Abstract
Background
Hospitalization is associated with negative clinical effects that last beyond discharge. This study aimed to determine whether hospitalization in the year before major oncologic surgery is associated with adverse outcomes.
Methods
Patients 18 years of age or older with stomach, pancreas, colon, or rectal cancer who underwent resection in California and New York (2008–2010) were included in the study. Patients with hospitalization in the year prior to oncologic resection (HYPOR) were identified. Multivariable logistic regression was used to examine the association of prior hospitalization with the following adverse outcomes: inpatient mortality, complications, complex discharge needs, and 90-day readmission. Subset analysis by cancer type was performed. Outcomes based on temporal proximity of hospitalization to month of surgical admission were evaluated.
Results
Of 32,292 patients, 16.3% (n = 5276) were HYPOR. Patients with prior hospitalization were older (median age, 72 vs 67 years; p < 0.001) and had more comorbidities (Elixhauser Index ≥3, 86.5 vs 75.3%; p < 0.001). In the multivariable analysis, HYPOR was associated with complications (odds ratio [OR], 1.28; 95% confidence interval [CI] 1.18–1.40), complex discharge (OR, 1.44; 95% CI 1.34–1.55), and 90-day readmission (OR, 1.45; 95% CI 1.35–1.56). The interval from HYPOR to resection was not associated with adverse outcomes.
Conclusions
Patients hospitalized in the year before oncologic resection are at increased risk for postoperative adverse events. Recent hospitalization is a risk factor that is easily ascertainable and should be used by clinicians to identify patients who may need additional support around the time of oncologic resection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients are seldom completely recovered when they are discharged from the hospital. In fact, it is increasingly recognized that hospitalization is associated with a depletion of physiologic reserve.1,2 This phenomenon, known as post-hospital syndrome, has been shown to place patients treated for medical conditions such as heart failure, myocardial infarction, and pneumonia at increased risk for death and subsequent readmissions for a full year after discharge.3
Post-hospital syndrome has gained attention in the medical literature as target for quality improvement measures,2 but little is known about its effect on patients who subsequently undergo surgery. In one of the only studies to date on post-hospital syndrome and surgical outcomes, Brownlee et al.4 showed that a hospital stay during the 90 days preceding outpatient hernia surgery increases the risk for postoperative adverse events including complications, emergency department visits, and need for inpatient admission. But the duration and effects of prior hospitalization for patients who undergo major inpatient surgery has not been defined to date.
This study aimed to examine the outcomes for patients hospitalized for an indication unrelated to their cancer diagnosis in the year before major oncologic resection for stomach, pancreas, colon, or rectal cancer. The authors hypothesized that the detrimental effects of post-hospital syndrome and the depleted patient status of those requiring hospitalization adversely affects surgical outcomes.
Methods
Selection of the Study Population
Data from the California State Inpatient Database (SID), through the Healthcare Cost and Utilization Project (HCUP) of the Agency for Healthcare Research and Quality,5 and the New York Statewide Planning and Research Cooperative System (SPARCS)6 from 2007 to 2011 were queried. California and New York data sets were chosen because they both collect inpatient discharge data from all payers in a uniform format that links patients across multiple admissions and facilitates multi-state comparisons.
Patients with a new diagnosis of stomach, pancreas, colon, or rectal cancer who underwent oncologic resection between 2008 and 2010 were identified for study inclusion. Patients were selected for inclusion using the International Classification of Diseases, Ninth Revision, Clinical Modifications (ICD-9-CM) cancer diagnosis and procedure codes.7 Patients undergoing resection for curative intent and patients with metastatic disease at the time of resection were included in the study.
Index surgical hospitalization was defined by the claim that first documented the cancer diagnosis together with the oncologic resection. A 1-year look back was performed to identify all hospitalizations during the year preceding the index hospitalization.
Hospitalization in the year prior to oncologic resection (HYPOR) status was defined for each patient (HYPOR or no-HYPOR). For patients who had multiple hospitalizations in the year before the oncologic resection, the hospitalization closest in time to the index surgical hospitalization was chosen to represent HYPOR. Patients with cancer (defined by a 3-digit ICD-9-CM code of 150–159, 230, 234, 235, or 239) as the principal diagnosis of their prior hospitalization were excluded from the study. This ensured that the reason for prior admission in the HYPOR cohort was not related to the cancer diagnosis.
The HYPOR patients were classified by the indication for the prior admission using the major diagnostic category (MDC) for the admission. In the claims reimbursement system, MDCs correspond to a single organ system. All diagnosis-related groups are mapped to MDCs for billing purposes. In addition, HYPOR patients were classified by the principal procedure performed during the prior admission using Agency for Healthcare Research and Quality (AHRQ) Clinical Classifications Software (CCS) procedure categories when a procedure was performed. The CCS categories collapse ICD-9-CM procedure codes into meaningful and manageable categories.8
Study Variables
Data were collected on patient demographics, clinical and hospital-level variables, and postoperative clinical outcomes. The patient demographics included age, sex, race (white, black, other), insurance type (Medicare, Medicaid, commercial, self, other) and within-state income quartile by county. The clinical variables included type of cancer, evidence of metastasis at the time of surgical resection, Elixhauser Comorbidity Index,9,10 route of hospital admission (emergency department, direct admission), and length of hospital stay during the index admission. Evidence of metastasis was determined by the presence of ICD-9-CM code 196.0–199.1 or 789.51. The hospital-level variables included hospital bed size (small, 0–99; medium, 100–299; large, >300) and hospital type (investor-owned, not-for-profit rural, not-for-profit urban).
The postoperative clinical outcomes included inpatient mortality during the index surgical admission, any postoperative complication, hospital discharge requiring health services, and readmission within 90 days after postoperative discharge. Postoperative complication was treated as a binary outcome and signified the presence of at least one of the following complications: cardiac, pulmonary embolus/deep vein thrombosis, stroke/transient ischemic attack, pneumonia, other respiratory, perforation/abscess/intestinal obstruction, gastrointestinal (GI) bleed/other bleed, sepsis, renal dysfunction, wound infection, genitourinary complication, return to the operating room, and other postsurgical complication. Discharge requiring health services included patients discharged to a skilled nursing facility or other similar level of care, as well as those discharged home with home health services.
Statistical Analysis
Descriptive comparisons between the HYPOR and no-HYPOR patients were examined using Chi square tests for categorical variables and Wilcoxon rank-sum tests for continuous variables, with significance set at a p value lower than 0.05. Factors significantly associated with HYPOR (p < 0.10) were included in the multivariable analysis. Four independent multivariable logistic regression models were built to identify associations between HYPOR and each of the defined surgical outcomes. Subgroup analysis by specific cancer was preformed to examine further the effect of HYPOR on study outcomes. Operation type (e.g., partial vs total colectomy in the colon cancer model) was controlled for in the cancer-specific analysis. Secondary analysis examining rates of each outcome by time from prior hospitalization (in months) to index surgical hospitalization was performed using statistical tests for trend.11 All analyses were performed using Stata/MP 13.1 statistical software (StataCorp, College Station, TX, USA).12 This study was deemed exempt from review by the University of Pennsylvania Institutional Review Board.
Results
Population Characteristics
Our study included 32,292 patients who underwent surgical resection in New York or California between 2008 and 2010. Of these patients, 70.1% (n = 22,636) had colon cancer, 18.6% (n = 6002) had rectal cancer, 6.5% (n = 2096) had stomach cancer, and 4.8% (n = 1538) had pancreas cancer. The HYPOR patients made up 16.3% (n = 5276) of the overall cohort. The HYPOR and no-HYPOR patient sociodemographic and clinical characteristics and the characteristics of the hospitals in which the oncologic resections occurred are compared in Table 1. The HYPOR patients were older (median, 72 vs 67 years; p < 0.001), had more comorbidities (Elixhauser Index ≥3, 86.5 vs 75.3%; p < 0.001), were more likely to show recent weight loss (8.1 vs 4.7%; p < 0.001) and were more likely to have Medicare (61.4 vs 49.5%; p < 0.001) than the no-HYPOR patients. The number of patients with metastatic disease did not differ between the two groups.
Outcomes
Before adjustment, postoperative complication rates were higher for the HYPOR patients than for the no-HYPOR patients (16.4 vs 11.0%; p < 0.001) (Table 2). The 90-day readmission rate was higher in the HYPOR cohort (24.5 vs 17.6%; p < 0.001), and more of the HYPOR patients were discharged to nursing facilities or required home health services at discharge (38.3 vs 26.3%; p < 0.001) (Table 2). The inpatient mortality rate also was higher for the HYPOR patients than for the no-HYPOR patients (0.8 vs 0.4%; p < 0.001).
In the multivariable analysis adjusted for potential confounders, HYPOR conferred a 28% greater risk for postoperative complications (odds ratio [OR], 1.28; 95% confidence interval [CI], 1.18–1.40), a 44% greater risk for discharge requiring health services (OR, 1.44; 95% CI 1.34–1.55), and a 45% greater risk for 90-day readmission (OR, 1.45; 95% CI 1.35–1.56) (Table 3).
In the subset analysis examining the cancer-specific association of HYPOR and outcomes, with control for type of operation preformed, HYPOR had the most significant impact on outcomes after colon and rectal resection. For the rectal resection patients, HYPOR was associated with inpatient mortality (OR, 4.17; 95% CI 1.38–12.60). For the colon resection patients, HYPOR was associated with postoperative complications (OR, 1.33; 95% CI 1.21–1.48), discharge requiring health services (OR, 1.61; 95% CI 1.47–1.75), and 90-day readmission (OR, 1.53; 95% CI 1.40–1.67). The cancer-specific subset analysis with control for type of operation is shown in Table 4.
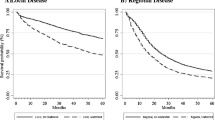
The interval between month of prior hospitalization and index surgical hospitalization for all the HYPOR patients can be seen in Fig. 1. The rates of each study outcome by month of prior hospitalization for the HYPOR patients compared with the point estimate percentage of each outcome for the no-HYPOR patients also are shown in Fig. 1. The secondary analysis showed no statistically significant trend in outcome rates by interval (in months) from prior hospitalization to index surgical admission.
Study outcome by month of prior hospitalization compared with outcome point estimate of non-hospitalized patients. a Number of patients hospitalized each month before oncologic resection. b–d Specific outcome rate by month of prior hospitalization among those hospitalized before oncologic resection compared with the point percentage estimate of those not hospitalized. Footnote The bold horizontal line marks the point percentage estimate of all patients not hospitalized before oncologic resection for each study outcome. The x-axis represents number of months between prior hospitalization and admission for oncologic resection. Royston test for trend by month for each outcome: any complication (p = 0.27), readmission within 90-days (p = 0.44), discharge requiring health services (p = 0.47)
The most common reasons for admission (by MDC) of the HYPOR cohort are shown in Table 5. Diagnoses related to the digestive system (27.9%) and circulatory system (16.4%) were the most frequent reasons for admission. Most of the HYPOR patients (69.7%; n = 3677) underwent at least one procedure during the prior admission. The most common procedures performed for the HYPOR patients during their prior admission (by CCS category) were blood transfusions (14%) and upper GI endoscopies (11.7%).
Discussion
This study showed that a hospital stay at any point in the year before oncologic resection for stomach, pancreas, colon, or rectal cancer increases the likelihood of postoperative complications, discharge requiring health services, and readmission within 90 days after surgical discharge. Cancer diagnosis-specific analysis also showed an association between prior hospitalization and inpatient mortality for rectal cancer patients. Whereas prior studies have examined the effect of prior hospitalization on outcomes of nonsurgical patients3,13 and surgical patients undergoing outpatient hernia repair,4 this study was the first to assess the effect of post-hospital syndrome on patients who undergo major inpatient cancer surgery.
It is important to note that the relationship between a prior admission and the study outcomes remained significant even after adjustment for all known comorbidities and risk factors. This suggests that the history of a prior admission captures an additional dimension of risk that is not included in the more standard preoperative comorbidities used for risk assessment.
One explanation for the poor outcomes in the HYPOR cohort is that patients who require hospitalization differ physiologically from those who do not. The most frequent MDCs of the HYPOR cohort, particularly those related to the circulatory and respiratory systems, often are disease processes that take a physiologic toll on the patient. Nutritional and physiologic derangements also are common after hospitalization,1 which is reflected in the fact that more HYPOR patients have recent weight loss than no-HYPOR patients. Also, HYPOR patients are older and have more comorbidities, both of which are known risk factors for poor postoperative outcomes in surgical patients.14,15 A prior hospitalization also could be a surrogate for patient frailty, which has been shown to have a negative impact on outcomes including mortality, complications, and length of hospital stay for surgical oncology patients.16
In a recent study of colorectal cancer patients undergoing resection, frail patients had an 80% 1-year postoperative survival rate compared with a 92% 1-year survival rate for non-frail patients.17 For patients undergoing pancreas resection, frailty conferred a 50% increased risk of 30-day mortality.18 Many different frailty evaluations currently exist,19,20,21 with varying levels of complexity in terms of patient assessment.18,22,23 The association between prior hospitalization and clinical outcomes seen in our study may make recent hospitalization a simple first screening question to prompt more formal frailty assessments before resection surgery.
For optimization of patients who require hospitalization in the year preceding cancer resection, specific preoperative interventions also may be needed. To that end, surgeons in multiple disciplines have begun to adopt preoperative rehabilitation (prehabilitation) strategies to improve physiology in vulnerable patient populations.24,25 A recent randomized control trial testing a 4-week prehabilitation program (composed of moderate-intensity exercise, nutritional counseling with protein supplementation, and anxiety-reduction strategies) compared with a postoperative rehabilitation regimen for colorectal cancer patients undergoing resection resulted in a shorter postoperative hospital stay and better postoperative functional capacity in the prehabilitation arm.26,27 Even a 7-day course of intensive prehabilitation has been shown to decrease the hospital stay and the postoperative complications of patients undergoing major thoracic surgery.28 Because major complications are among the most significant predictors of a downward trajectory after surgery,29 identification of a preoperative risk factor for postoperative complications such as recent hospitalization, which is easy to elicit as part of the patient’s history, could trigger enrollment in prehabilitation interventions. In turn, these programs may provide significant postoperative benefit for this vulnerable cohort.
Although evidence-based studies that support prehabilitation interventions for different patient populations have become more prevalent in the literature as prehabilitation medicine has become more accepted,30 we are unaware of any studies performed to date in the United States that specifically examined the role of prehabilitation for patients undergoing oncologic resection for GI malignancies. A single-institution randomized control trial performed in Brazil suggested that 2–3 weeks of preoperative physical therapy may decrease postoperative pulmonary complications for patients undergoing open GI surgery.31 A study based in the Netherlands assessing patients awaiting surgery for different cancers (including pancreatic, esophageal, lung cancers) showed that a short-duration prehabilitation program increased objective measures of physical fitness.32 This study did not investigate postoperative outcomes. More studies are needed to confirm the benefits of prehabilitation interventions for oncologic populations with identifiable risk factors such as our study cohort to direct best practice guidelines.
Because of limited data, specific recommendations are beyond the scope of this investigation. However, physicians are advised to familiarize themselves with the American College of Surgeons Strong for Surgery quality initiative,33 which provides clinicians with updates on prehabilitation information including preoperative screening tools to identify risk factors for complications. It also provides information on potential preoperative interventions (e.g., nutrition, glycemic control, smoking cessation) to improve outcomes. Knowledge of a prior hospitalization should be discussed with patients and may influence decisions regarding preoperative interventions as well as care during the perioperative or immediate post-discharge period.
Our study had some limitations, including its retrospective nature and the use of administrative claims data. Mortality was based on discharge status information, so we were unable to determine timing from operation to death for the patients who died and whether death was directly associated with surgery. The cohort had fewer pancreas and stomach cancer patients than colorectal cancer patients, so we may have been underpowered to identify outcomes differences in the cancer-specific analysis due to the small sample size. We were not able to control for the use of preoperative chemotherapy or radiation given in the outpatient setting. Given the common use of neoadjuvant therapy for rectal cancer, this may have contributed to the increased risk of death noted in the population of rectal cancer HYPOR patients.
Finally, this was an observational study, and we were therefore unable to identify the cause for the greater risk of poor outcomes in the setting of prior hospitalization. However, regardless of the cause, recognition of prior hospitalization as a risk factor may allow for better risk stratification of patients clinically. Hospitalization in the year preceding resection likely represents a measure of unadjusted severity of illness that should be considered for future outcome studies and ultimately used to identify patients that might benefit from additional care around an oncologic resection.
Conclusion
Patients hospitalized for any indication unrelated to their cancer diagnosis in the year before surgical resection for stomach, pancreas, colon, or rectal cancer are at increased risk for postoperative complications, discharge requiring health services, and subsequent hospital readmissions. This study identified cancer patients with an easily ascertainable preoperative risk factor that allows for better risk stratification before surgery and may be a place to focus preoperative interventions for improvement of postoperative outcomes.
References
Krumholz HM. Post-hospital syndrome: an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100–2.
Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311:2169–70.
Dharmarajan K, Hsieh AF, Kulkarni VT, Lin Z, Ross JS, Horwitz LI, et al. Trajectories of risk after hospitalization for heart failure, acute myocardial infarction, or pneumonia: retrospective cohort study. BMJ. 2015;350:h411.
Brownlee SA, Blackwell RH, Blanco BA, Zapf MA, Kliethermes S, Gupta GN, et al. Impact of post-hospital syndrome on outcomes following elective, ambulatory surgery. Ann Surg. 2017;266(2):274–9.
California State Inpatient Database (SID). Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality. Retrieved 10 April 2017 at www.hcup-us.ahrq.gov/db/state/siddbdocumentation.jsp.
Bureau of Health Informatics. Office of Quality and Health Safety. Statewide Planning and Research Cooperative System (SPARCS). Albany: New York State Department of Health; 2016. Retrieved 10 April 2017 at https://www.health.ny.gov/statistics/sparcs/.
Centers for Medicare & Medicaid Services. ICD-9-CM diagnosis and procedure codes: abbreviated and full code titles. Retrieved 10 April 2017 at http://www.cms.gov/Medicare/Coding/ICD9ProviderDiagnosticCodes/codes.html.
HCUP CCS. Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; 2017. Retrieved 10 June 2017 at http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp.
Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27.
Elixhauser ASC, Kruzikas D. Elixhauser Comorbidity Software. Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality. Retrieved 10 April 2017 at https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp.
Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–74.
StataCorp. Stata Statistical Software: Release 13. College Station, TX, StataCorp LP; 2013.
Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–63.
Massarweh NN, Legner VJ, Symons RG, McCormick WC, Flum DR. Impact of advancing age on abdominal surgical outcomes. Arch Surg. 2009;144:1108–14.
Sogaard M, Thomsen RW, Bossen KS, Sorensen HT, Norgaard M. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013;5:3–29.
Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and postoperative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16:157.
Ommundsen N, Wyller TB, Nesbakken A, Jordhoy MS, Bakka A, Skovlund E, et al. Frailty is an independent predictor of survival in older patients with colorectal cancer. Oncologist. 2014;19:1268–75.
Mogal H, Vermilion SA, Dodson R, Hsu FC, Howerton R, Shen P, et al. Modified frailty index predicts morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 2017;24(6):1714–1721.
Perna S, Francis MD, Bologna C, Moncaglieri F, Riva A, Morazzoni P, et al. Performance of Edmonton Frail Scale on frailty assessment: its association with multi-dimensional geriatric conditions assessed with specific screening tools. BMC Geriatr. 2017;17:2.
Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52:1929–33.
Soong JT, Poots AJ, Bell D. Finding consensus on frailty assessment in acute care through Delphi method. BMJ Open. 2016;6:e012904.
Hoogendijk EO, van der Horst HE, Deeg DJ, Frijters DH, Prins BA, Jansen AP, et al. The identification of frail older adults in primary care: comparing the accuracy of five simple instruments. Age Ageing. 2013;42:262–5.
Caillet P, Laurent M, Bastuji-Garin S, Liuu E, Culine S, Lagrange JL, et al. Optimal management of elderly cancer patients: usefulness of the Comprehensive Geriatric Assessment. Clin Interv Aging. 2014;9:1645–60.
Hulzebos EHJ, Smit Y, Helders PPJM, van Meeteren NLU. Preoperative physical therapy for elective cardiac surgery patients. Cochrane Database Syst Rev. 2012. doi:10.1002/14651858.CD010118.pub2.
Tegels JJW, De Maat MFG, Hulsewe KWE, Hoofwijk AGM, Stoot JHMB. Improving the outcomes in gastric cancer surgery. World J Gastroenterol. 2014;20:13692–704.
Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa A, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121:937–47.
Li C, Carli F, Lee L, Charlebois P, Stein B, Liberman AS, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc. 2013;27:1072–82.
Lai Y, Huang J, Yang M, Su J, Liu J, Che G. Seven-day intensive preoperative rehabilitation for elderly patients with lung cancer: a randomized controlled trial. J Surg Res. 2016;209:30–6.
Kassin MT, Owen RM, Perez SD, Leeds I, Cox JC, Schnier K, et al. Risk factors for 30-day hospital readmission among general surgery patients. J Am Coll Surg. 2012;215:322–30.
Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. 2013;92:715–27.
Soares SMdTP, Nucci LB, da Silva MMdC, Campacci TC. Pulmonary function and physical performance outcomes with preoperative physical therapy in upper abdominal surgery: a randomized controlled trial. Clin Rehab. 2013;27:616–27.
Timmerman H, de Groot J, Hulzebos H, de Knikker R, Kerkkamp H, Van Meeteren N. Feasibility and preliminary effectiveness of preoperative therapeutic exercise in patients with cancer: a pragmatic study. Physiother Theory Pract. 2011;27:117–24.
American College of Surgeons. Strong for Surgery. Retrieved 16 June 2017 at https://www.facs.org/quality-programs/strong-for-surgery.
Conflict of interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharoky, C.E., Collier, K.T., Wirtalla, C.J. et al. Hospitalization in the Year Preceding Major Oncologic Surgery Increases Risk for Adverse Postoperative Events. Ann Surg Oncol 24, 3477–3485 (2017). https://doi.org/10.1245/s10434-017-6032-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-6032-y