Abstract
Background
Hyperthermia enhances the cytotoxicity of chemotherapeutic agents used during cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemoperfusion (HIPEC). However, this may result in an elevated core body temperature (CBT), with unintended effects on surgical morbidity. This study evaluates the relationship of maximum CBT during CRS/HIPEC on postoperative outcomes.
Methods
A retrospective review of patients undergoing CRS/HIPEC from January 2011 to July 2017 was performed. Outcomes were stratified according to maximum CBT reached during HIPEC. Primary study endpoints were 30-day morbidity and 30-day complication severity.
Results
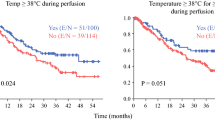
Overall, 135 consecutive CRS/HIPEC cases were reviewed; 36 (27%) had a maximum CBT ≥ 39.5 °C during the 90-min HIPEC. CBT ≥ 39.5 °C was associated with an increase in 30-day postoperative complications (58% vs. 34%, p = 0.01) and severe Clavien–Dindo grade III or higher complications (22% vs. 11%, p = 0.04). On multivariate analysis, the adjusted odds ratio of having any complication was 3.77 (95% confidence interval [CI] 1.56–9.14) and a Clavien–Dindo grade III or higher complication was 3.46 (95% CI 1.10–10.95) when maximum CBT reached 39.5 °C. Flow rates ≥ 2.35 L/min were associated with lower average CBT (p = 0.05) and improved peritoneal heating (p = 0.02).
Conclusion
Maximum CBT ≥ 39.5 °C is associated with an increased risk of postoperative morbidity. Higher flow rates are associated with improved intraperitoneal heating, lower CBT, and may contribute to optimizing the therapeutic benefit of HIPEC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In the management of peritoneal surface malignancy, cytoreductive surgery (CRS) effectively reduces the tumor burden of macroscopic disease, while the subsequent delivery of heated intraperitoneal chemotherapy (HIPEC) targets residual microscopic deposits.1,2,3,4,5,6,7 Hyperthermia functions as a chemosensitizing agent, with the magnitude of effect dependent on the chemotherapy agent utilized.8 Compared with fever, which is an elevation of the physiologic set point of core body temperature (CBT), hyperthermia differs fundamentally and refers instead to a rise in CBT resulting from an external heat load or failure of heat dissipation.9
Mechanistically, HIPEC induces a regional hyperthermia within the abdominal cavity. Ultimately, this functions to increase the permeability of the peritoneal lining and improve the delivery of chemotherapy to cancer cells.8 Rising temperatures induce vasodilation in healthy tissues, however this physiologic response is blunted in tumor, resulting in inefficient heat dissipation and a reduced pH, factors that synergistically increase cancer’s sensitivity to hyperthermia.10,11,12 Furthermore, hyperthermia has been linked to tumor-directed cytotoxic effects through induction of heat shock proteins, protein denaturization, and impairment of DNA repair mechanisms.10,11,12
The efficacy of regional hyperthermia during HIPEC is related to the target peritoneal temperature being achieved and subsequently maintained for the duration of chemoperfusion. In the setting of CRS/HIPEC, with the continuous infusion of heated perfusate directly into the abdomen, unwanted systemic hyperthermia may develop, as noted by a rise in CBT. Most data published on immunologic changes in humans exposed to systemic heat demonstrate a threshold CBT of 39–39.5 °C, at which point alterations of immune system function may be identified.13,14,15 Specifically, this degree of hyperthermia appears to induce a significant reduction in circulating CD4+ lymphocytes, reduce the ratio of CD4+ to CD8 + lymphocytes, and increase natural killer cell activity.13,15 These changes in the cellular immune response are relatively nonspecific, but may be interpreted as part of a general response to the major physiologic stress of systemic hyperthermia.
Overall, little is known about the impact of CBT during HIPEC on oncologic or surgical outcomes. The primary aim of this study was to evaluate the effect of induced systemic hyperthermia, defined as a CBT ≥ 39.5 °C, on 30-day morbidity and 30-day complication severity following CRS/HIPEC. Additionally, we sought to investigate the potential for optimization of chemoperfusion by adjusting flow rates as a means to maximize regional hyperthermia while limiting systemic hyperthermia.
Methods
Data Sources
This was a single-institution, retrospective cohort study. Baseline characteristics, intraoperative factors, chemoperfusion parameters, and postoperative complications were recorded prospectively. Complications were identified in accordance with definitions from the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) and severity graded according to the Clavien–Dindo classification system.16,17 The study was performed in compliance with the policies of our Institutional Review Board.
Patient Cohort
Consecutive patients who underwent CRS/HIPEC at our institution from January 2011 to August 2017 were identified and their medical records reviewed. Cases were divided into two groups according to the maximum CBT recorded during HIPEC, i.e. those with a documented temperature ≥ 39.5 °C (CBT–High) and those with a maximum temperature < 39.5 °C (CBT–Low).
Intraoperative Monitoring
Standard intraoperative monitoring devices were utilized per American Society of Anesthesiologists guidelines. During the 90-min HIPEC, perfusion parameters (perfusion flow rate [L/min], inflow temperature [°C], outflow temperature [°C], and esophageal temperature [°C]) were recorded at the start of chemoperfusion and every 15 min thereafter, for a total of seven time points. Inflow and outflow temperatures were monitored using temperature-sensing catheter probes. Esophageal temperature was measured by an esophageal temperature probe and used as a surrogate for CBT. Intraoperative management of physiologic aberrations related to CBT were made on a case-by-case basis with target outflow temperatures ≥ 40 °C and intraperitoneal temperatures of 40–42 °C.
Statistical Analysis
Descriptive statistics were calculated for the entire cohort. The distribution of all covariates were compared between study groups using Chi square tests for categorical variables, and Student’s t tests and Wilcoxon rank-sum tests for continuous variables. Statistical significance was assigned a threshold of p ≤ 0.05. The primary study endpoints were 30-day morbidity and 30-day complication severity. Secondary endpoints included 90-day mortality and average flow rates during HIPEC. Separate logistic regression models were used to estimate the odds of any postoperative complication or severe Clavien–Dindo grade III or higher complication associated with the CBT–High group in reference to the CBT–Low group. Both models were adjusted for age, sex, intraoperative estimated blood loss (EBL), operation time, Peritoneal Cancer Index (PCI) score, and completeness of cytoreduction (CC) score. Adjusted odds ratio (aOR) with 95% confidence intervals (CI) were reported. All analyses were performed using STATA software version 15.1 (StataCorp LLC, College Station, TX, USA).
Results
Study Subjects
A total of 135 consecutive patients undergoing CRS/HIPEC were included in this study. At the beginning of HIPEC after cytoreduction, mean CBT was 36.7 °C. During the 90-min chemoperfusion, all patients demonstrated some degree of systemic hyperthermia, with a maximum CBT range from 37.5 to 40.9 °C. The majority of patients (56%) had a documented maximum CBT between 38.5 and 39.5 °C. Ultimately, 36 patients (27%) had a documented maximum CBT ≥ 39.5 °C (CBT–High), while 99 patients (73.3%) had a documented maximum CBT < 39.5 °C (CBT–Low). No patients developed malignant hyperthermia or hypothermia during HIPEC.
Preoperative Characteristics
Median age was 56 years, and a minority of patients were male (34%). Medical comorbidities did not significantly differ between groups, except for body mass index (BMI) and hypertension (Table 1). The primary tumor histology leading to peritoneal dissemination was most commonly of appendiceal origin (67%), followed by colorectal origin (19%), but did not differ significantly between CBT groups (p = 0.63). The extent of disease as measured by the PCI was 17.9 for the entire study population.
Intraoperative Variables
The extent of cytoreduction was similar in the two groups, as evidenced by the number of organ resections, anastomoses, and peritonectomy procedures (Table 2). Operative time was unchanged between groups (9.3 vs. 9.0 h, p = 0.92), and provided further evidence of a comparable case complexity. All patients underwent a 90-min chemoperfusion. A 30–40 mg mitomycin C HIPEC was most common (92%), with the drug delivered intraperitoneally over three divided doses. The remaining cases were managed with an 800 mg/m2 carboplatin HIPEC (7%) or a 25 mg doxorubicin HIPEC (1%), again with drug delivery over three divided doses. No between-group differences were identified (p = 0.73). A complete cytoreduction (CC), defined as a CC score of 0 or 1, was achieved in approximately 80% of cases in both groups (p = 0.31).
Chemoperfusion Parameters
The median average flow rate was 2.35 L/min. Flow rates ≥ 2.35 L/min were associated with a reduced CBT and improved intraperitoneal heating, as defined by increased peritoneal outflow temperatures and a reduced inflow/outflow temperature differential (Table 3). Patients with a documented maximum CBT ≥ 39.5 °C maintained lower average flow rates (2.1 vs. 2.4 L/min, p = 0.10) compared with patients whose CBT remained < 39.5 °C.
Postoperative Outcomes
Patients who reached a maximum CBT ≥ 39.5 °C during HIPEC experienced more 30-day postoperative complications and severe Clavien–Dindo grade III or higher complications (Table 4). On univariate analysis, a CBT ≥ 39.5 °C suggested a potentially significant association with both 30-day morbidity (OR 3.00, 95% CI 1.37–6.61) and 30-day complication severity (OR 2.29, 95% CI 0.84–6.25). Multivariate analysis was used to account for both clinically relevant and statistically significant covariates. Controlling for age, sex, intraoperative EBL, operation time, PCI score, and CC score, a maximum CBT ≥ 39.5 °C demonstrated a statistically significant association with both 30-day morbidity (aOR 3.77, 95% CI 1.56–9.14) and Clavien–Dindo complication severity (aOR 3.46, 95% CI 1.10–10.95) [Table 5]. The 90-day mortality rate for the entire cohort was 0.8%. Mortality rates did not vary by maximum CBT (0% vs. 1%, p = 0.55).
Discussion
Regional therapy for the management of primary peritoneal disease or peritoneal metastasis was first described in the 1980s and remains a key component of present-day management.18,19,20,21,22,23 Herein, we report on the potential postoperative morbidity of systemic hyperthermia that develops to varying degrees during HIPEC. Patients whose CBT reached a threshold value of 39.5 °C were found to be at increased risk of developing a postoperative complication, and, if a complication did arise, it was more likely to be severe (Clavien–Dindo grade III or higher). Elevated CBT ≥ 39.5 °C did not correlate with improved intraperitoneal heating. The extent of cytoreduction did not appear to influence CBT during CRS/HIPEC, and, despite the varying degree of CBT elevation, all patients completed the intended 90-min chemoperfusion. Although there was no statistically significant difference in the average flow rate between the CBT–High and CBT–Low groups (2.1 vs. 2.4 L/min, p = 0.10), chemoperfusion flow rates ≥ 2.35 L/min were shown to improve intraperitoneal heating and reduce average CBT during HIPEC.
When utilizing hyperthermia as a therapeutic adjunct, such as with HIPEC, the two key components are time and temperature. It has been shown that a critical threshold of 40 °C must be reached for potentiation of intraperitoneal cytotoxic chemotherapy.24 Accordingly, intraperitoneal target temperatures during HIPEC typically range from 40 to 43 °C.21,22,23 To accomplish this, inflow temperatures may be increased to 45 °C over the course of the 90-min perfusion, particularly in the setting of a low flow rate. Given the peritoneum’s high potential for heat transfer, systemic hyperthermia may develop, as manifested by a rising CBT.
Little is known about the morbidity of systemic hyperthermia that develops during HIPEC, however our data suggest an association between maximum CBT ≥ 39.5 °C and 30-day postoperative complications. A recent study by Goldenshluger et al. investigated the effect of both CBT and intra-abdominal pressure in HIPEC.25 To the best of our knowledge, this was the first published study to evaluate the impact of rising CBT during HIPEC on postoperative outcomes. CBT was measured continuously and a calculated mean CBT was used for subsequent regression analyses. While our data support their ultimate conclusion that elevated CBT is associated with an increased risk of postoperative complications, there are fundamental differences to address. In our study, the mean CBT for the entire cohort was 38.2 °C, significantly higher than the mean CBT of 37.5 °C in the study by Goldenshluger et al. Furthermore, there was no report on maximum CBT reached during HIPEC, which we found to be the most predictive measure of 30-day morbidity. Most importantly, we believe that reporting results in reference to maximum CBT, as opposed to mean CBT, provides the opportunity to guide real-time intraoperative decision making and intervention. A clearly defined maximum CBT threshold has the value of being an easily understood and objective metric that can be assessed at any time during HIPEC. If CBT approaches that critical value of 39.5 °C, then passive and/or active cooling measures can be implemented.
Higher flow rates have been shown to improve heating during HIPEC by facilitating more rapid achievement of peritoneal target temperature and maintaining inflow/outflow temperature gradients.26 We observed similar findings in this study. Flow rates ≥ 2.35 L/min were associated with better peritoneal heating, as shown by increased outflow temperatures and a reduced inflow/outflow temperature differential, while generating less systemic hyperthermia. Mechanistically, it is possible higher chemoperfusion flow rates act to mitigate the effects of the body’s heat sink, thus minimizing systemic hyperthermia while maximizing locoregional hyperthermia. Achieving higher flow rates during HIPEC represents a potentially effective measure to decrease the risk of postoperative complications observed with an elevated maximum CBT, while optimizing the oncologic efficacy of hyperthermia within the peritoneal cavity.
Limitations
This study has the limitations inherent in a single-institution, retrospective review of observational data. During the study period, there was no defined CBT protocol. Rather, the objective was achievement and maintenance of regional peritoneal hyperthermia by increasing perfusion flow rates and inflow temperatures as needed. While CBT was monitored, there were no titratable endpoints to follow. Accordingly, all intraoperative management decisions related to an elevated CBT were made at the discretion of the attending surgeon and anesthesiologist based on a given patient’s hemodynamic status and overall clinical condition. The small sample size of the study limited the number of variables that could be controlled for and the overall power of the regression model, thus we cannot exclude the possibility of other relevant covariates. Lastly, it is important to note that our conclusions regarding CBT and flow rates are limited to short-term outcomes and cannot be extrapolated to long-term outcomes.
Conclusion
While locoregional hyperthermia is the goal during HIPEC, the oncologic impact of systemic hyperthermia is yet to be determined. Our findings suggest that reaching a maximum CBT ≥ 39.5 °C at any time during chemoperfusion is associated with an increased risk of both 30-day morbidity and grade III/IV complications. Accordingly, we recommend target outflow temperatures of at least 40 °C with an intraperitoneal target temperature between 40 and 42 °C. This approach should provide therapeutic intraperitoneal hyperthermia without excessive elevations in CBT, particularly if target temperatures are achieved through a combination of inflow temperature adjustment and increased flow rates.
References
Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi- institutional study. J Clin Oncol. 2004;22:3284–92.
Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116:5608–18.
Sugarbaker PH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: progress toward a new standard of care. Cancer Treat Rev. 2016;48:42–9.
Cao C, Yan TD, Black D, et al. A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2009;16:2152–65.
Smeenk RM, Verwaal VJ, Antonini N, et al. Survival analysis of pseudomyxoma peritonei patients treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2007;245:104-9.
Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6:727–31.
Elias D, Gilly F, Quenet F, et al. Pseudomyxoma peritonei: a French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol. 2010;36:456–62.
Sugarbaker PH. Laboratory and clinical basis for hyperthermia as a component of intracavitary chemotherapy. Int J Hyperthermia. 2007;23:431–42.
Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19(3):267–94.
Ohno S, Siddik ZH, Kido Y, Zwelling LA, Bull JMC. Thermal enhancement of drug uptake and DNA adducts as a possible mechanism for the effect of sequencing hyperthermia on cisplatin-induced cytotoxicity in L1210 cells. Cancer Chemother Pharmacol. 1994;34:302–6.
Song C, Park HJ, Lee CK, Griffin R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia 2005;21(8):761–768.
Horsman MR. Tissue physiology and the response to heat. Int J Hyperthermia. 2006;22(3):197–203.
Frey B, Weiss EM, Rubner Y, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia. 2012;28:528–42.
Skitzki JJ, Repasky EA, Evans SS. Hyperthermia as an immunotherapy strategy for cancer. Curr Opin Investig Drugs. 2009;10:550–8.
Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33–56.
User Guide for the 2016 ACS NSQIP Participant Use Data File. American College of Surgeons National Surgical Quality Improvement Program; Oct 2017. pp. 15–8.
Dindo D, Demartines N, Clavien P. classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients. Ann Surg. 2004;240(2):205–13.
Spratt JS, Adcock RA, Muskovin M, et al. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980;40:256–60.
Koga S, Hamazoe R, Maeta M, Shimizu N, Kanayama H, Osaki Y. Treatment of implanted peritoneal cancer in rats by continuous hyperthermic peritoneal perfusion. Cancer Res. 1984;44:1840–2.
Koga S, Hamazoe R, Maeta M, Shimizu N, Murakami A, Wakatsuki T. Prophylactic therapy for peritoneal recurrence of gastric cancer by continuous hyperthermic peritoneal perfusion with mitomycin C. Cancer. 1988;61:232–7.
Verwaal VJ, Bruin S, Boot H, et al. 8-Year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426–32.
Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009;27(36):6237–42.
Chua T, Moran B, Sugarbaker P, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–56.
Schaaf L, Kuip H, Zopf W, et al. A temperature of 40°C appears to be a critical threshold for potentiating cytotoxic chemotherapy in vitro and in peritoneal carcinomatosis patients undergoing HIPEC. Ann Surg Oncol. 2015;22:758–65.
Goldenshluger M, Zippel D, Ben-Yaacov A, et al. Core body temperature but not intraabdominal pressure predicts postoperative complications following closed system hyperthermic intraperitoneal chemotherapy (HIPEC) administration. Ann Surg Oncol. 2018;25:660–6.
Furman MJ, Picotte RJ, Wante MJ, et al. Higher flow rates improve heating during hyperthermic intraperitoneal chemoperfusion. J Surg Oncol. 2014;110:970–5.
Acknowledgment
The data for this research were provided by the Cancer Research Office of the University of Massachusetts Medical Center.
Funding
Funding for this study was provided by the Division of Surgical Oncology at the University of Massachusetts Medical School.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Ryan J. Hendrix, Jonathan P. Kassira, and Laura A. Lambert have no disclosures to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hendrix, R.J., Kassira, J.P. & Lambert, L.A. Elevated Maximum Core Body Temperature During Hyperthermic Intraperitoneal Chemoperfusion (HIPEC) is Associated with Increased Postoperative Complications. Ann Surg Oncol 27, 232–239 (2020). https://doi.org/10.1245/s10434-019-07495-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07495-5