Abstract
Background
Recent advances in imaging and the increasing use of neoadjuvant therapy puts the contemporary utility of staging laparoscopy for patients with pancreatic adenocarcinoma (PDAC) into question. This study aimed to develop a prognostic score to optimize prevention of an unnecessary laparotomy and minimize the rate for unnecessary laparoscopy.
Methods
Clinicopathologic data were evaluated for all patients undergoing surgical intervention for PDAC between 2001 and 2015, who were stratified into group 1 (2001–2008) and group 2 (2009–2014).
Results
The study identified 1001 patients eligible for analysis, 331 (33%) of whom underwent a staging laparoscopy before exploration. An unnecessary laparotomy was prevented for 44.4% of the patients in period 1 and for 24% of the patients in period 2 (p < 0.001). Male gender [odds ratio (OR), 1.8; p < 0.05], preoperative resectability (borderline resectable OR 2.1; p < 0.019; locally advanced OR 7.6; p < 0.001), CA 19-9 levels higher than 394 U/L (OR 3.1; p < 0.001), no neoadjuvant chemotherapy (OR 2.7; p = 0.012), and pancreatic body or tail lesions (OR 1.8; p = 0.063) were predictive of occult metastatic disease. The developed scoring index demonstrated a c-statistic of 0.729. The observed-to-expected ratio for the index at every score level validated the index’s model. A score cutoff at 4 was able to detect 76.1% of radiographically occult metastatic disease.
Conclusion
The rate for unnecessary laparotomy among patients with PDAC has decreased in contemporary times, but unnecessary laparotomy still occurs for 1 in 4 patients. Using our scoring system, a cutoff of 4 allows 76% of radiographically occult metastases to be predicted, thereby selecting high-risk patients for laparoscopic biopsy and potentially avoiding a non-therapeutic laparotomy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Pancreatic adenocarcinoma represents one of the most biologically aggressive solid organ tumors, with more than 70% of patients presenting with locally advanced or metastatic disease.1 The 5-year disease-specific survival rates for patients with pancreatic cancer are lower than 20%,2 with surgical resection in the absence of metastatic disease providing patients the best opportunity for long-term survival. However, as many as one third of patients harbor occult metastatic disease not detected on preoperative imaging, resulting in unnecessary morbidity from a non-therapeutic laparotomy.3–5
Staging laparoscopy is a minimally invasive method that allows for more accurate staging by detecting low-volume, radiographically occult metastatic disease not identified on preoperative imaging. It was first proposed by our institution as an initial staging method in 1986, when Warshaw et al.6 demonstrated that staging laparoscopy identified occult metastases and prevented an unnecessary laparotomy for 35% of patients. This incidence rate has been consistent throughout subsequent studies, which report a rate of 31–51% of all patients explored for pancreatic adenocarcinoma.5,7,8 Patients at higher risk for radiographically occult metastases include those with pancreatic tail lesions and elevated CA 19-9 levels.
However, the majority of studies reporting on the diagnostic utility of staging laparoscopy were conducted in the early 2000s.5,8–11 Since then, imaging technology has improved, with high-resolution computed tomography scans providing greater sensitivity.12 Additionally, the treatment paradigm for locally advanced and unresectable pancreatic cancer has changed, with neoadjuvant FOLFIRINOX demonstrating encouraging downstaging rates and margin-negative resections.13–15
This study aimed first to determine the utility of staging laparoscopy for patients with pancreatic adenocarcinoma in the contemporary setting of improved imaging and increased use of neoadjuvant chemotherapy. The second aim was to develop a novel index to identify patients at higher risk of having laparoscopically detectable metastases to minimize unnecessary laparotomies and the overuse of laparoscopy.
Methods
This study was approved by the Massachusetts General Hospital Institutional Review Board (protocol ID: 2014p001951). All patients who underwent a surgical exploration for pancreatic adenocarcinoma at Massachusetts General Hospital from 2001 to 2015 were identified via the Research Patient Data Registry (RPDR), our institution’s centralized clinical data registry/warehouse. Patients’ medical records from clinic visits, inpatient admissions, emergency room visits, operative notes, and pathology reports were linked to obtain longitudinal data. Patients who underwent a pancreatectomy were then defined by Current Procedural Terminology (CPT) codes for pancreaticoduodenectomy (48150, 48152), distal pancreatectomy (48140, 48145, 48146), and total pancreatectomy (48155) as a primary procedure in the database.
Patients were included in the study irrespective of age. Patients who underwent staging laparoscopy were identified via the CPT codes 49320 and 49321 and confirmed via medical record chart review. The staging laparoscopy group included patients who underwent laparoscopy before surgical exploration as a combined procedure or as a separate staging procedure at a date before exploration for curative intent. Patients who were explored for symptoms of biliary or gastric obstruction were excluded from the analysis to prevent allocation bias.
Patients receiving neoadjuvant treatment before resection were enrolled in a standardized neoadjuvant protocol, in which surveillance imaging is performed every 2 months throughout their treatment. Additionally, a final, preoperative staging scan was performed with a high-resolution pancreas protocol CT scan not more than 4 weeks before their date for surgical exploration.
Perioperative Variables
Patient demographics including age, sex, race, and comorbidities were extracted from RPDR. The Charlson comorbidity index was used to quantify patients’ comorbidities based on the presence or absence of International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes during episodes of care at the time of presentation for surgery or before.16
Tumor-specific variables and chemotherapy data were obtained via linkage with the Tumor Registry of the Massachusetts General Hospital. The tumor-specific variables included date of diagnosis, tumor size, location, lymph node involvement, surgical margins, date of recurrence or metastatic disease, and date of disease-specific death. Receipt of neoadjuvant chemotherapy was defined as a chemotherapy start date before the date of the operation and adjuvant chemotherapy as a chemotherapy start date after the date of the operation. The patient’s resectability was determined by the documentation in the surgeon’s preoperative note. For patients classified as borderline or locally advanced, the preoperative imaging was reviewed. Pretreatment tumor resectability was categorized as resectable, borderline, or unresectable as defined by the AHPBA/SSAT/SSO Expert Consensus Statement.17
Outcomes Definition
Avoidance of an unnecessary laparotomy was defined as radiographically occult metastatic disease found on staging laparoscopy, thus avoiding a laparotomy. Laparotomies were then classified as therapeutic if surgical extirpation of the tumor was performed or non-therapeutic if the resection was not performed because of metastases missed by laparoscopy due to local vascular invasion. All metastatic lesions were biopsied and confirmed via pathology analysis. The study period was divided into period 1 (2001–2008) and period 2 (2009–2015) for comparative analysis of staging laparoscopy’s yield.
Derivation of Index Predicting Laparoscopically Detectable Metastases
To determine the yield of staging laparoscopy for the entire population, we used a previously described technique to generate an index to identify independent predictors of laparoscopically detectable metastases.18 The predictors were identified by evaluating preoperative factors for patients who avoided an unnecessary laparotomy secondary to laparoscopically detected metastases, or who were found to have metastatic disease during exploratory laparotomy. Univariate analyses of laparoscopically detectable metastases were first performed using all preoperatively available variables. Variables with a p value lower than 0.05 and a frequency of occurrence greater than 20% were then included in a backward, stepwise logistic regression model with a p value threshold greater than 0.1 for variable exclusion. Then a preliminary index was generated by applying weighted coefficients to the variables based on the odds ratio (OR) of the variables (multiplier index). The coefficients then were divided by the lowest common denominator and rounded off to the nearest integer to develop the final index (rounded index) and thus generate a score that would be pragmatic and easy to use.
Performance Measures of Index
Discrimination, the ability of the index to distinguish patients who have laparoscopically detectable metastases from those who do not, was measured by the area under the receiver operating characteristic (ROC) curve (i.e., the c-statistic). The ROC is bounded by 0.50 and 1, with better discriminating scores having more area under the curve than poorer discriminating scores. Calibration (goodness of fit), the correlation between the predicted value of the index and the actual value, was measured by the model’s pseudo r 2. The pseudo r 2 is bounded by 0 and 1, and the greater the magnitude of calibration, the greater the value. The Youden index, which is the sum value of the sensitivity and specificity, was used to select an optimal cutoff. This approach assumes that the value of sensitivity and specificity is equal and allows selection of the highest sum value of both measures.
Statistical Analysis
Statistical analyses were performed using Intercooled Stata software, version 12.0 (StateCorp College Station, TX, USA). Categorical variables were analyzed using a Pearson chi-square test, and continuous variables were analyzed using the Wilcoxon rank-sum test. Certain continuous variables, such as age and CA 19-9 levels, were divided into quartiles and analyzed as ordinal variables to avoid assumption of linearity. All tests were performed two-sided, and statistical significance was accepted at a p value of 0.05 or less.
Results
Patient Demographics
We identified 1001 patients with pancreatic adenocarcinoma who underwent surgical exploration with curative intent throughout the study period. The exploration was performed for 493 of the patients during period 1 and for 508 of the patients during period 2. Staging laparoscopy was performed more frequently during period 2 (36.8 vs 29.2% during period 1; p = 0.01). Gender, age, race, and Charlson comorbidity index (CCI) score did not differ between the patients who underwent staging laparoscopy and those who did not (Table 1).
Tumor-Specific Variables
More tumors were located in the pancreatic body/tail in the laparoscopy group (29.3 vs 17.4% in the non-laparoscopy group; p < 0.001). The median tumor size for the entire cohort was 3 cm [interquartile range (IQR), 2.4–4.0 cm]. No difference was identified between the laparoscopy (3 cm) and no-laparoscopy cohorts (3.2 cm; p = 0.378). The preoperative CA 19-9 level of the patients in the staging laparoscopy group (median, 118 U/L; IQR, 28–631 U/L) was higher than that of the patients in the non-laparoscopy group (71 U/L; IQR 17–350 U/L; p = 0.0035).
The preoperative imaging showed a higher proportion of patients deemed to have locally advanced pancreatic adenocarcinoma in the staging laparoscopy group (18.8 vs 6.0% in the non-laparoscopy group; p < 0.001). Staging laparoscopy also was performed more frequently for the patients undergoing distal pancreatectomy (30.2 vs 17.8% in the non-laparoscopy group; p = 0.001, Table 1).
Time Period Analysis of the Benefits Conferred by Staging Laparoscopy
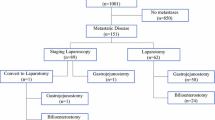
Figure 1 depicts the flow diagram of our study’s cohort in periods 1 and 2. In period 1, 144 patients underwent staging laparoscopy, and an unnecessary laparotomy was prevented in 44.4% (64/144) of all cases. Despite staging laparoscopy, 25 patients were not resected due to metastatic (n = 10) or locally advanced (n = 15) disease. In period 2, staging laparoscopy prevented an unnecessary laparotomy for 24% (45/187) of the patients, which was lower than the 44% observed in period 1 (p < 0.001). Despite staging laparoscopy, 13 patients (9%) were not resected due to metastatic (n = 4) or locally advanced (n = 9) disease in this period.
Of all the patients who did not undergo surgical resection secondary to metastatic disease, the long-term incidence of postoperative gastric outlet obstruction was 7% for the laparoscopy group and 6% for the laparotomy group (p = 0.210). Additionally, the incidence of biliary obstruction requiring biliary stenting did not differ between the laparoscopy group (35%) and the laparotomy group (24%) (p = 0.210).
Predictors of Laparoscopically Detectable Metastases
We identified 176 patients who presented with laparoscopically detectable occult metastatic disease. Most of the patients had metastases found on the liver (n = 113, 11.3%) or peritoneum (n = 56, 5.6%), followed by the root of the celiac artery (n = 20, 2.0%) and the superior mesenteric artery (n = 13, 1.3%). We then modeled a stepwise regression analysis to identify predictors of its occurrence. Preoperative resectability was the strongest predictor of laparoscopically detectable metastases. The patients with borderline resectable and locally advanced tumors were respectively 2.1 times [95% confidence interval (CI), 1.129–3.836; p = 0.019] and 7.6 times (95% CI 3.667–15.668; p < 0.001) more likely to have laparoscopically detectable metastases. Additionally, preoperative CA 19-9 levels higher than 394 U/L (OR 3.1; 95% CI 1.812–5.408; p < 0.001) and pancreatic body/tail lesions (OR 1.8; 95% CI 0.970–3.172; p = 0.063) also were associated with higher odds of patients having occult metastases that could be identified on laparoscopy. Neoadjuvant chemotherapy was associated with lower odds, so it was converted to a variable reflecting opposite directionality (no neoadjuvant chemotherapy: OR 2.7; 95% CI 1.242–5.796; p = 0.012) for ease of index generation. The original model had a c-statistic of 0.726 and a pseudo r 2 of 0.139. All predictors of laparoscopically detectable metastases had a frequency occurrence of at least 20% (Table 2). The multiplier index had a c-statistic of 0.723 and a pseudo r 2 of 0.135. The final index (rounded index) had a c-statistic of 0.729 and a pseudo r 2 of 0.137, both of which were similar to that of the original model. Table 2 depicts the c-statistics and pseudo r 2 of each index-generation step.
Final Index Performance Measures
The difference in the predicted laparoscopically detectable metastases rate versus the actual rate, the observed-to-expected (O:E) ratio, was examined to assess the validity of the index (Fig. 2). The O:E ratio had 95% CIs that crossed 1 at every score level, indicating no significant difference between the predicted and observed rates at all score levels.
The incidence of laparoscopically detectable metastases at each index score level as well as the trade-offs of selecting different cutoff values is depicted in Fig. 3. Assuming the value of sensitivity and specificity to be equal, a cutoff value is picked based on the highest Youden index, which puts the cutoff at 4 (sensitivity of 76.1%, specificity of 49.1%, Youden index of 125.2). A proposed index score cutoff of 4 detects up to 76.1% of all laparoscopically detectable metastases.
Probability of patients with pancreatic adenocarcinoma having laparoscopy detectable metastases (line) at each index score, together with the sensitivity (second row), specificity (third row), proportion of missed laparoscopically detectable metastases (fourth row), and proportion of unnecessary laparoscopies (fifth row) at each index score cutoff
Discussion
Staging laparoscopy can be used as a minimally invasive method to improve the staging of pancreatic adenocarcinoma. Laparoscopy can prevent an unnecessary laparotomy by detecting radiographically occult metastases, particularly in patients with pancreatic tail lesions and elevated CA 19-9 levels.
A recent meta-analysis analyzed 24 studies assessing the utility of staging laparoscopy for pancreatic cancer, which demonstrated sensitivities ranging from 44 to 93% and specificities ranging from 59 to 93%. However, most of the studies were performed in the early 2000s, with the most recent study published in 2011.3
The contemporary utility of staging laparoscopy for pancreatic cancer often is questioned due to improved imaging and increased use of neoadjuvant chemotherapy. The findings of this study demonstrated that staging laparoscopy prevented a laparotomy for 24.1% of the patients, a percentage significantly lower than the 35% reported by Warshaw et al.6 However, diagnostic laparoscopy adds time and cost to an operation. To improve the utility of laparoscopy, we developed a novel index with five variables to help predict which patients have a high likelihood of laparoscopically detectable metastases and therefore would benefit from a diagnostic laparoscopy.
The decreased number of unnecessary laparotomies in period 2 (24.1%) compared with period 1 (44.4%; p < 0.001) may reflect the improvement in imaging technology.12 Improved resolution of multidetector CTs has led to better radiographic detection of metastatic disease. However, staging laparoscopy still prevented an unnecessary laparotomy for almost 1 in 5 patients, which is significant enough to warrant its use in contemporary times. Additionally, modern-day laparoscopes have superior resolution, increased brightness and depth of field, and decreased distortion, allowing for fewer metastases to be missed.19,20 These improved cameras most likely contributed to fewer metastases being missed in period 2.
Recently, more neoadjuvant chemotherapy is being used for borderline resectable and locally advanced pancreatic adenocarcinomas. In particular, findings show that FOLFIRINOX radiographically downstages one third of patients who initially present with stage 3 disease.15 More importantly, neoadjuvant chemotherapy and radiation therapy lead to a desmoplastic response around the Tumor and conventional radiographic imaging is unable to distinguish between the desmoplasia and true tumor involvement of major visceral vessels.
Our institution analyzed 40 patients receiving neoadjuvant FOLFIRINOX and found that 92% had an R0 resection despite imaging suggesting continued unresectability, leading to our practice of exploring patients based on improvement in CA 19-9 levels.13 Our study demonstrated that the use of neoadjuvant chemotherapy was associated with a decreased incidence of laparoscopically detectable metastases, in theory because the patients had withstood the “test of time.”
It is interesting to note that male patients were 1.8 times more likely to have laparoscopically detectable metastases than female patients. It is well established that independent of body weight, males have more visceral adipose tissue volume, as measured by computed tomography, than females.21,22 This higher visceral adipose tissue volume in males may obscure the radiographic detection of intraabdominal metastases, given that up to 30% of metastases are on the omental and mesenteric fat. Additionally, tumor size was not predictive of radiographically occult metastatic disease, given that it was collinear with resectability and CA 19-9 levels in the multivariate analysis.
Finally, although the risk factors for patients with radiographically occult metastatic disease are well defined, no well-validated nomogram or scoring system exists to stratify these patients. We generated a practical index composed of five simple predictors selected on the basis of three concepts: predictive value, adequate frequency in the preoperative setting, and clinical face validity. The index underwent simplification procedures to allow its pragmatic use by clinicians, without compromise of its discrimination and calibration performances (Table 2).
It needs to be highlighted that parsimonious risk models should be created for better applicability in practice. Meguid et al.23 used the American College of Surgeons National Surgical Quality Improvement Program data set and demonstrated that the first 7 of 28 variables entered accounted for at least 99% of the c-index for the full model.
To summarize, patients at highest risk of harboring radiographically occult metastases who would benefit from staging laparoscopy include male patients, patients with borderline resectable and locally advanced lesions, patients who did not undergo neoadjuvant chemotherapy, patients who have a CA 19-9 level higher than 394 U/L, and patients with the lesion in the pancreatic body or tail. Figure 3 best depicts the trade-offs of each score cutoff if it were to be selected.
We propose an index score cutoff of 4 for selecting patients at higher risk for occult metastatic disease based on the Youden index, which values sensitivity and specificity equally.24 Whereas this score cutoff would detect a radiographically occult metastasis in 18% of patients, 76% of laparoscopies would be unnecessary (Fig. 3). However, we contest that the cost and additional operative time for a brief staging laparoscopy is a good trade-off if 1 in 5 patients can be spared of an unnecessary laparotomy. If it is deemed that sensitivity (benefits derived from an unnecessary laparotomy, namely, shorter stay, lower morbidity rates, improved quality of life, and quicker initiation of chemotherapy)25 carries more value than specificity (cost of performing a laparoscopy before laparotomy), the threshold should be moved downward, thus sacrificing specificity for sensitivity. It should be emphasized that the scoring system is intended to select high-risk patients for laparoscopy, and if a suspected metastatic lesion is found, a biopsy should be performed to confirm metastases, which will guide treatment planning in addition to avoiding a laparotomy. Furthermore, staging laparoscopy allows for intraoperative peritoneal washing to be performed, which may suggest metastatic spread and incipient peritoneal carcinomatosis when positive.26
Our study must be interpreted in the context of its design. The definition of our exposure—staging laparoscopy, included both staged procedures and single procedures. For some of the early neoadjuvant therapy clinical trials, we were required to perform laparoscopy at the time of diagnosis. However, because no change in the chemotherapeutic regimen occurred for patients with metastatic disease or locally advanced disease, we currently give these patients the “benefit of the doubt” and perform laparoscopy only at the time of resection.
Our definition of laparoscopically detectable metastases included patients in the no-laparoscopy group shown to have metastatic disease on laparotomy, presuming that they would have been detected if staging laparoscopy had been performed. However, previous studies have shown that the false-negative rate for laparoscopy is low, with rates reported to range from 4 to 7%.3,6 Our own series reported a rate of 4.2%, comprising mostly metastases located in the posterior segments of the liver. Additionally, our study represents an analysis of a single, high-volume referral center. Therefore, its generalizability may be limited and should be validated in other institutions.
Finally, opponents of staging laparoscopy would note that patients eventually require prophylactic bypass procedures and therefore would need a laparotomy. However, data suggest that almost none (2%) of the patients who present without obstruction eventually experience an indication for a bypass.27 Additionally, the advent of minimally invasive procedures for symptomatic and obstructive palliation such as endoscopic biliary stenting and laparoscopic gastrointestinal or biliary bypass further obviates the need for a laparotomy.
In the contemporary setting of improved imaging and increased use of neoadjuvant chemotherapy, our study demonstrated that staging laparoscopy would still prevent an unnecessary laparotomy for 24% of patients undergoing surgical exploration. We propose a practical scoring index for better selection of patients at higher risk for occult metastatic disease to further maximize the yield of staging laparoscopy for patients with pancreatic adenocarcinoma.
References
Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9:222–31.
Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7.
De Rosa A, Cameron IC, Gomez D. Indications for staging laparoscopy in pancreatic cancer. HPB Oxford. 2016;18:13–20.
Conlon KC, Dougherty E, Klimstra DS, et al. The value of minimal access surgery in the staging of patients with potentially resectable peripancreatic malignancy. Ann Surg. 1996;223:134–40.
Jimenez RE, Warshaw AL, Rattner DW, et al. Impact of laparoscopic staging in the treatment of pancreatic cancer. Arch Surg. 2000;135:409–14; discussion 414–5.
Warshaw AL, Tepper JE, Shipley WU. Laparoscopy in the staging and planning of therapy for pancreatic cancer. Am J Surg. 1986;151:76–80.
Stefanidis D, Grove KD, Schwesinger WH, et al. The current role of staging laparoscopy for adenocarcinoma of the pancreas: a review. Ann Oncol. 2006;17:189–99.
Liu RC, Traverso LW. Diagnostic laparoscopy improves staging of pancreatic cancer deemed locally unresectable by computed tomography. Surg Endosc. 2005;19:638–42.
Barreiro CJ, Lillemoe KD, Koniaris LG, et al. Diagnostic laparoscopy for periampullary and pancreatic cancer: what is the true benefit? J Gastrointest Surg. 2002;6:75–81.
Maithel SK, Maloney S, Winston C, et al. Preoperative CA 19-9 and the yield of staging laparoscopy in patients with radiographically resectable pancreatic adenocarcinoma. Ann Surg Oncol 2008;15:3512–20.
Pisters PW, Lee JE, Vauthey JN, et al. Laparoscopy in the staging of pancreatic cancer. Br J Surg. 2001;88:325–37.
Zamboni GA, Kruskal JB, Vollmer CM, et al. Pancreatic adenocarcinoma: value of multidetector CT angiography in preoperative evaluation. Radiology. 2007;245:770–8.
Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261:12–7.
Nitsche U, Wenzel P, Siveke JT, et al. Resectability after first-line FOLFIRINOX in initially unresectable locally advanced pancreatic cancer: a single-center experience. Ann Surg Oncol. 2015;22(Suppl 3):S1212–20.
Sadot E, Doussot A, O’Reilly EM, et al. FOLFIRINOX induction therapy for stage 3 pancreatic adenocarcinoma. Ann Surg Oncol. 2015;22:3512–21.
Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–33.
Easterlin MC, Chang DC, Wilson SE. A practical index to predict 30-day mortality after major amputation. Ann Vasc Surg. 2013;27:909–17.
Pierre SA, Ferrandino MN, Simmons WN, et al. High-definition laparoscopy: objective assessment of performance characteristics and comparison with standard laparoscopy. J Endourol. 2009;23:523–8.
Qin Y, Hua H, Nguyen M. Characterization and in vivo evaluation of a multi-resolution foveated laparoscope for minimally invasive surgery. Biomed Opt Express. 2014;5:2548–62.
Lemieux S, Prud’homme D, Bouchard C, et al. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr. 1993;58:463–7.
Grauer WO, Moss AA, Cann CE, et al. Quantification of body fat distribution in the abdomen using computed tomography. Am J Clin Nutr. 1984;39:631–7.
Meguid RA, Bronsert MR, Juarez-Colunga E, et al. Surgical Risk Preoperative Assessment System (SURPAS): II. Parsimonious risk models for postoperative adverse outcomes addressing need for laboratory variables and surgeon specialty-specific models. Ann Surg. 2016;264:10–22.
Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458–72.
Hashimoto D, Chikamoto A, Sakata K, et al. Staging laparoscopy leads to rapid induction of chemotherapy for unresectable pancreatobiliary cancers. Asian J Endosc Surg. 2015;8:59–62.
Hoshimoto S, Hishinuma S, Shirakawa H, et al. Prognostic significance of intraoperative peritoneal washing cytology for patients with potentially resectable pancreatic ductal adenocarcinoma. Pancreatology. 2017;17:109–14.
Espat NJ, Brennan MF, Conlon KC. Patients with laparoscopically staged unresectable pancreatic adenocarcinoma do not require subsequent surgical biliary or gastric bypass. J Am Coll Surg. 1999;188:649–55; discussion 655–7.
Disclosure
All authors have no relevant disclosures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fong, Z.V., Alvino, D.M.L., Fernández-del Castillo, C. et al. Reappraisal of Staging Laparoscopy for Patients with Pancreatic Adenocarcinoma: A Contemporary Analysis of 1001 Patients. Ann Surg Oncol 24, 3203–3211 (2017). https://doi.org/10.1245/s10434-017-5973-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-5973-5