Abstract
Background
Synchronous colorectal carcinomas (CRC) occur in 1–8% of patients diagnosed with CRC. This study evaluated treatment patterns and patient outcomes in synchronous CRCs compared with solitary CRC patients.
Methods
All patients diagnosed with primary CRC between 2008 and 2013, who underwent elective surgery, were selected from the Netherlands Cancer Registry. Using multivariable regressions, the effects of synchronous CRC were assessed for both short-term outcomes (prolonged postoperative hospital admission, anastomotic leakage, postoperative 30-day mortality, administration of neoadjuvant or adjuvant treatment), and 5-year relative survival (RS).
Results
Of 41,060 CRC patients, 1969 patients (5%) had synchronous CRC. Patients with synchronous CRC were older (mean age 71 ± 10.6 vs. 69 ± 11.4 years), more often male (61 vs. 54%), and diagnosed with more advanced tumour stage (stage III–IV 54 vs. 49%) compared with solitary CRC (all p < 0.0001). In 50% of the synchronous CRCs, an extended surgery was conducted (n = 934). Synchronous CRCs with at least one stage II–III rectal tumour less likely received neoadjuvant (chemo)radiation [78 vs. 86%; adjusted OR 0.6 (0.48–0.84)], and synchronous CRCs with at least one stage III colon tumour less likely received adjuvant chemotherapy [49 vs. 63%; adjusted OR 0.7 (0.55–0.89)]. Synchronous CRCs were independently associated with decreased survival [RS 77 vs. 71%; adjusted RER 1.1 (1.01–1.23)].
Conclusions
The incidence of synchronous CRCs in the Dutch population is 5%. Synchronous CRCs were associated with decreased survival compared with solitary CRC. The results emphasize the importance of identifying synchronous tumours, preferably before surgery to provide optimal treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In the western world, colorectal cancer (CRC) is the third and second most common cancer among men and women, and it is the second and third most common cause of cancer death.1 Patients with primary CRC can have more than one lesion at the time of initial presentation.2 Previous studies report a frequency of synchronous CRCs varying from 1 to 8%2,3,4,5,6,7 Part of this variation can be explained by differences in definitions, selection criteria, patient populations, and time periods studied. Risk factors for developing synchronous CRCs are largely unknown, although familial polyposis and ulcerative colitis with dysplasia have been suggested to influence synchronous CRC development.8,9
For stage I–III CRC patients, surgery of the primary treatment is the cornerstone of curative treatment. Neoadjuvant (chemo)radiation is recommended in Dutch and American treatment guidelines for patients with stage II–III rectal cancer, whereas adjuvant chemotherapy is recommended for patients with stage III colon cancer.10,11
A preoperative diagnosis of synchronous CRCs may modify the type of surgical procedure and influence clinical decision making on the use of additional treatments. Moreover, synchronous CRCs more often may require extended surgery and, if overlooked, may be diagnosed as early metachronous cancers, possibly at a more advanced stage.
Conflicting evidence exists about whether synchronous CRCs have the same prognosis in survival as solitary CRC patients.2,6,12,13 Many clinical series have been based on single-centre numbers and the analysis of less than 50 patients.4,5,14 The objectives of this study were to investigate, in depth, the various clinicopathological features of synchronous CRC patients compared with solitary CRC patients and its association with treatment patterns, short-term postoperative outcomes, and long-term survival.
Patients and Methods
Data Source
Data from the nationwide population-based Netherlands Cancer Registry (NCR), managed by the Netherlands Comprehensive Cancer Organisation (IKNL), were used. Information on patient and tumour characteristics, diagnosis, and treatment is routinely extracted from the medical records. The quality of the data is high due to thorough training of the registration team and computerised consistency checks at regional and national level.
Anatomical site of the tumour is registered according to the International Classification of Disease–Oncology (ICD-O).15 The TNM (tumour-node-metastasis) classification is used for stage notification of the primary tumour, according to the edition valid at time of cancer diagnosis.16 Furthermore, detailed information was available on: emergency resection (< 24 h after presentation) and anastomotic leakage as a surgical complication. Anastomotic leakage was recorded as such if a surgical intervention or readmission was necessary within 2 months after primary anastomosis. Data on prolonged postoperative hospital admission (> 14 days; yes/no) were available for patients diagnosed in 2012–2013. Prolonged postoperative hospital admission after surgery served as a proxy for a complicated postoperative period.
Definition of Synchronous Colorectal Cancer
A slightly modified version of the Warren and Gates criteria were used to define multiple CRC.17 Synchronous CRC was defined as two or more invasive tumours that are diagnosed simultaneously or within 6 months. Multiple independent tumours in the same segment of the colon and rectum are regarded as different malignancies and are counted as two or more primary cancers. For every synchronous CRC patient, the most extensive tumour according to TNM stage was designated as the index tumour in the analyses. When synchronous CRC patients were diagnosed with at least one of the lesions as stage IV, all tumours were classified as stage IV.
Study Population
All patients diagnosed with primary CRC between 2008 and 2013 were included (Fig. 1). Patients for whom tumour stage was unknown (n = 2379), who were not treated by resection (n = 9017), who underwent local tumour treatments (e.g., TEM, polypectomy; n = 1904), or who underwent emergency resection (n = 2709) were excluded. Synchronous CRC patients were excluded from the analyses if the dates of resection differed for the tumours (n = 130).
Patients were categorized into two groups accordingly: solitary or synchronous CRC. Patients were divided into age groups: < 65, 65–74, 75–84, and ≥ 85 years. Disease stage was based on the pathological TNM classification. Tumour localization was categorised into anatomical subsites: colon ascendens (C18.0–18.2); colon transversum (C18.3–18.5); colon descendens (C18.6–18.7); unknown or overlapping subsites of the colon (C18.8–18.9); and rectum (C19–20).
Patients’ vital status was obtained by linking the NCR to the Municipal Personal Records Database (GBA). Follow-up was completed until January 1, 2017.
Statistical Analyses
Differences in patient and tumour characteristics were evaluated using χ 2. A priori outcomes of interest were type of surgical procedure, prolonged postoperative hospital admission, anastomotic leakage, postoperative 30-day mortality, and administration of neoadjuvant or adjuvant treatment and were analysed between synchronous and solitary CRC patients using univariable (χ 2 tests) and multivariable analyses (logistic regression models).
Survival was defined as the time from the date of resection to death or last follow-up date for patients who were still alive. Relative survival (RS) was defined as the ratio of the survival observed among the cancer patients and the survival that would have been expected based on the corresponding (age, gender, and year) general population. RS was calculated using the Pohar Perme method.18 Relative excess risks of death (RER) were estimated using a multivariable generalized linear model with a Poisson distribution, based on collapsed relative survival data, using exact survival times, adjusting for age, gender, period of diagnosis, stage (most advanced tumour in sCRC), location of tumour (most advanced tumour in sCRC), type of surgical procedure, and (neo)adjuvant treatment.
P values < 0.05 were considered statistically significant. SAS/STAT® statistical software (SAS system 9.4, SAS Institute, Cary, NC) and STATA (version 13.0, Statcorp LP, College Station, TX) were used for all analyses.
Results
From 2008 to 2013, 41,060 patients were diagnosed with primary CRC and underwent elective surgery. Of these, 1969 (4.8%) patients met the definition for synchronous CRC. Patient and tumour characteristics are presented in Table 1. Synchronous CRC patients were slightly older (mean age 71 ± 10.6 vs. 69 ± 11.4 years), more often male, and diagnosed with more advanced tumour stage (stage III–IV) compared with solitary CRC patients (all p < 0.0001).
Patients with Two Synchronous CRCs
Of the synchronous CRC patients, the majority (n = 1865, 95%) had two tumours. Table 2 gives an overview of the anatomical and stage distribution of the first and second tumour of patients with two synchronous CRCs.
Most of the synchronous CRC patients were diagnosed with at least one stage III/IV tumour (n = 1009, 54%). Of these patients, 451 (43, 24% of total) were diagnosed with two stage III tumours and 241 (23, 13% of total) were diagnosed with two stage IV tumours.
Half of the synchronous tumours were located in similar segments of the large bowel (n = 922, 50%). In 1222 (66%) patients, both tumours were localized in the colon, of which 729 (60; 39% of total) were in one colon segment. In 193 (10%) patients, both tumours were situated in the rectum.
Figure 2 shows the type of surgical procedures for the different anatomical sites in patients with two synchronous CRCs compared with solitary CRC patients. As expected, (sub)total (proto)colectomy was most often performed if synchronous tumours were located in different segments of the colon. Extended surgery (e.g., (sub)total colectomy, proctocolectomy, or combined resections) was performed in 50% (n = 934) of the patients with two synchronous CRCs compared with 2% (n = 577) in solitary CRC (p < 0.0001).
Type of surgical resection for the different anatomical sites of solitary (n = 39,091) or two synchronous CRC (n = 1865). LAR low anterior resection; APR abdominal perineal resection; Other = resection not specified; Combined resections = when two synchronous tumours were removed using two different type of surgical procedures
Treatment Variables and Short-term Outcomes
Table 3 presents observed proportions and adjusted odds ratios of surgical approach, prolonged postoperative hospital stay, presence of anastomotic leakage, postoperative mortality, and administration of neoadjuvant or adjuvant treatment among solitary and synchronous CRC.
Synchronous CRC patients with at least one stage II–III rectal tumour (index tumour) were less likely to receive neoadjuvant treatment compared with solitary CRC patients (Table 3a). Of the synchronous CRC patients receiving neoadjuvant treatment, the majority (n = 185, 57%) was diagnosed with stage III rectal tumour as index tumour. In 88 patients (49%) with stage III rectal tumour as index tumour, both tumours were situated in the rectum. Of these patients, 45 patients (52%) were treated with neoadjuvant radiotherapy, and 42 patients (48%) received neoadjuvant chemoradiation. In 97 patients (51%) with stage III rectal tumour as index tumour, one tumour was located in the rectum and the other tumour was located in a colon segment. Of these patients, the majority (n = 56, 61%) was treated with neoadjuvant radiotherapy, whereas 41 patients (39%) received neoadjuvant chemoradiation. The administration of adjuvant chemotherapy was lower in synchronous CRC patients with at least one stage III colon tumour (index tumour), even after case-mix adjustment (Table 3f).
Survival
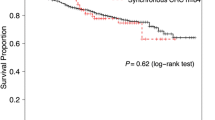
Median follow-up time for patients included was 60 months. For solitary CRC patients the 5-year RS rate was 77% (Supplementary Fig. 1a), whereas the proportion of 5-year RS for synchronous CRC patients was 71% (Supplementary Fig. 1b).
After case-mix adjustment, synchronous CRCs were associated with an increased RER of death [adjusted RER synchronous vs. solitary CRC 1.1 (1.01–1.23)]. In addition, after stratification by tumour stage, the association between increased risk of death and having more than one colorectal tumour remained in patients with stage I–III synchronous CRC [stage I RER 1.1 (95% confidence interval (CI) 1.01–1.58), stage II RER 1.2 (1.03–1.38), stage III RER 1.2 (1.03–1.20), and stage IV RER 1.1 (0.89–1.17)].
Discussion
In this nationwide population-based study, synchronous CRC was prevalent in 5% of patients with CRC. We evaluated treatment patterns, short-term patient outcomes and 5-year RS in synchronous and solitary CRC patients. We found a decreased use of neoadjuvant and adjuvant treatment in synchronous CRC patients. Furthermore, synchronous CRCs were independently associated with a decrease in survival.
Definitions of synchronous CRC tend to differ in the literature. The prevalence of synchronous CRC ranged from approximately 1–8%.2,3,4,5,6,7 Synchronous and metachronous cancers often were mixed together in previous studies.4,8,19,20,21,22,23 Synchronous CRC is generally defined as two or more distinct colorectal tumours diagnosed within 6 months after initial diagnosis.2,5,6,8,22,23,24,25 In other studies, CRCs diagnosed within a year of the initial diagnosis were classified as synchronous, and in others, those diagnosed simultaneously at time of surgery.4,7,26,27 We considered patients with two or more invasive CRCs, diagnosed simultaneously or within 6 months, as synchronous CRC.
In the present study, 61% of the synchronous CRC patients were male compared with 54% of the solitary CRC patients. Most other studies also reported that synchronous CRC were more frequent in men than women.2,5,6,7 However, some studies showed no association between gender and the presence of synchronous CRCs.23,26 It is unclear whether the male predominance reflects an increased risk factor to develop synchronous CRC or a greater exposure of men to environmental risk factors associated with synchronous CRC. Furthermore, we found that synchronous CRC patients were older and diagnosed with more advanced tumour stage compared with solitary CRC. These findings are comparable to other studies.5,6,7,26
In 50% of the synchronous CRC patients, tumours were located in similar segments of the large bowel. In line with previous Dutch studies of Van Leersum et al. and Mulder et al., we found that synchronous CRC patients were more likely to undergo extended surgery.6,7 We found that patients with synchronous tumours that were located in different segments of the large bowel, mostly required (sub)total (proto)colectomy or extended surgery. One could expect that when tumours were located in the same or adjacent segment, the choice for surgery will be simple, either a hemicolectomy or an extended hemicolectomy with the adjacent segment. However, if, for instance, one tumour is located in the right colon while the other tumour is simultaneously located in the rectum, either a (sub)total (proto)colectomy can be performed, or two separate resections with two anastomoses can be performed. The latter can result in a higher risk of anastomotic leakage. We found no associations between synchronous CRC and the presence of anastomotic leakage.
Remarkably, in this study, synchronous CRC patients with at least one stage II–III rectal tumour less often received neoadjuvant (chemo)radiation compared with those with solitary rectal tumours. At the same time, patients with a stage III rectal tumour as index tumour, who were eligible for neoadjuvant chemoradiation, and a tumour situated in a colon segment, more often received neoadjuvant radiotherapy compared to synchronous CRC patients in which both tumours were located in the rectum (61 vs. 52%). It is possible that patients with a stage III rectal tumour as index tumour and a tumour located in the colon were less often treated with neoadjuvant chemoradiation to avoid the postponement of surgery and treatment of the colon tumour.
Synchronous CRC patients with at least one stage III colon tumour were associated with a lower probability of receiving adjuvant chemotherapy compared with solitary colon tumours. We do not have an obvious explanation for this finding, and no data are available from previous studies. One might expect that synchronous CRC patients may be highly susceptible to adjuvant therapy. The proportion of solitary CRC patients who were treated with adjuvant chemotherapy was 63% compared with 49% in synchronous CRC patients. The relatively low utilization of adjuvant chemotherapy overall in the Netherlands could be carried over and be amplified in patients with synchronous CRC. Explanations could be that synchronous CRC patients were in worse general health or had more surgical complications of the extended surgery, although a prolonged hospital stay was not observed.
Five-year survival for synchronous CRC patients was worse than for solitary CRC patients (76 vs. 69%). Conflicting results have been reported regarding long-term prognosis of synchronous CRC patients. The majority of the studies showed no difference in survival rates between synchronous and solitary CRC.2,6,12,13,28,29 Some studies reported worse survival for synchronous CRC.26,30,31 A previous Dutch study by Liu et al. showed that overall survival of patients with one colon cancer was significantly better than those with two, irrespective of lag-time between the two colon cancers.32 Poor prognosis of synchronous CRC is thought to be caused by the relatively frequent distant metastasis that occur in synchronous CRC patients.26
The main strength of this study is the use of a large dataset, including approximately 2000 synchronous CRC patients. We believe that this is the largest cohort published on this subject. Moreover, the objectives of this study were to investigate, in depth, the effects of synchronous CRC on choice of treatment and short- and long-term patients’ outcomes. Clinical implications in terms of treatment and prognosis of synchronous CRC patients were seldom analyzed in large cohorts.4,5,8,23,24
Some limitations of this study should be acknowledged. The main limitation is the lack of data on the presence of inherited syndromes (Lynch, FAP, HNPCC). These inherited syndromes are known to be predisposing conditions for synchronous CRC.5 Although the NCR collects a huge variety of potential case-mix factors, we cannot exclude that other factors, such as functional status, patient preferences, and specific postoperative complications other than anastomotic leakage, may have influenced our results as well.
Information on differences in patient and treatment outcomes between solitary and synchronous CRC on a national level is relevant, because a preoperative diagnosis of synchronous CRCs may modify or extent the type of surgical procedure and influence clinical decision making of the use of additional treatments. It is important to identify the presence of synchronous tumours, preferably before surgery, to provide an optimal treatment. Preoperative total colonoscopy should be performed, if possible, in all patients with CRC to detect synchronous tumours. Moreover, results of this study showed that synchronous CRCs are an independent determinant of survival, indicating the relevance of this variable in case-mix adjustment models.
In conclusion, we showed that synchronous CRC occurred in 5% of patients with CRC. Synchronous CRC patients were associated with extended surgery, less (neo)adjuvant treatment, and a decrease in survival compared with solitary CRC.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Latournerie M, Jooste V, Cottet V, Lepage C, Faivre J, Bouvier AM. Epidemiology and prognosis of synchronous colorectal cancers. Br J Surg. 2008;95(12):1528–33.
Derwinger K, Gustavsson B. A study of aspects on gender and prognosis in synchronous colorectal cancer. Clin Med Insights Oncol. 2011;5:259–64.
Fante R, Roncucci L, Di Gregorio C, et al. Frequency and clinical features of multiple tumors of the large bowel in the general population and in patients with hereditary colorectal carcinoma. Cancer. 1996;77(10):2013–21.
Lam AK, Carmichael R, Gertraud Buettner P, Gopalan V, Ho YH, Siu S. Clinicopathological significance of synchronous carcinoma in colorectal cancer. Am J Surg. 2011;202(1):39–44.
Mulder SA, Kranse R, Damhuis RA, et al. Prevalence and prognosis of synchronous colorectal cancer: a Dutch population-based study. Cancer Epidemiol. 2011;35(5):442–7.
van Leersum NJ, Aalbers AG, Snijders HS, et al. Synchronous colorectal carcinoma: a risk factor in colorectal cancer surgery. Dis Colon Rectum. 2014;57(4):460–6.
Papadopoulos V, Michalopoulos A, Basdanis G, et al. Synchronous and metachronous colorectal carcinoma. Tech Coloproctol. 2004;8 Suppl 1:s97–100.
Greenstein AJ, Slater G, Heimann TM, Sachar DB, Aufses AH, Jr. A comparison of multiple synchronous colorectal cancer in ulcerative colitis, familial polyposis coli, and de novo cancer. Ann Surg. 1986;203(2):123–8.
Netherlands Comprehensive Cancer Organisation. National Evidence Based Guideline for Colon and Rectal Cancer. 2014. https://www.oncoline.nl/coloncarcinoom. Accessed 8 Mar 2017.
NCCN Clinical practice Guidelines in Oncology: colorectal cancer. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
Chen HS, Sheen-Chen SM. Synchronous and “early” metachronous colorectal adenocarcinoma: analysis of prognosis and current trends. Dis Colon Rectum. 2000;43(8):1093–9.
Passman MA, Pommier RF, Vetto JT. Synchronous colon primaries have the same prognosis as solitary colon cancers. Dis Colon Rectum. 1996;39(3):329–34.
Yeoman A, Young J, Arnold J, Jass J, Parry S. Hyperplastic polyposis in the New Zealand population: a condition associated with increased colorectal cancer risk and European ancestry. N Zealand Med J. 2007;120(1266):U2827.
Fritz A. PC, Jack A. et al. International Classification of Diseases for Oncology (ICD-O). Vol 3rd edn. Geneva: World Health Organisation; 2000.
Wittekind C GF, Hutter R, Klimpfinger M, Sobin L. TNM Atlas. Berlin: Springer; 2004.
Warren SG, O. Multiple primary malignanct tumors: a survery of the literature and a statistical study. Am J Cancer. 1932;16:1358–414.
Perme MP, Stare J, Esteve J. On estimation in relative survival. Biometrics. 2012;68(1):113–20.
Burns FJ. Synchronous and metachronous malignancies of the colon and rectum. Dis Colon Rectum. 1980;23(8):578–9.
Cunliffe WJ, Hasleton PS, Tweedle DE, Schofield PF. Incidence of synchronous and metachronous colorectal carcinoma. Br J Surg. 1984;71(12):941–3.
Kaibara N, Koga S, Jinnai D. Synchronous and metachronous malignancies of the colon and rectum in Japan with special reference to a coexisting early cancer. Cancer. 1984;54(9):1870–4.
Tziris N, Dokmetzioglou J, Giannoulis K, et al. Synchronous and metachronous adenocarcinomas of the large intestine. Hippokratia. 2008;12(3):150–2.
Wang HZ, Huang XF, Wang Y, Ji JF, Gu J. Clinical features, diagnosis, treatment and prognosis of multiple primary colorectal carcinoma. World J Gastroenterol. 2004;10(14):2136–9.
Evers BM, Mullins RJ, Matthews TH, Broghamer WL, Polk HC Jr. Multiple adenocarcinomas of the colon and rectum. An analysis of incidences and current trends. Dis Colon Rectum. 1988;31(7):518–22.
Takeuchi H, Toda T, Nagasaki S, et al. Synchronous multiple colorectal adenocarcinomas. J Surg Oncol. 1997;64(4):304–7.
Oya M, Takahashi S, Okuyama T, Yamaguchi M, Ueda Y. Synchronous colorectal carcinoma: clinico-pathological features and prognosis. Japanese J Clin Oncol. 2003;33(1):38–43.
Ueno M, Muto T, Oya M, Ota H, Azekura K, Yamaguchi T. Multiple primary cancer: an experience at the Cancer Institute Hospital with special reference to colorectal cancer. Int J Clin Oncol. 2003;8(3):162–7.
Adloff M, Arnaud JP, Bergamaschi R, Schloegel M. Synchronous carcinoma of the colon and rectum: prognostic and therapeutic implications. Am J Surg. 1989;157(3):299–302.
Heald RJ. Synchronous and metachronous carcinoma of the colon and rectum. Ann R Coll Surg Engl. 1990;72(3):172–4.
Travieso CR Jr, Knoepp LF Jr, Hanley PH. Multiple adenocarcinomas of the colon and rectum. Dis Colon Rectum. 1972;15(1):1–6.
Enker WE, Dragacevic S. Multiple carcinomas of the large bowel: a natural experiment in etiology and pathogenesis. Ann Surg. 1978;187(1):8–11.
Liu L, Lemmens VE, De Hingh IH, et al. Second primary cancers in subsites of colon and rectum in patients with previous colorectal cancer. Dis Colon Rectum. 2013;56(2):158–68.
Acknowledgement
The authors thank the registration team of the Netherlands Cancer Registry for their dedicated data collection.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bos, A.C.R.K., Matthijsen, R.A., van Erning, F.N. et al. Treatment and Outcome of Synchronous Colorectal Carcinomas: A Nationwide Study. Ann Surg Oncol 25, 414–421 (2018). https://doi.org/10.1245/s10434-017-6255-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-6255-y