Abstract
Background
Two prospective, randomized trials, TARGIT-A and ELIOT, have shown intraoperative radiation therapy to be a safe alternative, with a low-risk of local recurrence, compared with whole breast radiation therapy, following breast-conserving surgery, for selected low-risk patients. We report the first 1000 tumors treated with this modality at our facility.
Methods
A total of 1000 distinct breast cancers in 984 patients (16 bilateral) were treated with breast conserving surgery and X-ray IORT from June 2010 to August 2017. Patients were enrolled in an IORT registry trial. Local recurrence was the primary endpoint.
Results
There have been 28 ipsilateral local recurrences, ten DCIS and 18 invasive. Four local recurrences were within the IORT field, 13 outside of the IORT field but within the same quadrant as the index cancer, and 11 were new cancers in different quadrants. There have been four regional nodal recurrences and one distant recurrence. There have been no breast cancer related deaths and 14 non-breast cancer deaths. With a median follow-up of 36 months, Kaplan–Meier analysis projects 3.9% of patients will recur locally at 4 years. This includes all ipsilateral events in all quadrants.
Conclusions
The local, regional, and distant recurrence rates observed in this trial were comparable to those of the prospective randomized TARGIT-A and ELIOT trials. The low complication rates previously reported by our group as well as the low recurrence rates reported in this study support the cautious use and continued study of X-ray IORT in women with low-risk breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Intraoperative radiation therapy (IORT) is a form of accelerated partial breast irradiation (APBI) in which radiation therapy is delivered, in a single dose, directly to the tumor bed during surgery.1 IORT allows radiation to be delivered precisely to the area where recurrence is most likely while simultaneously reducing compliance issues, radiation exposure to normal tissues, and radiation-induced toxicity.2 IORT has been designed to reduce radiation-induced complications associated with whole breast radiotherapy (WBRT) without compromising oncologic or cosmetic outcomes.2,3,–4 If final histopathology reveals poor prognostic findings, WBRT can be added. IORT then becomes the boost. IORT given initially does not eliminate the potential use of excision and WBRT should there be a local recurrence in the future. Two prospective randomized trials, TARGIT-A and ELIOT, suggest that IORT is a safe alternative to WBRT for selected low-risk patients.3,5,6,7,–8
Minimal data are available about the clinical effectiveness of X-ray IORT using the Xoft® Axxent® Electronic Brachytherapy (eBx®) System®. We report our first 1000 tumors treated using this modality.
Methods and Materials
Patient Population
A total of 984 patients with 1000 unifocal breast tumors (16 bilateral) and a pathologic diagnosis of invasive ductal carcinoma, invasive lobular carcinoma, ductal carcinoma in situ (DCIS), or any combination of these diagnoses were accrued to a prospective IORT registry trial between June 2010 and August 2017 at Hoag Memorial Hospital Presbyterian (Newport Beach, CA). The trial protocol was approved by an institutional review board and met the guidelines of their responsible governmental agency. Patients ranged from 40 to 92 years of age.
Protocol Requirements
Prior to treatment, all patients were at least 40 years old and had tumor spans ≤ 30 mm in greatest extent as determined by mammography, ultrasonography and contrast-enhanced MRI, unless contraindicated. Final tumor extent was determined by the pathologist using serial sectioning and included all tumor foci. All patients with invasive breast cancer were required to have histopathologically negative axillary lymph nodes.
Eligibility Criteria
To be eligible for IORT as the sole adjuvant radiation therapy, final histopathology had to confirm tumor extent ≤ 30 mm, tumor margins ≥ 2 mm for both invasive and noninvasive disease, no extensive lymphovascular invasion, and negative axillary lymph nodes. Isolated tumor cells (N0i +) were acceptable. Patients that deviated from one or more protocol requirements were referred for additional surgery (re-excision or mastectomy) and/or WBRT with IORT becoming the boost. If a positive lymph node was discovered intraoperatively, IORT was not performed. These patients were not included in this analysis (N = 41).
Procedure
All patients underwent breast-conserving surgery and received IORT to the tumor bed. IORT consisted of 20 Gy delivered to the balloon surface using the Xoft® Axxent Electronic Brachytherapy (eBx®) System (Xoft, San Jose, CA, USA, a subsidiary of iCAD, Inc). The system delivers 50-kV X-ray radiation using a balloon applicator. Balloon sizes where chosen that best filled the excision cavity (75% 3–4 cm, 23% 4–5 cm, 2% 5–6 cm). Following IORT balloon placement, skin to balloon distance was measured with ultrasound. The minimum allowable distance for treatment was 8 mm. IORT was delivered to 947 tumors during the initial surgical procedure. Fifty-three patients underwent IORT during a separate delayed surgical procedure (26 patients elected reexcision for margins < 1 mm, 15 patients simply decided to have IORT after their initial excision, and 12 patients were referred from outside hospitals for IORT after their initial excision).
Data Collection and Analysis
Demographics and clinical information were collected during enrollment. Complication, recurrence, and outcome data were collected at 1 week, 1 month, 6 months, 1 year, and yearly thereafter. The primary endpoint was local recurrence. Kaplan–Meier analyses were used to estimate local recurrence probabilities. Curves were compared using the log-rank test.
Results
General Demographics
A total of 1000 breast cancers were treated with X-ray IORT. The cohort characteristics are shown in Table 1. The median follow-up was 36 months. Seventy-eight percent of tumors were invasive; 21% were pure DCIS; 94% of tumors were estrogen receptor (ER)-positive, and 83% were progesterone receptor (PR)-positive. 555 of 786 (71%) invasive tumors were luminal A, using immunohistochemical surrogates. Using 2017 American Society for Radiation Oncology (ASTRO) criteria, 415 tumors were suitable for APBI.9
Side Effects
Side effects were minimal as reported in a previous publication.10 In this series, 54 patients (5.4%) experienced grade 2 or 3 fibrosis or hyperpigmentation. Thirteen (1.3%) patients experienced minor wound healing problems, most likely related to oncoplastic surgery rather than IORT, and 12 (1.2%) patients experienced wound infections. There were no late toxicities. All side effects were seen within the first year.
Tumors that did not Deviate from Protocol Requirements
A total of 695 tumors did not deviate from any protocol criteria and met all study criteria after final histopathology examination. Of these, 693 were treated with IORT as their only form of local treatment; 2 of these patients elected to add WBRT to their IORT, despite meeting all study criteria.
Tumors that did Deviate from Protocol Criteria
Protocol deviations and treatment following deviations are summarized in Table 2.
305 tumors deviated from one or more trial requirements; 143 of these patients declined additional local treatment, bringing the total number of tumors treated with IORT alone to 836 (693 + 143). Of the 162 remaining patients (305–143) with protocol deviations, 109 received WBRT, 9 underwent reexcision followed by WBRT, 27 underwent reexcision alone, and 17 elected mastectomies. Local treatment and the number of recurrences in each treatment group are summarized in Table 3. Because 2 patients without protocol deviations added WBRT, the total number of patients receiving excision plus WBRT is 111.
Recurrences
There were 28 local recurrences. Eighteen were invasive; ten were pure DCIS. Seventeen of 28 (58%) local recurrences were in the same quadrant as the index cancer. Four of these were within the IORT field, and 13 were outside the IORT field but within the same quadrant as the index cancer. Eleven local recurrences were in quadrants different from the index cancer and were thought to be new cancers. Average time to local recurrence was 28 (range 7–55) months. It was 31 months for same quadrant recurrences and 23 months for different quadrant recurrences.
Two patients with local recurrences had axillary metastases at the time of local recurrence. Two additional patients presented with axillary metastases without having had a local recurrence. All four patients with axillary recurrence received IORT only as their initial local treatment. One of these patients also developed metastatic disease in a cervical lymph node. Another patient with a local recurrence had metastases to two vertebrae. Overall, there were 28 local recurrences, 4 regional nodal recurrences, and 1 patient with bone metastases (33 events) among 30 patients.
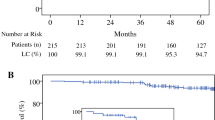
Table 4 shows the 4-year probability of local recurrence for a range of different study subsets. For the entire cohort of 1000 tumors, the Kaplan–Meier probability of a local recurrence at 4 years was 3.9% (Fig. 1). This includes all recurrences (invasive and DCIS) in all quadrants. If the analysis is limited to patients with 2 years or more of follow-up (n = 652) and all 28 local recurrences are included, the 4-year probability of local recurrence increases only 0.4 to 4.3%. The median follow-up for this subgroup increases to 44 months.
When DCIS is not considered as an endpoint, the probability of local invasive recurrence in any quadrant at 4 years decreases to 2.6%. When same quadrant invasive recurrences are considered as the endpoint, the probability of local invasive recurrence at 4 years decreases even more to 2.0%.
We compared tumors that received IORT as their only local treatment with those that received IORT followed by WBRT. There were 26 local recurrences among 836 tumors that received IORT only and 1 local recurrence among 111 tumors that received IORT plus WBRT. The probability of local recurrence at 4 years was 4.3% for IORT only versus 1.0% for IORT plus WBRT (p = 0.21).
There was a significant difference in local recurrence probability for luminal A invasive cancers versus nonluminal A invasive tumors. There were 11 local recurrences among 555 luminal A and 11 among 231 nonluminal A. The probability of local recurrence at 4 years was 3.0% for luminal A tumors versus 6.8% for nonluminal A (p = 0.04). The remaining six recurrences developed from noninvasive index lesions whose initial biologic subtype was unknown.
There have been no breast cancer-related deaths. Fourteen patients, ranging in age from 53 to 94 years, have died from non-breast cancer causes. Three of these patients experienced a local recurrence without metastatic disease.
Discussion
IORT as an alternative treatment option for low-risk patients is based,on results of the prospectively randomized TARGIT-A and ELIOT trials, both of which compared the outcome of IORT to WBRT.3,5,6,7,–8,11 ELIOT employed megavoltage electrons while TARGIT-A employed low-energy, X-ray IORT, similar to that utilized in our study.3,8
Patients in the TARGIT-A trial received either 50-kV X-ray IORT to a dose of 20 Gy at the surface of the tumor bed or 50 Gy of WBRT plus or minus a boost, depending on institutional preference.3,5,11 When IORT patients had poor prognostic factors on final histopathologic examination, they received an additional 50 Gy equivalent of WBRT and the IORT dose was considered a radiation boost.3,5,11 The addition of WBRT to higher-risk tumors was termed “risk-adapted” and occurred in 15% of all patients and 22% of pre-pathology patients. The 5-year probability of recurrence for X-ray IORT versus WBRT in the TARGIT-A Trial was 3.3 and 1.3% respectively (p = 0.042).3,5,8
Kaplan–Meier analysis of the first 204 tumors treated at our institution with X-ray IORT showed results similar to TARGIT-A, predicting a 2.9% 4-year probability of local recurrence.12 In the current study, we present recurrence data for the first 1000 tumors treated with X-ray IORT. To our knowledge, this is the largest single-institution report of x-ray IORT recurrence data in the United States.
Most tumors in this trial were biologically favorable, comparable to those included in the TARGIT-A trial.3,5,11 Of all tumors, 94% were estrogen receptor (ER)-positive and 83% were progesterone receptor (PR)-positive. Of the invasive tumors, 96% were HER2/neu-negative and 71% were luminal A. The majority of tumors in the current study would be stage IA according to the new AJCC 8th edition staging system.13
We analyzed our data by 2017 ASTRO APBI recommendations, comparing all possible combinations of ASTRO subgroups (suitable, cautionary, and unsuitable).9 We found no significant difference in the probability of local recurrence between any two subgroups or combinations of subgroups. For example, there were 10 local recurrences among 415 ASTRO suitable tumors compared with 18 local recurrences among 585 not suitable tumors (354 cautionary + 231 unsuitable). At 4 years, the probability of an ASTRO suitable tumor recurring locally was 3.3% compared with 4.4% for a nonsuitable tumor (p = 0.62).
The 2017 ASTRO guidelines include ER among the criteria to be considered for patients receiving APBI.9 We looked at hormone receptors as a predictor of the probability of local recurrence at 4 years (Table 4). While both ER and PR receptor-negative patients recurred at a slightly higher rate than receptor-positive patients, the differences were not significant.
The 2017 ASTRO guidelines do not include biologic subtyping in the determination of a patient’s suitability for APBI.9 However, when luminal A invasive tumors were compared with nonluminal A invasive tumors, there was a significant difference. At 4 years, the probability of a luminal A tumor recurring locally was 2.8% compared with 6.5% for a nonluminal A tumor (p = 0.04). These data suggest that additional biologic subtyping may allow refinement of the suitability guidelines for IORT.
There have been 33 events among 30 patients: 28 local recurrences, 4 regional nodal recurrences, and 1 patient with bone metastases. No patient has died from breast cancer. Seventeen of 28 (58%) local recurrences were in the same quadrant as the original cancer. Four were within the IORT field and were considered true IORT failures; 13 were outside the IORT field but in the same quadrant as the index cancer and were considered marginal misses. Eleven local recurrences were in quadrants different from the index cancer.
X-ray IORT using a balloon catheter delivers a therapeutic dose to a sphere approximately 1 cm beyond the excision site.14 IORT cannot be expected to control the development or progression of new or previously existing cancers in other quadrants. It also cannot be expected to control cancers in the same quadrant that are more than 1 cm from the balloon surface. If we consider only in-field local recurrences, there have been only four true failures of IORT in this series. It is possible that WBRT with a standard boost would have controlled some, but not all, of these out-of-field recurrences.
A total of 18 recurrences were invasive while 10 were DCIS. If invasive recurrence is used as the endpoint rather than all recurrences, the predicted 4-year probability of local recurrence decreases from 3.9 to 2.6% (Table 4). Because DCIS recurrences are not life-threatening and can sometimes be treated with excision alone, perhaps they should not be considered treatment failures, particularly if the index tumor was invasive. This belief has generated the development of three prospective, randomized trials that are currently underway, in which patients with needle biopsy-proven low- and intermediate-grade DCIS are randomized to surveillance alone versus standard treatment.15,16,–17 If we accept this concept and consider only invasive recurrences in the same quadrant as treatment failures (n = 12), then the 4-year probability of a local failure in our series decreases to 2.0%.
In the current study, 109 patients who deviated from the trial protocol, plus 2 who did not deviate, subsequently received WBRT. Nine additional patients underwent reexcision and received WBRT, bringing the total number of patients treated with WBRT to 120. Among this group, there was only one local recurrence, and it was in a different quadrant from the index cancer. The fact that only 1 of 120 patients who received IORT plus WBRT has recurred is encouraging. It supports the concept of utilizing IORT as a planned boost during initial surgery. The 4-year Kaplan–Meier probability of a local recurrence among 120 patients in this trial who received IORT plus WBRT was only 0.9%, even though those patients violated one or more protocol criteria and therefore had worse prognostic factors and a higher probability of local recurrence than the remainder of the cohort.
Evidence from the International Society of Intraoperative Radiotherapy (ISIORT) European pooled analysis has demonstrated breast tumor control rates of 99.2% at 73 months when using electron IORT as a boost to WBRT.18 Favorable toxicity and cosmesis using IORT as a boost treatment also was reported.19 The prospective multicenter TARGIT-B, using X-ray IORT as a planned boost, is currently underway.20
Whole breast radiation therapy is an integral part of breast conservation. Multiple prospective and retrospective trials have proven that it works, yielding survival rates equivalent to mastectomy but with far better cosmetic and emotional results.21,22,–23 The radiation component takes expensive equipment and a high degree of medical expertise, leading to a lack of availability in rural areas.24,25 Many women with early breast cancer who are excellent candidates for breast conservation find that they live too far from a radiation therapy center to make the treatment practical or they discover that 30–35 treatments are simply too inconvenient and/or too costly.24,25 This may lead to unnecessary mastectomy and, in some cases, bilateral mastectomies. This happens too frequently when excision plus IORT could have been a simple, outpatient, 1-day, less expensive, breast-conserving solution.
Data from our trial, which includes 1000 tumors, suggest that X-ray IORT is a viable alternative to WBRT for a select subset of low-risk women with early-stage breast cancer. Additionally, biologic subtyping data may allow refining suitability guidelines for IORT. The excellent results achieved in patients who received WBRT after IORT supports further exploration of IORT as a planned boost to WBRT in higher-risk patients. IORT is profoundly convenient. Its increased use would make breast conservation available to many women who now must undergo mastectomy, because standard WBRT is simply too costly, too far away, or too unavailable.
References
Harris EER, Small W Jr. Intraoperative radiotherapy for breast cancer. Front Oncol. 2017;7:317.
Polgar C, Fodor J, Major T, Sulyok Z, Kasler M. Breast-conserving therapy with partial or whole breast irradiation: ten-year results of the Budapest randomized trial. Radiother Oncol. 2013;108(2):197–202.
Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet. 2010;376(9735):91–102.
Corica T, Nowak A, Saunders C, et al. Cosmesis and breast-related quality of life outcomes after intraoperative radiation therapy for early breast cancer: a substudy of the TARGIT-A Trial. Int J Radiat Oncol. 2016;96(1):55–64.
Vaidya J, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet. 2014;383:603–13.
Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol. 2013;14(13):1269–77.
Silverstein MJ, Fastner G, Maluta S, et al. Intraoperative radiation therapy: a critical analysis of the ELIOT and TARGIT trials. Part 1–ELIOT. Ann Surg Oncol. 2014;21(12):3787–92.
Silverstein MJ, Fastner G, Maluta S, et al. Intraoperative radiation therapy: a critical analysis of the ELIOT and TARGIT trials. Part 2–TARGIT. Ann Surg Oncol. 2014;21(12):3793–9.
Correa C, Harris EE, Leonardi MC, et al. Accelerated Partial Breast Irradiation: Executive summary for the update of an ASTRO Evidence-Based Consensus Statement. Pract Radiat Oncol. 2017;7(2):73–9.
Epstein M, Silverstein M, Lin K, et al. (2016) Acute and chronic complications in breast cancer patients treated with intraoperative radiation therapy. Ann Surg Oncol; 23:3304–3309.
Vaidya JS, Wenz F, Bulsara M, et al. An international randomised controlled trial to compare TARGeted Intraoperative radioTherapy (TARGIT) with conventional postoperative radiotherapy after breast-conserving surgery for women with early-stage breast cancer (the TARGIT-A trial). Health Technol Assess. 2016;20(73):1–188.
Silverstein MJ, Epstein MS, Lin K, et al. Intraoperative radiation using low-kilovoltage x-rays for early breast cancer: a single site trial. Ann Surg Oncol. 2017;24(10):3082–7.
Giuliano AE, Connolly JL, Edge SB, et al. Breast cancer: major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(4):290-303
Hensley FW. Present state and issues in IORT physics. Radiat Oncol. 2017;12(1):37.
Francis A, Fallowfield L, Rea D. The LORIS Trial: addressing overtreatment of ductal carcinoma in situ. Clin Oncol (R Coll Radiol). 2015;27(1):6–8.
Elshof LE, Tryfonidis K, Slaets L, et al. Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ—the LORD study. Eur J Cancer. 2015;51(12):1497–510.
Youngwirth LM, Boughey JC, Hwang ES. Surgery versus monitoring and endocrine therapy for low-risk DCIS: The COMET Trial. Bull Am Coll Surg. 2017;102(1):62–3.
Fastner G, Sedlmayer F, Merz F, et al. IORT with electrons as boost strategy during breast conserving therapy in limited stage breast cancer: long term results of an ISIORT pooled analysis. Radiother Oncol. 2013;108(2):279–86.
Lemanski C, Azria D, Thezenas S, et al. Intraoperative radiotherapy given as a boost for early breast cancer: long-term clinical and cosmetic results. Int J Radiat Oncol Biol Phys. 2006;64(5):1410–5.
Vaidya JS, Tobias JS. Targeted intraoperative radiotherapy tumour bed boost during breast-conserving surgery after neoadjuvant chemotherapy. Breast Care (Basel). 2017;12(5):314–6.
Veronesi U, Banfi A, Salvadori B, et al. Breast conservation is the treatment of choice in small breast cancer: long-term results of a randomized trial. Eur J Cancer. 1990;26:668–70.
Veronesi U, Saccozzi R, Del Vecchio M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection and radiotherapy in patients with small cancers of the breast. N Engl J Med. 1981;305:6–10.
Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–32.
Athas WF, Adams-Cameron M, Hunt WC, Amir-Fazli A, Key CR. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. J Natl Cancer Inst. 2000;92(3):269–71.
Ballard-Barbash R, Potosky AL, Harlan LC, Nayfield SG, Kessler LG. Factors associated with surgical and radiation therapy for early stage breast cancer in older women. J Natl Cancer Inst. 1996;88(11):716–26.
Acknowledgments
The authors thank Cristina De Leon, R.N., January Lopez, M.D., and Ralph MacKintosh, Ph.D. for their contribution to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silverstein, M.J., Epstein, M., Kim, B. et al. Intraoperative Radiation Therapy (IORT): A Series of 1000 Tumors. Ann Surg Oncol 25, 2987–2993 (2018). https://doi.org/10.1245/s10434-018-6614-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-6614-3