Abstract
Objective
We aimed to compare the clinicopathological features and survival after surgery of patients with intrahepatic cholangiocarcinoma (ICC) according to the patterns of distribution of hepatic nodules.
Methods
A retrospective analysis of a multi-institutional series of 259 patients with resected ICC was carried out. Patients were further classified according to the pattern of distribution of hepatic nodules: single tumors (type I), single tumors with satellites in the same liver segment (type II), or multifocal tumors (type III).
Results
Overall, 64.5% of patients had type I, 21.9% had type II, and 13.5% had type III. The 5-year overall survival rate was 49.4, 34.2, and 9.9% for types I, II, and III, respectively (p < 0.001). A multivariate survival analysis identified the following independent prognostic factors: pattern types II and III (p = 0.001 and p = 0.001, respectively), size ≥ 50 mm (p = 0.021), lymph node (LN) metastases (p = 0.005), and R1 resections (p = 0.019). We stratified survival for each type of pattern according to the other prognostic factors identified in the multivariate analysis. N0 and R0 patients with type II and III tumors had encouraging long-term results. Conversely, patients with LN metastases and R1 resections had poor prognosis, particularly patients with type III tumors.
Conclusion
ICC has distinct patterns of distribution with different prognoses that should be considered when making therapeutic decisions. Patients with type III tumors had a significantly worse prognosis, and the benefits of upfront surgery should be carefully evaluated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Intrahepatic cholangiocarcinoma (ICC) is an aggressive neoplasm that arises from the epithelium of the intrahepatic bile ducts,1 accounting for 10–15% of all primary liver malignancies.2,3 The incidence of ICC has increased during the last few decades, likely due to both an escalation in the prevalence of viral and chronic hepatitis and improved clinical-pathological knowledge of the disease.4,5,–6 The treatment of patients with ICC often represents a challenging issue for hepato-pancreatico-biliary (HPB) surgeons and radical surgery remains the only chance for long-term survival.7,8 The prognosis of patients with ICC after surgery has improved in the last few decades, with an overall 5-year survival rate ranging from 25 to 40%.9,10,–11 Tumor size, lymph node (LN) metastasis, vascular invasion, radicality of surgery, and the presence of multiple nodules are the main clinicopathological factors that have a prognostic role after surgery.5,9,10,12 Some authors consider the presence of multifocal tumors a contraindication to liver resection;13 however, the presence of multiple nodules varies in surgical series, ranging from 20 to 32%,6,14 but the data show that different definitions and terminology have been used, including terms such as ‘satellite nodules’, ‘multifocal tumor’, and ‘intrahepatic metastases’. For these reasons, differentiation between ‘multiple nodules’, which may correspond to a multifocal carcinogenesis or to nodules that spread from the primary tumor, remains debatable. Moreover, in the recently released 8th edition of the American Joint Committee on Cancer (AJCC) staging system, ICC patients with vascular invasion or multiple nodules are both considered to be in the same T2 category, without any further classification.15 A recent radiological study, based on computed tomography (CT) scans, identified and proposed three different patterns of ICC at the time of presentation, according to the distribution of hepatic nodules (single tumor, single with satellites, and multifocal tumors), and each had a progressively worse prognosis, regardless of the type of treatment.16
The current multi-institutional study aimed to compare the clinical-pathological characteristics and to evaluate the long-term outcomes of patients who underwent surgery for ICC according to the pattern of distribution of hepatic nodules.
Patients and Methods
Patients
From January 1995 to December 2015, a total of 282 consecutive patients who underwent surgery for ICC at three hepatobiliary Italian tertiary referral centers (the Division of General and Hepatobiliary Surgery, G.B. Rossi Hospital, University of Verona, Verona; the Division of Hepatobiliary and General Surgery, Humanitas Clinical and Research Center, Humanitas University, Rozzano and Milan; and the Department of General and Emergency Surgery and Organ Transplantation, S. Orsola-Malpighi Hospital, University of Bologna, Bologna) were considered for this study. Of these patients, 13 did not undergo resection (exploratory or palliative surgery) due to advanced disease. The remaining 269 patients underwent resection, of whom 10 (3.7%) had a residual macroscopic tumor after surgery (R2 resection) and were therefore excluded. Moreover, a total of eight patients who were classified as having pM1 disease were also excluded—six patients who had non-regional LN metastases (the celiac artery, the superior mesenteric artery, and/or the periaortic LN) and two patients with a resectable hepatic lesion and a single peritoneal nodule of carcinomatosis. Thus, the remaining 251 patients who underwent curative resection were enrolled in this study. The distribution of hepatic nodules was collected based on preoperative imaging and pathological evaluation. The pattern of distribution of hepatic nodules for the analysis was defined according to the final pathological evaluation. Patients were further classified as follows: single tumor (type I), single tumor with satellite nodules in the same Couinaud liver segment (type II), and multifocal scattered tumors in different Couinaud liver segments (type III).16 A serum cancer antigen (CA) 19-9 level > 55 U/mL was considered abnormally high.
Clinical data were retrieved from each center’s prospectively collected database, pathological data in each institution were retrospectively reviewed according to the proposed classification, and the analysis was performed retrospectively. Data collection and analysis were performed according to the institutional guidelines and conformed to the ethical standards of the World Medical Association (Declaration of Helsinki). The study was approved by each local ethical committee, and signed consent was obtained from all subjects.
To analyse the prognostic role of the association of the pattern of distribution of hepatic nodules with other prognostic factors, a subgroup analysis was performed that stratified each pattern of distribution according to the main prognostic factors identified by the multivariate analysis.
Statistical Analysis
Numerical variables were summarized using the mean and standard deviation, or the median and interquartile range (IQR), as appropriate. Student’s t test, and the Chi square and Fisher tests were used to compare means and proportions, respectively, between independent groups. CA19-9 levels were compared between groups using the non-parametric Kruskal–Wallis test and non-parametric Mann–Whitney U test.
Overall survival (OS) was calculated from the date of surgery to the date of death from any cause or last follow-up, whichever came first. The median follow-up period for surviving patients was 38.6 months. Eight patients with 90-day postoperative mortality were excluded from the survival analysis. The OS curves were estimated according to the Kaplan–Meier method and were compared using the log-rank test. Multivariable Cox modeling was used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs), adjusting for potential confounders, and the pattern of distribution was considered a categorical variable. Differences were considered statistically significant at p values ≤ 0.05.
Results
Patient Characteristics
Clinical and pathological characteristics of the study population are summarized in Table 1.
Comparison of Clinical and Pathological Characteristics According to the Pattern of Distribution of Hepatic Nodules
Comparison of the clinical and pathological characteristics according to the different patterns of distribution are summarized in Table 2. Compared with patients with type I tumors, patients with type II and III tumors had significantly larger tumors (p = 0.019 and p = 0.007, respectively), a greater number of nodules (p < 0.001 and p < 0.001, respectively) and a higher serum CA19-9 level (p = 0.035 and p = 0.018, respectively). Preoperative chemotherapy was performed in eight (4.9%) patients with type I, eight (14.5%) patients with type II, and five (14.7%) patients with type III (type I vs. type II, p = 0.024 and, type I vs. type III p = 0.037, respectively). In 30 (54.5%) patients with type II and six (17.6%) patients with type III, preoperative imaging identified only one lesion (p < 0.001), and the diagnosis of multiple nodules (satellites or multifocal lesions) was made based on pathology. The sensitivity and accuracy of preoperative imaging in identifying the correct pattern, using pathology as the reference, were 45.5 and 88.1% for type II, and 82.3 and 97.6% for type III. The rate of LN metastases increased according to the pattern of distribution, from 21.6% (n = 35) in patients with single tumors, to 34.5% (n = 19) in patients with satellites, and to 35.2% (n = 12) in patients with multifocal tumors, but the differences were not statistically significant. The rate of R0 resection significantly decreased from 76.5% in type I, to 74.5% in type II, and to 52.9% in type III.
Prognostic Factors for Overall Survival
The 5-year OS rate and median OS of the entire cohort were 40.6% and 45.9 months, respectively. Table 3 shows the univariate and multivariate analysis of the prognostic factors for OS.
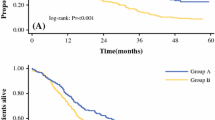
Based on univariate analysis, sex (male), CA19-9 level (≥ 55 U/mL), tumor size (≥ 50 mm), LN metastases, vascular invasion, radicality of surgery (R1), and pattern of distribution of hepatic nodules were identified as prognostic factors for OS. The 5-year OS rate was 49.4% in patients with single tumors (type I), 34.2% in patients with satellites (type II), and 9.9% in patients with multifocal tumors (type III) [p < 0.001; Fig. 1]; however, only one patient in the type III group survived more than 3 years (patient alive at 66.8 months). Based on multivariate analysis, a tumor size ≥ 50 mm (HR 2.539, 95% CI 1.150–5.606, p = 0.021), presence of LN metastases (HR 2.482, 95% CI 1.323–4.653, p = 0.005), and radicality of surgery (HR 2.101, 95% CI 1.129–3.910, p = 0.019) were confirmed to be the main prognostic factors for OS, but the pattern of distribution of hepatic nodules was the strongest prognostic factor (type II: HR 3.101, 95% CI 1.552–6.197, p = 0.001; type III: HR 4.064, 95% CI 1.825–9.049, p = 0.001).
The 5-year OS rate according to the preoperative imaging-based pattern of distribution of hepatic nodules was 47.9% in patients with single tumors (preoperative type I), 21.7% in patients with satellites (preoperative type II), and 0.0% in patients with multifocal tumors (preoperative type III) [p < 0.001; electronic supplementary Figure 1].
The 5-year recurrence-free survival rate was 37.1% in patients with single tumors (type I), 16.7% in patients with satellites (type II), and 0% in patients with multifocal tumors (type III) [p < 0.001; electronic supplementary Table 1]. Univariate and multivariate analysis of the prognostic factors for recurrence-free survival are shown in electronic supplementary Table 1.
Prognostic Impact of the Association of the Pattern of Distribution of Hepatic Nodules and Other Prognostic Factors
Figure 2 shows the survival curves of patients with a single tumor (type I, 2A), single tumors with satellites (type II, 2B), and multifocal tumors (type III, 2C) stratified by the other prognostic factors identified in the multivariate analysis, including the size of the tumor, LN status, and the radicality of surgery.
Overall survival according to the type I (a), II (b), and III (c) pattern of distribution of hepatic nodules stratified by the other prognostic factors identified at multivariable analysis: a1, b1, c1 size (< 50 or ≥ 50 mm); a2, b2, c2 lymph node status (N0 or N+); a3, b3, c3 radicality of surgery (R0 or R1 resection)
The size (≥ or < 50 mm) of the main hepatic lesion seems to have a prognostic role in patients with type I tumors, but not in patients with type II and III tumors (p = 0.048, p = 0.457, and p = 0.930, respectively). Conversely, patients without LN metastases and those who underwent R0 resection had better long-term survival in both the patients with satellites (p = 0.049 and p = 0.047, respectively) and patients with multifocal tumors groups (p = 0.001 and p = 0.048, respectively). Patients with LN metastases and R1 resection had very poor prognosis in association with multifocal tumors (type III).
Discussion
Improvements in surgical techniques and perioperative care have enhanced the feasibility and safety of liver resection, with satisfactory long-term results in patients with ICC.8,17,18
The current study represents a large surgical series that focuses on this topic and specifically analyses the clinical, pathological, and prognostic impact of the different patterns of distribution of hepatic lesions in patients who underwent surgery for ICC.
Of the 251 patients included in the study, 21.9% (n = 55) had a type II pattern (single tumor with satellite nodules) and 13.5% (n = 34) had a type III pattern (multifocal tumors). A recent study based on an international multi-institutional cohort of over 1100 patients reported the presence of satellite nodules and intrahepatic metastasis in 22 and 7% of patients, respectively, even in the absence of a clear definition of multiple nodules. Moreover, the authors considered both satellite nodules and intrahepatic metastases to be multifocal tumors in the survival analysis.19
A radiological study by Baheti et al. defined and proposed three different patterns of ICC according to the distribution of hepatic nodules (single tumor, single with satellites, and multifocal tumors) identified, at the time of presentation, on CT scans, which had different impacts on survival.16 However, preoperative imaging may have underestimated the real presence and distribution of multiple nodules. In our series, patients with satellite nodules (type II) or multifocal tumors (type III) appeared to have a single nodule on preoperative imaging in 54.5 and 17.6% of cases, respectively, leading the surgeon into an unexpected and critical decision-making situation during surgery. In case of intraoperative discovery of lesions that were not detected at preoperative imaging, the presence of other prognostic factors, such as the presence of LN metastases, may guide the therapeutic choice, therefore the hepatic pedicle LN sampling with frozen section pathological analysis could be suggested prior to aggressive surgery.
In our study, patients with satellite nodules (type II) and multifocal tumors (type III) had some differences in their clinical characteristics (see Table 2). These aspects suggest different biological behaviors of single tumors with satellite nodules compared with multifocal tumors. This hypothesis is also supported by the survival results (Fig. 1). In multivariate analysis, the pattern of distribution of hepatic nodules was the strongest predictor of OS.
Therefore, the prognosis in patients with multifocal tumors seems to be poor after surgical resection. Nevertheless, some long-term survivors exist in this subgroup of patients.
Uenishi et al. analyzed the prognostic impact of LN metastases in patients with single or multifocal ICC in a surgical series of 133 patients. The study reported no survivors at 3 years following surgery when the multifocal appearance of the hepatic lesions was associated with LN metastases. However, in patients without LN metastases, the 5-year survival rates were 54 and 26% in patients with single and multifocal tumors, respectively. According to these results, the authors concluded that surgery alone cannot prolong survival in patients with both LN metastases and multifocal tumors.20
We performed a similar subgroup analysis in our multi-institutional series. Patients with LN metastases and R1 resection had poor prognosis, particularly if associated with multifocal tumors (type III). Conversely, the long-term survival of patients with satellite nodules (type II) and multifocal tumors (type III) improves in the absence of other prognostic factors (N0 and R0 resection).
According to these results, surgery alone should not be recommended in patients with multifocal tumors (type III) when LN metastases are present and when R0 resection could not be achieved. A recent study of 116 patients with multifocal ICC comparing the survival outcomes of patients who underwent surgery with patients submitted to intra-arterial therapy (TACE and hepatic arterial infusion pump) shows promising results for non-surgical treatment.13
The retrospective nature of the current study should be considered a limitation that resulted in selection bias of patients who underwent surgery based on tumor characteristics and the hepatic distribution of lesions. Moreover, due to the low incidence and resectability rate of ICC, the multi-institutional design over a long period of time should also be considered a limitation of this study. External validation and further study are needed to confirm our results.
Conclusions
ICC has distinct patterns of distribution with peculiar clinical characteristics and different prognoses that should be considered when making therapeutic decisions. The prognosis of patients with multifocal tumors (type III) is significantly worse than the prognosis of patients with other patterns of nodule distribution. More efforts should be made to identify type III patients who may benefit from upfront surgery. In the remaining patients, multimodal treatments should be explored.
References
Miyazaki M, Ohtsuka M, Miyakawa S et al. Classification of biliary tract cancers established by the Japanese Society of Hepato-Biliary-Pancreatic Surgery: 3rd English edition. J Hepatobiliary Pancreat Sci. 2015;22(3):181–96.
Aljiffry M, Abdulelah A, Walsh M, Peltekian K, Alwayn I, Molinari M. Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature. J Am Coll Surg. 2009;208(1):134–47.
Herszenyi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci. 2010;14(4):249–58.
Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37(6):806–13.
Njei B. Changing pattern of epidemiology in intrahepatic cholangiocarcinoma. Hepatology. 2014;60(3):1107–8.
Zhang H, Yang T, Wu M, Shen F. Intrahepatic cholangiocarcinoma: epidemiology, risk factors, diagnosis and surgical management. Cancer Lett. 2016;379(2):198–205.
Ribero D, Pinna AD, Guglielmi A et al. Surgical approach for long-term survival of patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis of 434 patients. Arch Surg. 2012;147(12):1107–13.
Ruzzenente A, Conci S, Valdegamberi A, Pedrazzani C, Guglielmi A. Role of surgery in the treatment of intrahepatic cholangiocarcinoma. Eur Rev Med Pharmacol Sci. 2015;19(15):2892–900.
Guglielmi A, Ruzzenente A, Campagnaro T et al. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg. 2009;33(6):1247–54.
Jonas S, Thelen A, Benckert C et al. Extended liver resection for intrahepatic cholangiocarcinoma: a comparison of the prognostic accuracy of the fifth and sixth editions of the TNM classification. Ann Surg. 2009;249(2):303–9.
Spolverato G, Kim Y, Alexandrescu S et al. Is hepatic resection for large or multifocal intrahepatic cholangiocarcinoma justified? Results from a multi-institutional collaboration. Ann Surg Oncol. 2015;22(7):2218–25.
Hwang S, Lee YJ, Song GW et al. Prognostic impact of tumor growth type on 7th AJCC staging system for intrahepatic cholangiocarcinoma: a single-center experience of 659 cases. J Gastrointest Surg. 2015;19(7):1291–304.
Wright GP, Perkins S, Jones H et al. Surgical resection does not improve survival in patients with multifocal Intrahepatic Cholangiocarcinoma: a comparison of surgical resection with intra-arterial therapy. Ann Surg Oncol. 2018;25(1):83–90.
Bagante F, Gani F, Spolverato G et al. Intrahepatic Cholangiocarcinoma: Prognosis of Patients Who Did Not Undergo Lymphadenectomy. J Am Coll Surg. 2015;221(6):1031-40.e1-4.
Amin MB, Edge SB, Green FL, et al. editors. American Joint Committee on Cancer (AJCC). Cancer staging manual. 8th edn. New York: Springer; 2017.
Baheti AD, Tirumani SH, Shinagare AB, Rosenthal MH, Hornick JL, Ramaiya NH, et al. Correlation of CT patterns of primary intrahepatic cholangiocarcinoma at the time of presentation with the metastatic spread and clinical outcomes: retrospective study of 92 patients. Abdom Imaging. 2014;39(6):1193–201.
Ruzzenente A, Conci S, Ciangherotti A, et al. Impact of age on short-term outcomes of liver surgery: lessons learned in 10-years’ experience in a tertiary referral hepato-pancreato-biliary center. Medicine (Baltimore). 2017;96(20):e6955.
Doussot A, Lim C, Gomez Gavara C, et al. Multicentre study of the impact of morbidity on long-term survival following hepatectomy for intrahepatic cholangiocarcinoma. Br J Surg. 2016;103(13):1887–1894.
Reames BN, Bagante F, Ejaz A, et al. Impact of adjuvant chemotherapy on survival in patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis. HPB (Oxford). 2017;19:901–909.
Uenishi T, Kubo S, Yamazaki O, Yamada T, Sasaki Y, Nagano H, Monden M. Indications for surgical treatment of intrahepatic cholangiocarcinoma with lymph node metastases. J Hepatobiliary Pancreat Surg. 2008;15:417–422.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Simone Conci, Andrea Ruzzenente, Luca Viganò, Giorgio Ercolani, Andrea Fontana, Fabio Bagante, Francesca Bertuzzo, Andrea Dore, Antonio Daniele Pinna, Guido Torzilli, Calogero Iacono and Alfredo Guglielmi have no conflict of interest to declare.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Conci, S., Ruzzenente, A., Viganò, L. et al. Patterns of Distribution of Hepatic Nodules (Single, Satellites or Multifocal) in Intrahepatic Cholangiocarcinoma: Prognostic Impact After Surgery. Ann Surg Oncol 25, 3719–3727 (2018). https://doi.org/10.1245/s10434-018-6669-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-6669-1