided in the NCDB. Missing data for each variable were included as a separate category for analyses.
Statistics
Statistical analyses were performed using R v3.5.1.18 All tests were two-sided. P values < 0.05 were considered statistically significant. Descriptive statistics are presented as frequencies for categorical variables and medians with interquartile ranges (IQRs) for continuous variables. Statistical analyses were performed using Pearson’s χ2 test and analysis of variance or Wilcoxon rank-sum test, respectively.
OS was estimated using the Kaplan–Meier method and compared using the log-rank test. The Bonferroni method was used to adjust for multiple testing in stepdown pairwise analyses by diagnosis period. Factors associated with OS were determined using the Cox proportional hazards model. Multivariable analyses adjusted for demographic, clinicopathologic, and treatment characteristics available in the NCDB. Associations between OS and prognostic factors used in the Cox model are described using hazard ratios (HRs) with 95% confidence intervals (CIs).
Results
Patient Characteristics
Of 3720 study patients, 525 (14%) were diagnosed in P1, 1375 (37%) in P2, and 1820 (49%) in P3 (Table 1). Median age at diagnosis increased from 58 (IQR 47–71) years in P1 to 61 (IQR 50–71) years in P3 (P = 0.004). Treatments largely did not differ except in the increased utilization of radiation therapy (P1 15.0%; P2 17.9%; P3 21.0%, P < 0.001). Administration of immunotherapy as first-line treatment did not change (P1 26.5%; P2 27.6%; P3 27.6%, P = 0.14). Notably, ipilimumab was not approved in the adjuvant setting until 2015.14
Survival in the Novel Therapeutic Era
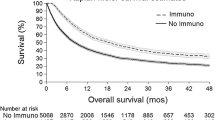
Median follow-up was 44.1 (IQR 17.6–110.2) months for patients diagnosed in P1, 41.4 (IQR 17.2–80.9) months for P2, and 33.4 (19.0–42.5) months for P3 (P < 0.001). OS differed significantly by diagnosis period, with median OS of 50.5 (95% CI 43.8–62.1), 49.3 (95% CI 43.3–57.5), and 58.2 (95% CI 51.7 to not reached) months, respectively (log-rank P < 0.001) (Fig. 1a). In stepdown analyses, OS was longer for patients diagnosed in P3 than P2 (Bonferroni-corrected log-rank P < 0.001), but a difference in OS did not reach significance compared with P1 (Bonferroni-corrected log-rank P = 0.11). Comparing P1 and P2, OS did not differ (Bonferroni-corrected log-rank P > 0.99). The 3-year OS rate was higher for patients diagnosed in P3 (64.9%, 95% CI 62.6–67.2%) than in P1 (58.1%, 95% CI 53.9–62.6%, Bonferroni-corrected log-rank P = 0.018) and P2 (56.8%, 95% CI 54.2–59.5%, Bonferroni-corrected log-rank P < 0.001). In contrast, 3-year OS rates did not differ between P1 and P2 (Bonferroni-corrected log-rank P > 0.99).
Overall survival (OS) and adjusted hazard ratios (HRs) in patients newly diagnosed with clinical stage III melanoma, stratified by diagnosis period: a Kaplan–Meier estimates of OS curves, b multivariable Cox proportional hazards analyses of OS, adjusted for demographic and clinicopathologic factors. HR < 1.0 is favorable and > 1.0 is unfavorable compared with P1 (2004–2005). Error bars indicate 95% confidence intervals (CIs)
The survival benefit observed for P3 persisted after adjusting for other prognostic factors (Fig. 1b, Table 2). Specifically, diagnosis in P3 (HR 0.76, 95% CI 0.66–0.88, P < 0.001), but not P2 (HR 0.95, 95% CI 0.83–1.09, P = 0.46), was associated with improved OS compared with P1. Other factors associated with OS included age, sex, Charlson–Deyo score, primary payer type, unknown primary site, N stage, and treatments received (Table 2).
Trends in Survival by Clinical N Stage
When stratified by N stage, median OS was 79.9 (95% CI 70.4–89.6) months for N1, 47.6 (95% CI 41.5–56.9) months for N2, and 28.2 (95% CI 26.5–35.2) months for N3 disease (log-rank P < 0.001). The 3-year OS rates were 66.1% (95% CI 64.1–68.1%), 58.8% (95% CI 55.4–62.5%), and 45.5% (95% CI 41.9–49.6%), respectively (log-rank P < 0.001).
Within each N stage, OS improved in P3 for patients with N2 (log-rank P = 0.023) and N3 (log-rank P = 0.036) disease, but not N1 disease (log-rank P = 0.20) (Fig. 2a–c). On multivariable analysis, improved OS for patients diagnosed in P3 compared with P1 was observed for all N stages, with an increasing hazard reduction from N1 (HR 0.81, 95% CI 0.67–0.99, P = 0.035) to N3 stage (HR 0.65, 95% CI 0.48–0.87, P = 0.004) (Fig. 2d).
Overall survival (OS) and adjusted hazard ratios (HRs) in patients newly diagnosed with clinical stage III melanoma by N stage: Kaplan–Meier estimates of OS curves, stratified by diagnosis period, for patients with a N1, b N2, and c N3 disease; d multivariable Cox proportional hazards analyses of OS, adjusted for demographic and clinicopathologic factors. HR < 1.0 is favorable and > 1.0 is unfavorable compared with P1 (2004–2005). Error bars indicate 95% confidence intervals (CIs)
Trends in Survival by Extent of Lymphadenectomy
The regional LN basin involved was not available in the NCDB. When stratified by primary site, the median (IQR) number of LNs resected was 24 (8–41) for head/neck primaries, 16 (5–24) for upper extremity primaries, 10 (5–16) for lower extremity primaries, and 17 (9–26) for truncal or unknown/unspecified primary sites. The adjuvant trials of immune checkpoint inhibitors in patients with stage III melanoma typically included only those who underwent complete LN dissection prior to therapy. OS was therefore further evaluated in subgroups of patients who underwent resection of < 5 (N = 727) and ≥ 10 LNs (N = 2547). The proportion of patients by extent of lymphadenectomy did not change over time (≥ 10 LNs: P1 67.6%, P2 68.4%, P3 68.7%, P = 0.63). Compared with those who had < 5 LNs resected, patients who had ≥ 10 LNs resected were younger (median [IQR] 58 [47–69] versus 65 [53–76] years, P < 0.001), more likely treated at an academic center (47.1% versus 35.5%, P < 0.001), and more likely classified as having N3 disease (21.2% vs. 9.2%, P < 0.001) (Supplementary Table 1). Patients who underwent resection of ≥ 10 LNs were also more likely to receive radiation therapy (21.2% vs. 12.2%, P < 0.001) and immunotherapy (31.1% vs. 16.9%, P < 0.001).
The OS trend for patients who underwent resection of ≥ 10 LNs was similar to that for the entire study population. Median OS increased from 50.5 (95% CI 43.0–65.9) months in P1 and 46.2 (95% CI 39.9–57.2) months in P2 to 58.2 (95% CI 51.7 to not reached) months in P3 (log-rank P < 0.001) (Fig. 3a). The 3-year OS rates by diagnosis period were 58.6% (95% CI 53.6–64.0%), 55.6% (95% CI 52.4–58.9%), and 65.9% (95% CI 63.2–68.7%), respectively (log-rank P < 0.001). On multivariable analysis, P3 (HR 0.73, 95% CI 0.62–0.87, P < 0.001), but not P2 (HR 0.95, 95% CI 0.81–1.12, P = 0.56), was associated with improved OS compared with P1 (Fig. 3c).
Overall survival (OS) and adjusted hazard ratios (HRs) in patients newly diagnosed with clinical stage III melanoma by extent of lymphadenectomy: Kaplan–Meier estimates of OS curves, stratified by diagnosis period, for patients who underwent resection of a ≥ 10 LNs and b < 5 LNs; c multivariable Cox proportional hazards analyses of OS, adjusted for demographic and clinicopathologic factors. HR < 1.0 is favorable and > 1.0 is unfavorable compared with P1 (2004–2005). Error bars indicate 95% confidence intervals (CIs)
A survival benefit in P3 was not observed for the subgroup of patients who had < 5 LNs resected (log-rank P = 0.40) (Fig. 3b). While the 3-year OS rate was higher for patients diagnosed in P3 (62.6%, 95% CI 57.6–68.1%) than P1 (58.1%, 95% CI 49.0–68.9%) and P2 (55.5%, 95% CI 49.7–62.0%), this was not statistically significant (log-rank P = 0.22). Adjusting for other prognostic factors, diagnosis period was not associated with OS in this subgroup (P3 vs. P1, HR 0.81, 95% CI 0.58–1.13, P = 0.21).
Discussion
Immune checkpoint and BRAF/MEK pathway inhibitors have transformed the treatment options for advanced melanoma. These therapies have demonstrated significant survival benefits within the context of well-designed clinical trials, but their impact on outcomes and melanoma care at a national population level is not fully understood. This study demonstrated a significant improvement in OS at a population level in patients diagnosed with clinical stage III melanoma in the novel therapeutic era. Historical survival data for this patient population are outdated.
Patients with stage III melanoma represent a heterogeneous group with respect to survival outcomes. Patients presenting with clinically apparent macroscopic LN metastases experience significantly worse survival than those with microscopic nodal disease.19,20 Furthermore, survival decreases with increasing number of involved LNs.2,3,19,20 Historically, patients with clinical N1 disease had median melanoma-specific survival time of about 60 months, while those with four or more metastatic LNs (clinical N3) had median survival time of only 24 months.20
The survival benefit of immune checkpoint inhibitors and BRAF/MEK pathway-targeted therapies in the treatment of advanced melanoma has been well reported in randomized trials. The phase 3 trial of adjuvant ipilimumab in patients with resected stage III melanoma reported a hazard reduction of 28% for all-cause death (HR 0.72, 95% CI 0.58–0.88) after median follow-up time of 5.3 years.14 For patients with unresectable melanoma, clinical trials have reported an even greater survival benefit with HRs ranging from 0.37 to 0.69, although the majority of patients in these studies had distant metastases.6,7,8,9,21
The findings in the current study comparing diagnosis in P3 versus P1 (HR 0.76, 95% CI 0.66–0.88) mirror the results from the ipilimumab trial for resected stage III melanoma. Patients diagnosed in P3 experienced a median survival time that was about 8 months longer than those diagnosed in P1 or P2. Specifically, in the subgroup of patients who underwent regional LN dissection (defined as resection of ≥ 10 LNs), diagnosis in P3 compared with P1 was associated with a hazard reduction of 0.73 (95% CI 0.62–0.87). The fact that P3, and not P2, was associated with improved survival suggests that the advent of novel therapies in 2011 likely played a role. Although immune checkpoint and BRAF/MEK pathway inhibitors were not approved for adjuvant therapy during the timeframe of the study, patients may nevertheless have benefited from participation in clinical trials or in the setting of recurrent disease.
An association between OS and diagnosis period was not observed for the subgroup of patients with < 5 LNs resected. This patient population was much less likely than those who underwent resection of ≥ 10 LNs to receive adjuvant treatments, including immunotherapy. Therefore, they may not have received treatment with the novel systemic therapies. Additionally, the reason these patients received nonstandard care is unknown. Since the outcome available and evaluated through the NCDB was OS, not melanoma-specific survival, patients may have experienced non-melanoma-related deaths.
Consistent with literature, the current study identified younger age19,20,22,23,24 female sex,22,24 thinner melanomas,22 fewer nodal metastases,19,20,22 and unknown primary site,25,26,27 to be independently associated with improved survival. The increased utilization of radiation therapy over time observed in the current study may be related to the 2012 publication of results from the randomized trial in clinical stage III melanoma demonstrating improved locoregional disease control with adjuvant radiation therapy.28 In contrast to the trial results, an association between radiation therapy and decreased OS was observed in our retrospective study, which may be due to the inability to account for important indications for radiation that also carry prognostic significance, such as larger LN size, matted LNs, and extracapsular extension.28
There are several study limitations that should be noted, including potential biases inherent to a retrospective study design. While the multivariable analyses took into consideration many patient and clinical factors, residual confounders are likely present. Because the NCDB identifies treatment categories and not specific agents, analyses for specific treatments could not be performed. Additionally, it should be emphasized that the first adjuvant indication for immune checkpoint inhibitors (ipilimumab) was not obtained until 2015.14 Therefore, the observed results are unlikely to reflect the effect of adjuvant treatment for study patients diagnosed in 2012–2013. Rather, these patients likely benefited from treatment of recurrent disease. The effect of adjuvant utilization of these treatments at a population level will require evaluation in future studies. Furthermore, patient cohorts differed in size and also in follow-up times. Statistical analyses took cohort size into consideration, and time-to-event statistical methods (Kaplan–Meier and Cox proportional hazards) mitigated potential biases inherent to differing follow-up times. Finally, recurrence data and melanoma-specific survival were not available in the NCDB and could not be evaluated.
Patients newly diagnosed with clinical stage III melanoma in the early years following the advent of novel immune checkpoint and BRAF/MEK pathway inhibitors have experienced significant improvements in OS compared with historical cohorts. Population-level survival outcomes will likely continue to evolve as the uptake of these agents in the adjuvant setting increases. The discussion of prognosis with patients newly diagnosed with clinical stage III melanoma should take into consideration the survival benefits of novel therapies.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. https://doi.org/10.3322/caac.21442
Balch CM, Buzaid AC, Soong S-J, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19(16):3635–48. https://doi.org/10.1200/jco.2001.19.16.3635
Balch CM, Gershenwald JE, Soong S-J, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–206. https://doi.org/10.1200/jco.2009.23.4799.
Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eight edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–92. https://doi.org/10.3322/caac.21409.
Bartlett EK, Karakousis GC. Current staging and prognostic factors in melanoma. Surg Oncol Clin N Am. 2015;24(2):215–27. https://doi.org/10.1016/j.soc.2014.12.001.
Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16. https://doi.org/10.1056/nejmoa1103782.
Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. https://doi.org/10.1056/nejmoa1003466.
Hauschild A, Grob J-J, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–65. https://doi.org/10.1016/s0140-6736(12)60868-x.
Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. https://doi.org/10.1056/nejmoa1412082.
Eggermont AM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–801. https://doi.org/10.1056/nejmoa1802357.
Wolchok JD, Kluger H, Callahan MK, et al. Safety and clinical activity of combined PD-1 (nivolumab) and CTLA-4 (ipilimumab) blockade in advanced melanoma patients. N Engl J Med. 2013;369(2):122–33. https://doi.org/10.1056/nejmoa1302369.
Eggermont AM, Chiarion-Sileni V, Grob J-J, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–30. https://doi.org/10.1016/s1470-2045(15)70122-1.
Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–35. https://doi.org/10.1056/nejmoa1709030.
Eggermont, Alexander M., Chiarion-Sileni V, Grob J-J, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845–55. https://doi.org/10.1056/nejmoa1611299.
Sinnamon AJ, Neuwirth MG, Gimotty PA, et al. Association of first-in-class immune checkpoint inhibition and targeted therapy with survival in patients with stage IV melanoma. JAMA Oncol. 2018;4(1):126–8. https://doi.org/10.1001/jamaoncol.2017.3462.
Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–90. https://doi.org/10.1245/s10434-007-9747-3.
About the National Cancer Database. National Cancer Database. https://www.facs.org/quality-programs/cancer/ncdb/about. Accessed 1 Oct 2018.
R Core Team (2018). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing https://www.R-project.org/. Accessed 1 July 2018.
Balch CM, Soong S-J, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19(16):3622–34. https://doi.org/10.1200/jco.2001.19.16.3622.
Balch CM, Gershenwald JE, Soong S-J, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28(14):2452–9. https://doi.org/10.1200/jco.2009.27.1627.
Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367(2):107–114. https://doi.org/10.1056/nejmoa1203421.
White RR, Stanley WE, Johnson JL, Tyler DS, Seigler H. Long-term survival in 2,505 patients with melanoma with regional lymph node metastasis. Ann Surg. 2002;235(6):879–887.
Balch CM, Soong S-J, Gershenwald JE, et al. Age as a prognostic factor in patients with localized melanoma and regional metastases. Ann Surg Oncol. 2013;20(12):3961. https://doi.org/10.1245/s10434-013-3100-9.
Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83(8):1664–78.
Cormier JN, Xing Y, Feng L, et al. Metastatic melanoma to lymph nodes in patients with unknown primary sites. Cancer. 2006;106(9):2012–20. https://doi.org/10.1002/cncr.21835.
Lee CC, Faries MB, Wanek LA, Morton DL. Improved survival after lymphadenectomy for nodal metastasis from an unknown primary melanoma. J Clin Oncol. 2008;26(4):535–41. https://doi.org/10.1200/jco.2007.14.0285.
Bae JM, Choi YY, Kim DS. Metastatic melanomas of unknown primary show better prognosis than those of known primary: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2015;72(1):59–70. https://doi.org/10.1016/j.jaad.2014.09.029.
Burmeister BH, Henderson MA, Ainslie J, et al. Adjuvant radiotherapy versus observation alone for patients at risk of lymph-node field relapse after therapeutic lymphadenectomy for melanoma: a randomised trial. Lancet Oncol. 2012;13(6):589–97. https://doi.org/10.1016/s1470-2045(12)70138-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
There are no relevant conflicts of interest or disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Song, Y., Tieniber, A.D., Gimotty, P.A. et al. Survival Outcomes of Patients with Clinical Stage III Melanoma in the Era of Novel Systemic Therapies. Ann Surg Oncol 26, 4621–4630 (2019). https://doi.org/10.1245/s10434-019-07599-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07599-y