Abstract
Introduction
Breast-conserving therapy is the standard of care for early-stage breast cancer. In the era of multimodality therapy, the debate on the value of revision surgery for compromised margins continues, and high re-excision rates persist despite updated guidelines. Our study sought to identify the local re-excision rate for compromised margins after lumpectomy, and identify predictors of residual disease at re-excision.
Methods
This population-based retrospective cohort study included women with breast cancer who underwent a lumpectomy between 2009 and 2012 in Manitoba, with close (≤ 2 mm) or positive margins that led to re-excision. Patient demographics and tumor characteristics were identified through provincial cancer registries and chart reviews. For patients with invasive cancer, the six anatomical margins were reported for margin status, width, and pathology type at the margin.
Results
Of the 2494 patients identified, 556 women underwent re-excision, yielding a re-excision rate of 22.29%. Of our 311 patients with invasive cancer who underwent re-excision, 62.7% had residual disease identified on revision. On univariable analysis, the size and grade of the invasive component, nodal stage, and the number of positive margins were associated with residual disease on re-excision (p < 0.05). With the exception of nodal stage, the same variables remained statistically significant on multivariable analysis.
Conclusions
Our results suggest that even in the absence of ‘no ink on tumor’, the cancer size and grade in lumpectomy specimens are high-risk factors for residual disease, and this subgroup of patients may benefit from re-excision. Long-term follow-up of this cohort is required to determine their risk of recurrence after adjuvant treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Breast-conserving therapy (BCT) has been the standard of care for early-stage invasive breast cancer for many years. It is estimated that 20–60% of women who undergo BCT require additional breast surgery due to positive or inadequate margins after the initial lumpectomy.1,2,–3 Previous lack of consensus on what constitutes adequate negative margins in BCT has led to variability in practice between centers and surgical oncologists.4 Unfortunately, this controversy has resulted in increased rates of re-excision, as well as increased costs, risk of complications, stress to the patient, poor cosmetic outcome, and the delay of necessary adjuvant treatment.1,5
Current literature has extensively examined breast cancer recurrence rates in relation to resection margin width.6,7,8,9,10,–11 A 2014 meta-analysis by Houssami et al. specifically assessed the evidence on surgical margins for BCT in the era of multidisciplinary therapy.11 The study confirmed that while negative margins reduced the odds of local recurrence, increasing the width of the resection margin was not associated with reduced recurrence rates. Later that year, the Society of Surgical Oncology (SSO) developed consensus guidelines for BCT, including margin recommendations, based on the 2014 meta-analysis. The guidelines definitively recommended the use of ‘no ink on tumor’ as the standard margin for adequate resection of invasive breast cancer.12
Although endorsed by the American Society of Clinical Oncology (ASCO), flexibility in the application of these guidelines was advocated given their basis lies in the analysis of retrospective studies where heterogeneous definitions of close margins and significant selection bias limit the strength of the evidence.13 The decision whether or not to re-excise compromised margins should be made in a multidisciplinary fashion, and each individual patient’s clinical and pathologic features should guide the decision to perform re-excision in selected patients.14 A recent update of the 2014 meta-analysis by Houssami et al. was presented in December 2017 and cast doubt on the recommendation made by the SSO of ‘no ink on tumor’.15 The updated analysis added more studies, with a total of 55,302 patients. This study failed to confirm that ‘no ink on tumor’ is optimal. The crude rates of local recurrence decreased as margin distance increased: 7.2% for patients with margins < 2 mm, 3.6% for margins 2–5 mm (3.6%), and 3.2% for margins > 5 mm (p < 0.001 for each). Therefore, it is reasonable to hypothesize that with wider margins, we clear the remaining residual disease, leading to less recurrence. Consequently, predictors of residual disease or lack thereof should be identified to help rationalize the need for re-excision.
The objective of our study was to identify the local rate of re-excision for compromised margins after lumpectomy, and to identify characteristics on initial operation that are predictive of finding residual tumor on re-excision pathology. Such characteristics may help clinicians direct the need for re-excision, even in the absence of ink on the margin.
Methods
Our study was a retrospective cohort analysis of all women in Manitoba diagnosed with invasive or non-invasive breast cancer who underwent a lumpectomy between January 2009 and December 2012, with a resultant close (≤ 2 mm) or positive margin that led to a re-excision procedure. Patients were identified through the Manitoba Cancer Registry (MCR), a population-based registry covering a province of approximately 1.32 million. The MCR contains information regarding all Manitobans diagnosed with cancer since 1956 and has among the highest levels of completeness for cancer reporting databases in North America.16,17 Charts were reviewed after obtaining the required ethical approval.
Patient characteristics were obtained from the MCR, and cancer staging was determined according to the American Joint Committee on Cancer (AJCC) guidelines. For assessment of re-excision rate and predictors of residual cancer, we excluded patients with missing or incomplete data (patients without a pathology report for their first and/or second surgery), rare histological subtypes (including phyllodes), and patients who received neoadjuvant or adjuvant treatment prior to or between surgeries. Routine pathological data were identified from pathology reports for patients with mammary carcinomas. Each of the six anatomical margins was reported for margin status, margin width (if close), and pathology type found at that margin. A close margin was defined as a margin width ≤ 2 mm. Synoptic reporting of breast cancer specimens gives details of any margins ≤ 2 mm. In cases where multiple foci of cancer were apparent, the largest size and highest grade were reported.
For analysis of the impact of tumor size, we chose the intermediate size (0 to < 10 mm) as our reference group. Breast tumors that are < 1 cm in size are likely to be non-palpable, and thus are more likely to be excised via a needle localization procedure. When compared with excising a small but palpable tumor, this technique could carry a higher rate of positive margins as the intraoperative assessment of the adequacy of surgical resection by the surgeon is more difficult. On the other hand, larger tumors tend towards positive margins based on their tendency to have more diffuse, stellate or multifocal growth patterns, and may also be associated with a more extensive in situ component.14 As such, small palpable lesions are likely to have the lowest risk of residual disease, and served best as the reference group for size.
Univariable and multivariable logistic regression analyses were conducted using variables of interest identified through a literature review of the significant factors associated with residual disease. Cramer’s V was used as a post-test to determine strengths of association between the chosen variables (after Chi-square has determined significance), in order to avoid having variables that are highly related in the model.18 In our final multivariable model, no associations between the variables of interest were identified. A sensitivity analysis was conducted to confirm our complete case analysis. Rather than excluding the cases with missing or unknown grade, we included an ‘unknown’ category for grade in the multivariable model, which reached the same conclusions as the model without this category, thus confirming our multivariable model. p-values < 0.05 were considered statistically significant. All statistical analyses were conducted using SAS software (SAS Institute, Inc., Cary, NC, USA).
Results
Re-excision Rate
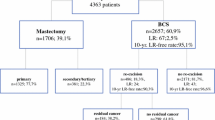
From 2009 to 2012, 4010 cases of breast cancer were identified in Manitoba, including 3523 patients with a diagnosis of invasive cancer and 487 patients with in situ only disease. A total of 2494 women underwent a lumpectomy as their initial breast cancer surgery, and 556 of those patients underwent a re-excision within 1 year of their original operation (426 patients with invasive disease and 130 with in situ only disease). This resulted in a lumpectomy re-excision rate of 22.29% (Fig. 1).
Study Cohort Description
Overall, 426 patients with a diagnosis of invasive breast cancer who underwent re-excision within 1 year of the original lumpectomy were identified. After excluding patients with missing or incomplete data, rare histological subtypes, and those who received neoadjuvant or adjuvant treatment prior to or between surgeries, the final analyses of rates and predictors of residual disease were performed on 311 patients. The frequencies of common tumor characteristics following initial surgical resection are summarized in Table 1.
Rate of Residual Cancer
Residual disease was identified in re-excision specimens in 195 patients of the 311 included in the study cohort (62.7%). In relation to the procedure type performed at the time of revision surgery, 50% of the lumpectomy patients (96/192) and 83% of the mastectomy patients (99/119) had evidence of residual disease on re-excision pathology (Fig. 2).
Statistical Analysis
Univariable logistic regression was conducted on clinical and pathological characteristics noted in the study cohort following the primary surgery. Our analysis identified four variables associated with the presence of residual disease at the time of second surgery: the size of the invasive component; nodal stage; grade of invasive component; and the number of positive margins (invasive or non-invasive). The results are reported in Table 2. With the exception of nodal stage, the same variables remained statistically significant in the multivariable logistic regression model. Molecular classification based on estrogen receptor 2 (ER2), progesterone receptor 2 (PR2) and human epidermal growth factor receptor 2 (HER2) was also studied but did not show a statistically significant correlation with the status of residual disease.
Discussion
Re-excision Rate
In our study cohort, the overall re-excision rate within 1 year of the primary lumpectomy procedure was 22.29%. This is consistent with the lower end of the recently published rates in the meta-analysis by Houssami et al., which reported a re-excision rate of 22–56% across the 17 studies included.11
Manitoba’s low rate of re-excision, which continues to be the lowest in Canada, can be explained by some key clinical factors.19 First, the majority of breast cancer surgery in Manitoba is performed by surgeons involved with The Breast Health Centre, a comprehensive provincial program. Extensive clinical experience combined with higher operative volumes undoubtedly leads to improved surgical technique, and may result in decreased positive margin rates.20 Second, it is important to recognize that not all compromised margins are re-excised. Patient choice and poor fitness for revision surgery are two common reasons margin re-excision does not occur. Third, Manitoba has a relatively high mastectomy rate, as well as the highest rate of breast reconstruction in Canada, most of which is completed in an immediate fashion.19 The relative ease with which patients can access a mastectomy with concurrent reconstruction may encourage its use when a surgeon feels a lumpectomy may result in an increased risk of positive margins. This potential for selection bias of lumpectomy candidates may affect both the re-excision rates and the rate of residual disease in our population.
Rate and Predictors of Residual Cancer
Overall, 62.7% of the cases in our final cohort (195/311) who underwent a second operation showed evidence of residual disease on their final pathology report. These results are consistent with the literature, which reports a rate of residual disease found on re-excision specimens of between 21 and 77%.6,7,10,21,22,23,–24
The recent recommendations by the SSO in 2014 on margins after lumpectomy produced positive impacts. For example, Merrill et al. showed that the new resection margin guidelines would have reduced their reoperation rate for BCT by half;21 however, residual disease was still present in a significant number of patients who would not have been recommended re-excision under the new guidelines.21 In addition, a recent economic analysis has also shown a substantial cost savings to the Canadian healthcare system if surgeons practice to this guideline.25
An updated meta-analysis of the 2014 work by Houssami et al. was presented in 2017 and included a larger number of studies (38 vs. 33), with a total of 55,302 patients, and more rigorous inclusion criteria.15 This updated meta-analysis showed that rates of local recurrence decreased as the margin distance increased. These results question the SSO recommendations and establish the observation that with wider clear margins there is less recurrence, which could be explained by reducing the chance of residual disease by having a wider resection margin.
Patient age, the presence of multiple positive margins,6 tumor multifocality,23,26 clinical tumor size,27 nodal status,26 and the presence of extensive intraductal subtype present at the margin10,23 are factors previously associated with residual disease after breast conservation surgery (BCS). Our study tried to identify predictors of residual disease after lumpectomy for invasive breast cancer (Table 2) so that patients with a high risk of residual disease may be considered for re-excision, even in the case of ‘no ink on tumor’.
First, we identified a statistically significant association between the number of positive margins on the initial surgical specimen and the presence of residual tumor on reoperation. Two or more positive margins, invasive or non-invasive pathology, were more likely to yield residual disease on re-excision (odds ratio [OR] 3.01; p = 0.0028), a finding consistent with previous reports in the literature.6,7 It has previously been shown that there is a linear association between positive margins and residual disease; with each additional margin involved, the risk of residual disease on re-excision increases.6 In essence, the number of close or positive margins acts as a surrogate for the extent of the tumor burden.
Second, the size of the invasive tumor component identified at first surgery was also found to be a statistically significant predictor of residual disease. Invasive tumor size ≥ 20 mm had greater odds of residual disease compared with tumors < 20 mm (OR 2.17, p = 0.0084). As early as 1993, it was reported that clinical tumor size may predict residual disease in a re-excision specimen.27,28 A more recent study has shown pathologic tumor size to be associated with compromised margins.23 Our study is the first to identify pathologic primary tumor size as a strong predictor of residual disease on re-excision.
Finally, our research is the first to show that grade II or III invasive breast cancer on primary tumor pathology is associated with an increased likelihood of identifying residual tumor in patients undergoing re-excision for positive margins. Much of the literature on BCT and breast cancer recurrence has included tumor grade in the assessment of pathology characteristics associated with positive margins or residual tumor; however, previous studies have been less discerning with their inclusion criteria, including in their analysis in situ only cases and cases with undetermined margins.23,24 By excluding patients with ductal carcinoma in situ or undocumented margins, we were able to increase the homogeneity of our cohort, thus enhancing the validity of our results.
In this manner, we feel our data support the SSO/American Society for Radiation Oncology (ASTRO) guideline on margin re-excision, in that a negative margin is generally adequate but must be cautiously viewed in concert with other factors, such as tumor size and grade, as presented in our study. Any exercise that better delineates who does or does not require further surgery is certainly to the benefit of our patients. Moving forward, we would like to identify predictors of the absence of residual disease even in the presence of a positive margin. In this way, a subgroup of patients may be saved an unnecessary operation. We would also like to assess if predictors of residual disease could also be linked to local recurrence on long-term follow-up.
Study Limitations
Missing patient data is a common concern in retrospective studies. Our relatively small sample size may also limit the strength of our results. The variability of margin reporting is another common issue seen in breast cancer surgery research; however, with the advent of synoptic reporting, consistency in pathology reports continues to improve. Our time frame for re-excision within 1 year of the initial lumpectomy procedure was chosen to include those who received adjuvant chemotherapy and/or radiation before undergoing re-excision in our analysis of the rate of re-excision in the province. This 1-year re-excision cut-off could potentially introduce bias by including tumor recurrence as re-excision. Finally, this work represents a single institutional experience.
Conclusions
With new updated meta-analysis data casting doubt on the strength of the SSO guidance for margins after lumpectomy, it is important to identify predictors of residual disease so that a rational decision could be made on who will benefit from re-excision. Our study has shown that predictors of residual disease after primary BCS include not only the number of positive margins but also the size and grade of invasive cancer.
References
Azu M, Abrahamse P, Katz SJ, Jagsi R, Morrow M. What is an adequate margin for breast-conserving surgery? Surgeon attitudes and correlates. Ann Surg Oncol. 2010; 17:558–63.
Morrow M, Harris JR, Schnitt SJ. Surgical margins in lumpectomy for breast cancer—bigger is not better. N Engl J Med. 2012; 367(1): 79–82.
Houssami N, Macaskill P, Marinovich ML, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer. 2010; 46(18): 3219–32.
McCahill LE, Single RM, Aiello Bowles EJ, et al. Variability in reexcision following breast conservation surgery. JAMA. 2012; 307(5): 467–75.
Adams BJ, Zoon CK, Stevenson C, Chitnavis P, Wolfe L, Bear HD. The role of margin status and reexcision in local recurrence following breast conservation surgery. Ann Surg Oncol. 2013; 20(7): 2250–5.
Fitzgerald S, Romanoff A, Cohen A, et al. Close and positive lumpectomy margins are associated with similar rates of residual disease with additional surgery. Ann Surg Oncol. 2016; 23(13): 4270–6.
Hadzikadic Gusic L, Mcguire KP, Ozmen T, et al. Margin width is not predictive of residual disease on re-excision in breast conserving therapy. J Surg Oncol. 2014; 109: 426–30.
Margenthaler JA, Gao F, Klimberg VS. Margin index: a new method for prediction of residual disease after breast-conserving surgery. Ann Surg Oncol. 2010; 17:2696–701.
Skripenova S, Layfield LJ. Initial margin status for invasive ductal carcinoma of the breast and subsequent identification of carcinoma in reexcision specimens. Arch Pathol Lab Med. 2010; 124: 109–14.
Jaffré I, Campion L, Dejode M, et al. Margin width should not still enforce a systematic surgical re-excision in the conservative treatment of early breast infiltrative ductal carcinoma. Ann Surg Oncol. 2013; 20(12): 3831–8.
Houssami N, Macaskill P, Marinovich ML, Morrow M. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann Surg Oncol. 2014; 21(3): 717–30.
Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol. 2014; 32(14): 1507–15.
Buchholz TA, Somerfield MR, Griggs JJ, et al. Margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: American Society of Clinical Oncology endorsement of the Society of Surgical Oncology/American Society for Radiation Oncology consensus guideline. J Clin Oncol. 2014; 32(14): 1502–6.
Hunt KK, Sahin AA. Too much, too little, or just right? Tumor margins in women undergoing breast-conserving surgery. J Clin Oncol. 2014; 32(14): 1401–6.
Shah C, Verma V, Sayles H, Recht A, Vicini. Appropriate margins for breast conserving surgery in patients with early stage breast cancer: a meta-analysis. Oral presentation at the 2017 San Antonio Breast Cancer Symposium; 5–9 Dec 2017; San Antonio, TX.
Minister of Health. Reporting of diseases and conditions—règlement sur la déclaration de maladies et d’affections; 2009: 1–24.
Singh H, De CC, Shu E, et al. Wait times from presentation to treatment for colorectal cancer: a population-based study. Can J Gastroenterol. 2010; 24(1):33–9.
Cramer Harald. Mathematical methods of statistics. Chapter 21: the two-dimensional case. Princeton: Princeton University Press; 1946. p. 282.
Canadian Institute for Health Information. Breast Cancer Surgery in Canada, 2007–2008 to 2009–2010. Ottawa: Canadian Institute for Health Information; 2012.
Aguilar B, Sheikh F, Pockaj B, Wasif N, Gray R. The effect of junior residents on surgical quality: a study of surgical outcomes in breast surgery. Am J Surg. 2011; 202: 654–8.
Merrill AL, Coopey SB, Tang R, et al. Implications of new lumpectomy margin guidelines for breast- conserving surgery: changes in reexcision rates and predicted rates of residual tumor. Ann Surg Oncol. 2016; 23: 729–34.
Sabel MS, Rogers K, Griffith K, et al. Residual disease after re-excision lumpectomy for close margins. J Surg Oncol. 2009; 99:99–103.
Dillon MF, Hill ADK, Quinn CM, McDermott EW, O’Higgins N. A pathologic assessment of adequate margin status in breast-conserving therapy. Ann Surg Oncol. 2006; 13(3): 333–9.
Cellini C, Hollenbeck ST, Christos P, et al. Factors associated with residual breast cancer after re-excision for close or positive margins. Ann Surg Oncol. 2004; 11(10): 915–20.
Baliski CR, Pataky RE. Influence of the SSO/ASTRO margin reexcision guidelines on costs associated with breast-conserving surgery. Ann Surg Oncol. 2017; 24(3): 632–7.
Coopey S, Smith BL, Hanson S, Buckley J, Hughes KS, Gadd M, et al. The safety of multiple re-excisions after lumpectomy for breast cancer. Ann Surg Oncol. 2011; 18: 3797–801.
Gwin JL, Eisenberg BL, Hoffman JP, Ottery FD, Boraas M, Solin LJ. Incidence of gross and microscopic carcinoma in specimens from patients with breast cancer after re-excisions lumpectomy. Ann Surg. 1993; 218(6): 729–34.
Jardines L, Fowble B, Schultz D, et al. Factors associated with a positive re-excision after excisional biopsy for invasive breast cancer. Surgery. 1995; 118(5): 803–9.
Acknowledgements
The authors thank the University of Manitoba and the Cancer Registry at CancerCare Manitoba for their support to accomplish this work. A special thank you to Pascal Lambert, a Health Outcomes Analyst in the Epidemiology Department at CancerCare Manitoba, for his guidance with the statistical analysis.
Disclosures
Lisa Findlay-Shirras, Oussama Outbih, Charlene Muzyka, Katie Galloway, Pamela Hebbard, and Maged Nashed have no commercial interests or financial/material support to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Findlay-Shirras, L.J., Outbih, O., Muzyka, C.N. et al. Predictors of Residual Disease After Breast Conservation Surgery. Ann Surg Oncol 25, 1936–1942 (2018). https://doi.org/10.1245/s10434-018-6454-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-6454-1