Abstract
Background
The outcome of gastric cancer patients with peritoneal metastasis remains poor. We treated these patients with intraperitoneal and intravenous paclitaxel plus oral S-1 (tegafur/gimeracil/oteracil), followed by gastrectomy in responders. We evaluated the clinical significance of peritoneal lavage carcinoembryonic antigen (CEA) messenger RNA (mRNA) levels as a biomarker for indication of conversion gastrectomy.
Methods
The peritoneal lavage of 68 patients who received the above regimen as induction chemotherapy was repeatedly collected via intraperitoneal access ports. Gastrectomy was considered when improvement of peritoneal metastasis was confirmed by a second laparoscopic examination with negative peritoneal cytology. CEA and porphobilinogen deaminase mRNAs were chronologically quantified using the transcription reverse-transcription concerted reaction method. The CEA mRNA index (CmRI) was calculated as CEA mRNA/porphobilinogen deaminase mRNA × 10,000.
Results
Thirty-nine patients underwent gastrectomy and 29 patients did not (median survival time, 27.8 vs. 10.7 months, respectively; P < 0.001). In gastrectomy-positive patients, the outcome largely differed according to CmRI values immediately prior to surgery. Patients with a preoperative CmRI value <100 (n = 20) were associated with a significantly longer survival than those with a preoperative CmRI value >100 (n = 19) (41.8 vs. 20.1 months, respectively; P < 0.001). A preoperative CmRI value <100 was confirmed as an independent predictor of survival for gastrectomy-positive patients in the multivariate analysis.
Conclusions
The CmRI reflects the response of peritoneal metastases to induction intraperitoneal chemotherapy. It may be a useful biomarker for indicating gastrectomy in gastric cancer patients with peritoneal metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Despite recent advances in chemotherapy agents, systemic chemotherapy alone has limited clinical effects on peritoneal metastases in gastric cancer patients.1 – 3 Recent reports have revealed that the intraperitoneal administration of taxanes (e.g., paclitaxel and docetaxel) can exhibit significant effects on peritoneal metastases in gastric cancer patients.4,5 Induction chemotherapy with intraperitoneal taxanes, followed by gastrectomy, has shown promise as a potential therapeutic strategy for this dismal disease. We and others have performed phase II studies targeting gastric cancer patients with peritoneal metastasis and have demonstrated a median survival time (MST) of 16.2–22.6 months and a 1-year overall survival (OS) rate of 70.0–78.0%.6 – 9 Additional retrospective analyses have shown that patients who undergo gastrectomy following a good response tend to exhibit a better outcome than those who do not undergo surgery.4,5
However, it remains to be determined whether gastrectomy contributes significantly to the survival benefit in such good responders. It also is unclear how and when gastrectomy should be performed. Hence, biomarkers are needed to predict the outcome of gastrectomy. We chronologically measured the relative expression of carcinoembryonic antigen (CEA) messenger RNA (mRNA) against an internal control [CEA mRNA index (CmRI)] in the peritoneal lavage of patients treated with induction intraperitoneal chemotherapy and evaluated the clinical significance of the CmRI in the decision to perform gastrectomy in these patients.
Patients and Methods
Patients and Treatments
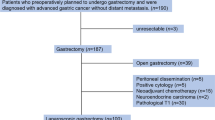
In total, 103 gastric cancer patients underwent staging laparoscopy under general anesthesia and peritoneal metastasis was confirmed at The University of Tokyo Hospital (Tokyo, Japan) between July 2009 and October 2013. Peritoneal access ports were implanted subcutaneously and patients received combination chemotherapy, including intraperitoneal paclitaxel, via the access port. Peritoneal lavage CEA mRNA levels were repeatedly quantified over time in 72 patients and the patients’ clinical records were retrospectively reviewed. In four patients, CEA mRNA levels were not positively detected (calculated as 0), despite positive cytology. These patients were excluded from further analysis.
A chemotherapy regimen comprising intravenous and intraperitoneal paclitaxel plus oral S-1 (tegafur/gimeracil/oteracil) was administered as described previously.10 Briefly, paclitaxel was administered intravenously (50 mg/m2) on Day 1 and intraperitoneally (20 mg/m2) on Day 8. Oral S-1 (80 mg/m2) was administered daily (Days 1–14 with a 1-week rest). Paclitaxel was diluted in 1.0 L of normal saline and administered to the peritoneal cavity through the implanted peritoneal access port. The treatment course was repeated every 3 weeks until disease progression or intolerable toxicity occurred.
Gastrectomy was considered when intraperitoneal chemotherapy exhibited a good response that facilitated macroscopically curative resection. The eligibility criteria for gastrectomy were as follows: no distant metastases detectable by computed tomography, except in the peritoneal cavity; cytologically negative peritoneal lavage; and the disappearance or marked shrinkage of peritoneal metastasis on second-look laparoscopy. Persistent peritoneal white nodules or scar-like lesions were resected as much as possible.11 In patients who did not undergo gastrectomy, the same combination chemotherapy regimen was continued until disease progression or intolerable toxicity occurred.
This study was approved by the Ethical Review Board Committee of The University of Tokyo (Tokyo, Japan). All participants provided informed, written consent, and research was conducted in accordance with the Declaration of Helsinki.
Peritoneal Lavage Sampling and the CEA mRNA Index
At initial staging laparoscopy, 100 mL of saline was administered into the Douglas pouch and collected through a suction and irrigation device. Half of the lavage was used for cytological examinations and the remainder for measurements of CEA mRNA levels. Peritoneal lavage was repeatedly collected every 3 weeks on the first day of each treatment course via the peritoneal access port after the injection of 100 mL of saline into the intraperitoneal space. Peritoneal lavage also was collected at the time of second-look laparoscopy, using the same procedure as that performed at the time of the initial staging laparoscopy.
CEA mRNA levels were measured using the transcription reverse-transcription concerted reaction (TRCR) method as described previously.12,13 Briefly, total cellular RNA was extracted from peritoneal lavage cell pellets using ISOGEN RNA extraction buffer (catalogue no.: #311-02501; Nippon Gene, Tokyo, Japan). The TRCRtest CEA-m (catalogue no.: #051009; Tosoh Corp., Tokyo, Japan) was used to measure CEA mRNA levels and the TRCRtest PBGD-m (catalogue no.: #051010; Tosoh Corp., Tokyo, Japan) was used to measure porphobilinogen deaminase (PBGD) mRNA levels as the internal control. The CmRI was calculated as CEA mRNA/PBGD mRNA × 10,000. The CmRI of each patient was measured 3–45 times. The CmRI of the samples taken 0–28 days before to surgery was adopted as the preoperative value in patients who had undergone conversion gastrectomy.
Statistical Analyses
Chi square tests were used to determine the significance of associations between different clinicopathological variables. The Wilcoxon rank-sum test was used to analyze the relationship of the CmRI in the peritoneal lavage of gastric cancer patients with peritoneal metastasis. OS was calculated using the Kaplan–Meier method and compared using the log-rank test. A Cox proportional hazards regression model was used to determine the significance of prognostic factors. Variables with a P < 0.05 in the univariate analysis were included in the multivariate analysis. Statistical analyses were conducted using JMP software version 12.2.0 (SAS Institute Inc., Cary, NC).
Results
Patient Characteristics
Thirty-nine of the 68 gastric cancer patients with peritoneal metastasis who were administered induction chemotherapy with intravenous and intraperitoneal paclitaxel plus oral S-1 met the criteria for surgery and underwent gastrectomy. The remaining 29 patients failed to meet the criteria (n = 20) or refused surgery (n = 9), even though they met the eligibility criteria.
The characteristics of gastrectomy-positive and gastrectomy-negative patients at the time of the initial staging laparoscopy are summarized in Table 1. Statistically significant differences in Eastern Cooperative Oncology Group performance status, the presence or absence of malignant ascites, and serum cancer antigen-125 (CA-125) levels were observed between the groups. The Peritoneal Cancer Index scores of the gastrectomy-negative group tended to be higher than those of the gastrectomy-positive group, although this could not be confirmed statistically, due to a lack of available data on seven patients in the gastrectomy-negative group.
Peritoneal Lavage CEA mRNA Index Values
Peritoneal lavage CmRI values of gastrectomy-positive and gastrectomy-negative patients at the time of the initial staging laparoscopy, prior to induction chemotherapy, and the CmRI values of gastrectomy-positive patients for the preoperative period after induction chemotherapy are displayed in Fig. 1. Initial peritoneal lavage CmRI values were not statistically significant between the groups, although a trend was observed for higher CmRI values in the gastrectomy-negative group (P > 0.05). In the majority of the gastrectomy-positive patients (n = 39), preoperative CmRI values were reduced compared to those at the time of the initial staging laparoscopy (P < 0.0001).
Carcinoembryonic antigen (CEA) messenger RNA (mRNA) index values at initial staging laparoscopy in patients who received induction chemotherapy with intravenous and intraperitoneal paclitaxel plus oral S-1 (tegafur/gimeracil/oteracil) (n = 68). Preoperative CEA mRNA index values are also expressed for patients who underwent conversion gastrectomy (n = 39)
Perioperative Characteristics of Conversion Gastrectomy Patients
The perioperative characteristics of the 39 patients who underwent conversion gastrectomy are summarized in Table 2. The median and maximum number of preoperative chemotherapy cycles was 6 and 31, respectively. In the majority of patients, serum levels of tumor markers reduced to within normal limits, even in patients whose values were elevated before induction chemotherapy. In fact, the levels of CEA, cancer antigen 19-9 (CA19-9), and CA-125 were normalized in 4 of 7 (57.1%), 4 of 12 (33.3%), and 11 of 17 (64.7%) patients who had exhibited elevated levels of each marker, respectively. A histological response of Grade IA was observed in 25 patients after induction chemotherapy, while Grade III was only observed in 1 patient.
Overall Survival and Preoperative CEA mRNA Index Values
Gastrectomy-positive patients (n = 39) exhibited a significantly better outcome than gastrectomy-negative patients (n = 29) (MST, 27.8 vs. 10.7 months, respectively; P < 0.001; Fig. 2a). In the gastrectomy-positive group, preoperative CmRI values were <100 in 20 patients and >100 in 19 patients. The MST of the former 20 patients was 41.8 months versus 20.1 months for the latter 19 patients. A significant difference in OS was observed between the groups (P < 0.01; Fig. 2b).
Kaplan–Meier curves of overall survival. a Patients were stratified according to those who underwent conversion gastrectomy (n = 39) versus those who did not (n = 29). b Patients who underwent conversion gastrectomy were stratified according to a preoperative carcinoembryonic antigen messenger RNA index (CmRI) value of <100 (n = 20) versus a preoperative CmRI value >100 (n = 19). c Patients who experienced CmRI values <100 on at least one occasion during induction chemotherapy (n = 44) were stratified according to those who underwent conversion gastrectomy versus those who did not. d Patients with CmRI values >100 during induction chemotherapy (n = 24) were stratified according to those who underwent conversion gastrectomy versus those who did not. P values were calculated using the log-rank test
In the univariate analysis, OS was not associated with any factors of preinduction chemotherapy but was significantly associated with preoperative serum CA19-9 (P < 0.05) and CA-125 (P < 0.05) levels and CmRI values (P < 0.01). In the multivariate analysis of prognostic factors, preoperative serum CA19-9 levels (P < 0.05) and CmRI values of <100 (P < 0.05) were confirmed as independent risk factors for OS (Table 3).
Overall Survival and CEA mRNA Index Values during Induction Chemotherapy
Changes in CmRI values during induction chemotherapy were subsequently examined in each patient. Among patients with preoperative CmRI values <100 (n = 20), the CmRI value was initially reduced to <100 at 22–161 days after commencing induction chemotherapy (Supplementary Fig. S1a). Moreover, among patients with preoperative CmRI values >100 (n = 19), the CmRI value was reduced to <100 on at least one occasion at 28–221 days in 12 patients, whilst never reducing to <100 in the remaining seven patients (Supplementary Fig. S1b). Supplementary Fig. S1c displays the tentative graph of CmRI values until the shift to second-line treatment in patients who did not undergo gastrectomy (n = 29). During induction chemotherapy, CmRI values of >100 were maintained in 17 patients. In the remaining 12 patients, CmRI values were reduced to <100 on at least one occasion.
Finally, the outcome of patients stratified according to CmRI values of <100 or >100 were compared. In the former patients who experienced CmRI values of <100 during induction chemotherapy, the MST of the gastrectomy-positive group (n = 32) was significantly longer than that of the gastrectomy-negative group (n = 12) (35.5 vs. 13.0 months, respectively; P < 0.001; Fig. 2c). In contrast, in the latter patients who did not experience CmRI values of <100, a survival benefit was not apparent in the gastrectomy-positive group [MST, 15.0 (n = 7) vs. 9.0 (n = 17) months, respectively; P > 0.05; Fig. 2d].
Discussion
Generally, intraperitoneal taxanes are highly effective for peritoneal metastasis, although they appear to be less effective for primary tumors and other metastases.4 – 6 In fact, the histological response of the resected gastric tumor was Grade IA in 25 of 39 patients, with a complete response observed in only one patient, although there were no residual tumor cells in the majority of the resected peritoneal metastases. Therefore, our rationale for performing gastrectomy after induction chemotherapy was to remove the primary lesion to prevent the development of new metastases and refractory hemorrhagic or stenotic complications caused by the regrowth of the primary tumor. However, open surgery frequently results in the progression of peritoneal micrometastases because of the perioperative pausing of induction chemotherapy and immunosuppression accompanying surgical intervention. Therefore, it is best to perform gastrectomy when peritoneal metastases are adequately controlled.
Peritoneal cytology is widely used to detect tumor cells in the abdominal cavity, which reportedly reflects the response of peritoneal metastasis to induction chemotherapy. Thus, we considered negative cytology to be one of the criteria for gastrectomy. However, previous studies have suggested that conventional cytological examinations of peritoneal lavage specimens lack sensitivity.14 – 16 Moreover, the molecular detection of cancer-specific peritoneal lavage CEA mRNA levels using reverse transcription polymerase chain reaction has superior prognostic value for postoperative peritoneal recurrence in patients with advanced gastric cancer.
In the present study, we calculated the CmRI as the relative expression of CEA mRNA levels by normalizing to PBGD mRNA. We subsequently evaluated whether CmRI values could serve as a biomarker to determine the outcomes of gastrectomy in a cohort of 68 gastric cancer patients with peritoneal metastasis. We applied a cutoff value of 100 for the CmRI based on the findings of a previous study.13 Among patients (n = 39) who underwent conversion gastrectomy, we found that the OS of patients with a preoperative CmRI value <100 was significantly better than that of patients with a preoperative CmRI value >100. Multivariate analysis identified preoperative serum CA19-9 levels and a CmRI value <100 as independent predictors of a better OS in patients who underwent conversion gastrectomy. Furthermore, conversion gastrectomy significantly improved the OS of patients with CmRI values that had once reduced to <100. However, it did not improve the OS of patients with CmRI values that had never reduced to <100. These findings indicate that CmRI values more accurately reflect the response of peritoneal metastasis to induction chemotherapy than conventional cytological examinations and suggest that CmRI values during induction chemotherapy could predict the benefit of conversion gastrectomy.
Interestingly, initial CmRI values were not associated with the OS of conversion gastrectomy patients. Additionally, OS was not associated with the presence of malignant ascites, Peritoneal Cancer Index scores, or tumor marker CEA, CA19-9, and CA-125 levels at the time of the initial staging laparoscopy prior to induction chemotherapy. Conversely, OS was positively associated with preoperative serum CA19-9 and CA-125 levels, as well as, CmRI values after induction chemotherapy. These findings suggest that patient outcomes are not affected by the initial tumor burden of the peritoneal metastases but are largely dependent on the tumor response to induction chemotherapy.
In a previous study, we reported that the sensitivity of serum CEA levels for the detection of peritoneal metastasis in gastric cancer patients was only 18.6%, whereas that of serum CA-125 levels was 46.1%.17 In the present study, we measured the CEA mRNA levels of epithelial cells in peritoneal lavage to detect carcinoma cells. According to the Spearman’s rank correlation test, there was no correlation between preoperative serum CEA levels and preoperative CmRI values of peritoneal lavage in patients (n = 39) who underwent conversion gastrectomy (ρ = 0.21). There also was no correlation between these variables at the time of the initial staging laparoscopy in patients (n = 68) who underwent induction chemotherapy (ρ = 0.17).
One possible limitation of our study is the variability in CEA mRNA expression levels among gastric cancer cells. In fact, CmRI values at the time of the initial staging laparoscopy did not correlate with Peritoneal Cancer Index scores and CEA mRNA levels were not detected in four patients, despite positive peritoneal lavage cytology. Given that CmRI values did not exceed 100, tumor cells in the peritoneal cavity were thought to express insufficient quantities of CEA mRNA. Previous studies have identified peritoneal lavage cytokeratin 20, matrix metalloproteinase-7, cluster of differentiation 44 antigen, and insulin-like growth factor 2 mRNA-binding protein 3 mRNA levels as being clinically useful for the detection of peritoneal micrometastases.18 – 21 Genetic detection of these components with the same methodology may be necessary for these patients.
In conclusion, our retrospective data suggest that the CmRI in peritoneal fluids is a reliable biomarker for predicting gastrectomy outcomes after induction chemotherapy in gastric cancer patients with peritoneal metastasis. Conversion gastrectomy should be recommended if peritoneal lavage CmRI values are reduced to <100.
References
Yamao T, Shimada Y, Shirao K, et al. Phase II study of sequential methotrexate and 5-fluorouracil chemotherapy against peritoneally disseminated gastric cancer with malignant ascites: a report from the Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group, JCOG 9603 Trial. Jpn J Clin Oncol. 2004;34:316–22.
Imazawa M, Kojima T, Boku N, et al. Efficacy of sequential methotrexate and 5-fluorouracil (MTX/5FU) in improving oral intake in patients with advanced gastric cancer with severe peritoneal dissemination. Gastric Cancer. 2009;12:153–7.
Oh SY, Kwon HC, Lee S, et al. A Phase II study of oxaliplatin with low-dose leucovorin and bolus and continuous infusion 5-fluorouracil (modified FOLFOX-4) for gastric cancer patients with malignant ascites. Jpn J Clin Oncol. 2007;37:930–5.
Kitayama J, Ishigami H, Yamaguchi H, et al. Salvage gastrectomy after intravenous and intraperitoneal paclitaxel (PTX) administration with oral S-1 for peritoneal dissemination of advanced gastric cancer with malignant ascites. Ann Surg Oncol. 2014;21:539–46.
Yamaguchi H, Kitayama J, Ishigami H, et al. Breakthrough therapy for peritoneal carcinomatosis of gastric cancer: intraperitoneal chemotherapy with taxanes. World J Gastrointest Oncol. 2015;7:285–91.
Ishigami H, Kitayama J, Kaisaki S, et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol. 2010;21:67–70.
Fushida S, Kinoshita J, Kaji M, et al. Phase I/II study of intraperitoneal docetaxel plus S-1 for the gastric cancer patients with peritoneal carcinomatosis. Cancer Chemother Pharmacol. 2013;71:1265–72.
Fujiwara Y, Takiguchi S, Nakajima K, et al. Intraperitoneal docetaxel combined with S-1 for advanced gastric cancer with peritoneal dissemination. J Surg Oncol. 2012;105:38–42.
Yamaguchi H, Kitayama J, Ishigami H, Emoto S, Yamashita H, Watanabe T. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer. 2013;119:3354–8.
Ishigami H, Kitayama J, Otani K, et al. Phase I pharmacokinetic study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer. Oncology. 2009;76:311–4.
Ishigami H, Yamaguchi H, Yamashita H, Asakage M, Kitayama J. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer. 2017;20:128–34.
Ishii T, Fujiwara Y, Ohnaka S, et al. Rapid genetic diagnosis with the transcription-reverse transcription concerted reaction system for cancer micrometastasis. Ann Surg Oncol. 2004;11:778–85.
Murono K, Kazama S, Yamaguchi H, et al. Detection of carcinoembryonic antigen mRNA in peritoneal lavage by the transcription-reverse transcription concerted method indicates poor prognosis in patients with stage II and III colon cancer. Surgery. 2015;157:322–30.
Kodera Y, Nakanishi H, Ito S, et al. Quantitative detection of disseminated cancer cells in the greater omentum of gastric carcinoma patients with real-time RT-PCR: a comparison with peritoneal lavage cytology. Gastric Cancer. 2002;5:69–76.
Wang JY, Lin SR, Lu CY, et al. Gastric cancer cell detection in peritoneal lavage: RT-PCR for carcinoembryonic antigen transcripts versus the combined cytology with peritoneal carcinoembryonic antigen levels. Cancer Lett. 2005;223:129–35.
Fujiwara Y, Doki Y, Taniguchi H, et al. Genetic detection of free cancer cells in the peritoneal cavity of the patient with gastric cancer: present status and future perspectives. Gastric Cancer. 2007;10:197–204.
Emoto S, Ishigami H, Yamashita H, Yamaguchi H, Kaisaki S, Kitayama J. Clinical significance of CA125 and CA72-4 in gastric cancer with peritoneal dissemination. Gastric Cancer. 2012;15:154–61.
Takata A, Kurokawa Y, Fujiwara Y, et al. Prognostic value of CEA and CK20 mRNA in the peritoneal lavage fluid of patients undergoing curative surgery for gastric cancer. World J Surg. 2014;38:1107–11.
Li Z, Zhang D, Zhang H, Miao Z, Tang Y, Sun G, Dai D. Prediction of peritoneal recurrence by the mRNA level of CEA and MMP-7 in peritoneal lavage of gastric cancer patients. Tumour Biol. 2014;35:3463–70.
Horikawa M, Iinuma H, Inoue T, Ogawa E, Fukushima R. Clinical significance of intraperitoneal CD44 mRNA levels of magnetically separated CD45-negative EpCAM-positive cells for peritoneal recurrence and prognosis in stage II and III gastric cancer patients. Oncol Rep. 2011;25:1413–20.
Okada K, Fujiwara Y, Nakamura Y, et al. Oncofetal protein, IMP-3, a potential marker for prediction of postoperative peritoneal dissemination in gastric adenocarcinoma. J Surg Oncol. 2012;105:780–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors have nothing to disclose.
Funding
This research was partially funded by a Grant from the Japan Agency for Medical Research and Development, and a KAKENHI Grant-in-Aid for Scientific Research (No. 15K10086) from the Japan Society for the Promotion of Science.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (TIFF 6079 kb)
Alterations in carcinoembryonic antigen (CEA) messenger RNA (mRNA) index values during induction chemotherapy in conversion gastrectomy patients with a preoperative CEA mRNA index value of (a) <100 (n = 20) or (b) >100 (n = 19). The x-axis represents the number of days since commencing induction chemotherapy. c Alterations in CEA mRNA index values during first-line chemotherapy up until the point of switching to second-line treatment in patients who did not undergo conversion gastrectomy (n = 29)
Rights and permissions
About this article
Cite this article
Yamaguchi, H., Satoh, Y., Ishigami, H. et al. Peritoneal Lavage CEA mRNA Levels Predict Conversion Gastrectomy Outcomes after Induction Chemotherapy with Intraperitoneal Paclitaxel in Gastric Cancer Patients with Peritoneal Metastasis. Ann Surg Oncol 24, 3345–3352 (2017). https://doi.org/10.1245/s10434-017-5997-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-5997-x