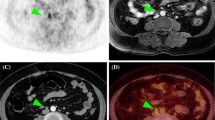
Abstract
Objective
The aim of this study was to investigate the value of [18F]fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) in predicting lymph node status in node-negative endometrial cancer on preoperative magnetic resonance imaging (MRI).
Methods
Patients with endometrial cancer who underwent both preoperative MRI and FDG-PET/CT followed by hysterectomy and lymphadenectomy were initially included. We then enrolled patients with MRI-defined node-negative disease (lymph nodes <1 cm in the short-axis diameter, or no visible lymph node). Histologic examination was the gold standard for lymph node metastasis diagnosis. The diagnostic performance of FDG-PET/CT in predicting lymph node metastasis was calculated in patient-by-patient and lymph node station-by-station analyses.
Results
On preoperative MRI, 362 patients had no lymph node metastasis. All patients underwent pelvic lymph node dissection and 118 patients underwent further para-aortic lymph node dissection. From 2099 lymph node stations, 10,238 lymph nodes were retrieved. Twenty-seven patients (7.5%) had lymph node metastasis in 49 lymph node stations (2.3%) on pathologic examination. FDG-PET/CT identified lymph node metastasis in five patients (18.5%) and eight lymph node stations (16.3%). The median diameter of false-negative metastatic lymph nodes was 6 mm (range 1–22) in the long axis and 3 mm (range 1–11) in the short axis. For para-aortic lymph nodes, FDG-PET/CT diagnosed 2 of 11 patients (18.1%) with para-aortic lymph node metastasis, and 3 of 12 para-aortic lymph node stations (25%) with metastasis.
Conclusion
Preoperative FDG-PET/CT has low value in predicting lymph node metastasis in node-negative endometrial cancer on preoperative MRI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Endometrial cancer is the most common gynecologic malignancy in North America and Europe.1,2 In addition, the incidence of endometrial cancer has rapidly increased in Asian countries in recent years.3,4,5 The standard management of endometrial cancer is surgery, which includes hysterectomy, bilateral salpingo-oophorectomy, and pelvic and/or para-aortic lymph node dissection.6 Two randomized controlled trials suggested that routine lymph node dissection does not improve the survival outcomes of endometrial cancer;7,8 therefore, the main role of lymph node dissection is in staging, specifically to guide postoperative adjuvant therapy and predict endometrial cancer prognosis.9 Because lymph node dissection causes morbidity in a significant number of patients, and decreases their quality of life, it is important to accurately predict lymph node status preoperatively to avoid futile lymph node dissection if possible.9
Enhanced magnetic resonance imaging (MRI) is the best preoperative diagnostic tool to predict lymph node status in endometrial cancer;6 however, because MRI is based on a change in lymph node morphology, its diagnostic performance in small metastatic lymph nodes <1 cm is not reliable. [18F]Fluorodeoxyglucose-positron emission tomography (FDG-PET) is a metabolic imaging modality that can detect early changes in morphologically normal organs. The integration of FDG-PET and computed tomography (CT) has improved the anatomic localization of metabolic changes in PET. Therefore, FDG-PET/CT is expected to have better diagnostic performance for small metastatic lymph nodes without morphological changes on MRI. However, the performance of FDG-PET/CT in patients with negative findings on MRI has not yet been evaluated. The aim of our present study was to evaluate the diagnostic performance of preoperative FDG-PET/CT in predicting lymph node status in node-negative endometrial cancer on MRI.
Materials and Methods
Study Patients
We identified patients with endometrial cancer who were treated at the Asan Medical Center, Seoul, Korea, between 2007 and 2011, using the electronic database and electronic medical records of the institution. MRI of the abdomen and pelvis, as well as FDG-PET/CT, were recommended to all patients with histologically confirmed endometrial cancer prior to surgery at the Asan Medical Center during the study period. Patients meeting the following criteria were included: (1) previously untreated endometrial cancer; (2) one of the following histological subtypes: endometrioid, mucinous, serous, clear cell, undifferentiated, mixed, or carcinosarcoma; (3) primary surgery that included hysterectomy and pelvic and/or para-aortic lymph node dissection at the Asan Medical Center; and (4) MRI of the abdomen and pelvis, as well as FDG-PET/CT, at the Asan Medical Center within 4 weeks prior to surgery. This study was approved by the Institutional Review Board of the Asan Medical Center, Seoul, Korea.
Pelvic and/or Para-Aortic Lymph Node Dissection
Bilateral pelvic and/or para-aortic lymph node dissection was performed and the lymph node stations were defined as previously reported.10 No patient underwent sentinel lymph node mapping during surgery. Pelvic and para-aortic lymph node areas were divided into eight lymph node stations during surgery in each patient (Fig. 1). In brief, lymph nodes over the external iliac vessels between the deep circumflex iliac vein and the bifurcation of the iliac vessels were removed and marked as external iliac lymph nodes; lymph nodes in the obturator area between the lower border of the external iliac vessels and lateral border of the internal iliac vessels and lymph nodes, which are located over, and medial to, the internal iliac vessels, were removed and marked as obturator/internal iliac lymph nodes; lymph nodes around the common iliac vessels between the bifurcation of the common iliac vessels and aortic bifurcation were removed and marked as common iliac lymph nodes; lymph nodes around the inferior vena cava and aorto-caval area between the aortic bifurcation and the origin of the left gonadal vessels were removed and marked as right para-aortic lymph nodes; and lymph nodes located in the left side of the aorta between the aortic bifurcation and left renal vein were removed and marked as left para-aortic lymph nodes.
Pelvic and para-aortic lymph node stations. 1 Right para-aortic lymph node station; 2 left para-aortic lymph node station; 3 right common iliac lymph node station; 4 left common iliac lymph node station; 5 right external iliac lymph node station; 6 left external iliac lymph node station; 7 right obturator/internal iliac lymph node station; 8 left obturator/internal iliac lymph node station
All of the retrieved lymph nodes were evaluated pathologically, and were bisected along the long axis and cut perpendicular to the long axis at 1-mm intervals for pathologic examination. Histological sections of lymph nodes were stained with hematoxylin and eosin. The results of the pathologic examination were reported for each lymph node station and included the number of lymph nodes retrieved, number of metastatic lymph nodes, and size of metastatic lymph nodes. For our present study, the size of the metastatic lymph nodes in both the long and short axes was measured by two pathologists who specialized in gynecologic oncology.
Preoperative Magnetic Resonance Imaging of the Abdomen and Pelvis
The protocol of MRI of the abdomen and pelvis is described in electronic supplementary File 1. Two radiologists who were blinded to the patients’ clinical information, as well as the FDG-PET/CT and pathologic results for the lymph nodes, reviewed all MRI images together, and the results were recorded according to their consensus. Lymph nodes <1 cm in diameter in the short axis, or no visible lymph nodes on MRI, were defined as negative lymph nodes on MRI. The definitions of lymph node stations for the MRI review were the same as those of the lymph node dissection discussed above.
Preoperative [18F]Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography
The protocol of FDG-PET/CT is described in electronic supplementary File 1. Two board-certified nuclear medicine physicians who were also blinded to the patients’ clinical information, as well as the MRI and pathologic results for the lymph nodes, reviewed all FDG-PET/CT images together and reached a consensus. Lymph nodes that had a higher uptake than the surrounding tissues were regarded as positive for malignancy, regardless of size. Equivocal uptakes that were similar to blood pool activity were regarded as negative. Physiologic uptake at the bowel, ureter, or ovaries was excluded. Lymph node stations were determined by referring to the combined CT. The definitions of lymph node stations for the FDG-PET/CT review were the same as those of the lymph node dissection discussed above.
Statistical Analysis
The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of FDG-PET/CT in predicting lymph node metastasis were assessed in patient-by-patient and lymph node station-by-station analyses with 95% confidence interval (CI). The mean values were compared using Student’s t-test, and the sensitivity and specificity between two independent groups were compared using the Chi square test or Fisher’s exact test. A p-value <0.05 in a two-sided test were regarded as statistically significant. All statistical analyses were performed using SPSS software, version 21.0 (IBM Corporation, Armonk, NY, USA).
Results
Study Patients
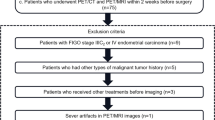
During the study period, 498 patients with endometrial cancer underwent primary surgery at the Asan Medical Center (Fig. 2). We excluded 83 of these patients who refused preoperative MRI of the abdomen and pelvis and/or FDG-PET/CT, and 22 patients who refused lymph node dissection and did not undergo lymph node dissection. The remaining 393 patients underwent both MRI and FDG-PET/CT prior to surgery and underwent bilateral pelvic and/or para-aortic lymph node dissection. Of these patients, 362 did not have a positive lymph node on preoperative MRI and were included in the analysis. Patient characteristics are listed in Table 1.
Lymph Node Dissection
All 362 study patients underwent pelvic lymph node dissection and 118 patients further underwent para-aortic lymph node dissection. Lymph node dissection was performed in 2099 lymph node stations. A total of 10,238 lymph nodes were retrieved, and 83 lymph nodes had a metastasis on pathologic examination.
Patient-by-Patient Analysis
Twenty-seven patients had lymph node metastasis on pathologic examination. The characteristics of 27 patients with lymph node metastasis are shown in Table 2. Five cases (2/27, 18.5%) were diagnosed with lymph node metastasis using FDG-PET/CT (see Table 3 for the FDG-PET/CT and pathologic results of the patients). The sensitivity, specificity, negative predictive value, positive predictive value, and accuracy of FDG-PET/CT in predicting lymph node metastasis were 18.5% (95% CI 7.0–38.7), 94% (95% CI 90.8–96.2), 20% (95% CI 7.6–41.3), 93.5% (95% CI 90.1–95.8), and 88.4% (95% CI 85.1–91.7), respectively, in patient-by-patient analysis.
Lymph Node Station-by-Station Analysis
Lymph node metastasis was found in 49 lymph node stations on pathologic examination. Eight of 49 lymph node stations (16.3%) were diagnosed with lymph node metastasis using FDG-PET/CT (see Table 3 for the FDG-PET/CT and pathologic results of the lymph node stations). The sensitivity, specificity, negative predictive value, positive predictive value, and accuracy of FDG-PET/CT in predicting lymph node metastasis were 16.3% (95% CI 7.8–30.2), 98.8% (95% CI 98.2–99.2), 25% (95% CI 12.1–43.8), 98% (95% CI 97.3–98.6), and 96.9% (95% CI 96.2–97.6), respectively, in lymph node station-by-station analysis.
Characteristics of False-Negative Metastatic Lymph Nodes
FDG-PET/CT detected 19 of 83 metastatic lymph nodes. The median diameter of the 19 true-positive metastatic lymph nodes was 8 mm (range 2–41) in the long axis and 5 mm (range 1–24) in the short axis. The remaining 64 metastatic lymph nodes were false-negative on FDG-PET/CT. The median diameters of false-negative metastatic lymph nodes were 6 mm (range 1–22) in the long axis and 3 mm (range 1–11) in the short axis. There was no statistically significant difference in the long-axis diameter between true-positive metastatic lymph nodes and false-negative metastatic lymph nodes (p = 0.070). However, the short-axis diameter of false-negative metastatic lymph nodes was significantly shorter than the short-axis diameter of true-positive metastatic lymph nodes (p = 0.043).
Diagnostic Performance in Predicting Para-Aortic Lymph Node Metastasis
Para-aortic lymph node dissection was performed in 118 patients, and 907 para-aortic lymph nodes were retrieved from 190 para-aortic lymph node stations. Twenty-eight metastatic lymph nodes were revealed on pathologic examination of 12 para-aortic lymph node stations in 11 patients. FDG-PET/CT was able to diagnose 2 of 11 patients (18.1%) with para-aortic lymph node metastasis and 3 of 12 para-aortic lymph nodes stations (25%) with metastasis. The sensitivity, specificity, negative predictive value, positive predictive value, and accuracy of FDG-PET/CT in predicting para-aortic lymph node metastasis were 18.2% (95% CI 3.2–52.2), 98.1% (95% CI 92.8–99.7), 92.1% (95% CI 85.1–96.1), 50% (95% CI 9.2–91.0), and 90.7% (95% CI 85.5–95.9%), respectively, in patient-by-patient analysis, and 25% (95% CI 6.7–57.2), 98.9% (95% CI 95.6–99.8), 95.1% (95% CI 90.7–97.6), 60% (95% CI 17.0–92.7), and 94.2% (95% CI 90.9–97.5), respectively, in lymph node station-by-station analysis (Table 3). The median diameters of false-negative metastatic para-aortic lymph nodes were 5.5 mm (range 1–12) in the long axis and 2.8 mm (range 1–7) in the short axis.
Subgroup Analysis According to Risk Group and Body Mass Index
Patients were divided into two risk groups: low-risk group versus high-risk group. Patients with grade 1–2, endometrioid histology, and myometrial invasion less than half were classified as the low-risk group (n = 253), and patients with papillary serous histology, clear cell histology, carcinosarcoma histology, grade 3 endometrioid histology, or myometrial invasion more than half were classified as the high-risk group (n = 109). The sensitivity, specificity, and accuracy of FDG-PET/CT in predicting lymph node metastasis were 0% (0/7), 96.7% (238/246), and 94.1% (238/253), respectively, for the low-risk group, and 25% (5/20), 86.5% (77/89), and 75.2% (82/109), respectively, for the high-risk group. There was no statistically significant difference in sensitivity (p = 0.238) between the low- and high-risk groups; however, the specificity was significantly better in the low-risk group (p < 0.001).
The mean body mass index (BMI) of patients was 25.09 kg/m2 (range 16.79–43.85), and patients were divided into normal-BMI (BMI <25 kg/m2, n = 198) and high-BMI (BMI ≥25 kg/m2, n = 164) groups. The sensitivity, specificity, and accuracy of FDG-PET/CT in predicting lymph node metastasis were 17.6% (3/17), 93.9% (170/181), and 87.4% (173/198), respectively, for the normal-BMI group, and 20% (2/10), 94.2% (145/154), and 89.6% (147/164), respectively, for the high-BMI group. There was no statistically significant difference in sensitivity (p > 0.999) and specificity (p = 0.928) between the normal- and high-BMI groups.
Discussion
The role of MRI and PET/CT in preoperative evaluation of lymph node metastasis in endometrial cancer is still controversial. The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of preoperative MRI in predicting lymph node status have been reported to be 50, 95, 90, 50, and 95%, respectively.11 Thus, MRI can predict only 50% of patients with lymph node metastasis. The diagnostic performance of MRI in detecting lymph node metastasis is limited in normal-sized metastatic lymph nodes. In the present study, lymph node metastasis was histologically identified in 7.5% (27/362) of patients without metastasis on MRI. The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of preoperative FDG-PET or PET/CT in predicting lymph node status have been reported to be 63, 95, 43, 97, and 90%, respectively.12,13 Some studies suggested the superiority of FDG-PET/CT to MRI in endometrial cancer.14,15 FDG-PET/CT is expected to detect early metabolic changes in normal-sized metastatic lymph nodes that cannot be detected on MRI.16 However, in our present study of patients with negative lymph nodes on MRI, FDG-PET/CT was able to diagnose only five patients (5/27, 18.5%) with lymph node metastasis, and only eight lymph node stations (8/49, 16.3%) with lymph node metastasis. Thus, FDG-PET/CT also had a low diagnostic value in normal-sized metastatic lymph nodes.
The diagnostic value of FDG-PET or PET/CT in patients with MRI-defined negative lymph nodes has not been well-evaluated in most malignant diseases, including endometrial cancer. Chou et al.17 reported the diagnostic value of FDG-PET in 60 cervical cancer patients with MRI-defined negative lymph node metastasis. In that prospective study, 10 patients had pelvic lymph node metastasis and one patient had para-aortic lymph node metastasis histologically.17 However, FDG-PET detected only one pelvic lymph node metastasis (1/10, 10%) and para-aortic lymph node metastasis.17 The sensitivity and false-negative rates were 10% and 90%, respectively. These results are similar to those of our present study.
FDG-PET/CT predicted only 16% of metastatic pelvic lymph nodes, but predicted 25% of metastatic para-aortic lymph nodes, in the present study. This may be due to the proximity of the ureter to the pelvic nodal basins. Because we used non-contrast-enhanced CT, the physiologic uptake of the ureter may be mistaken for pelvic lymph nodes in some cases.
In predicting lymph node metastasis of endometrial cancer, neither MRI nor FDG PET-CT are highly sensitive. In patients with negative nodes on MRI, FDG-PET/CT has low sensitivity in predicting lymph node metastasis. The sensitivity of FDG-PET/CT in these patients was not different between the low- and high-risk groups, and was not different between the normal- and high-BMI groups. However, the specificity of FDG-PET/CT was significantly better in the low-risk group compared with the high-risk group. Therefore, FDG-PET/CT may guide treatment properly, especially in medically compromised, inoperable patients with low-risk histology.
In our study, we used a 1 cm criterion for negative lymph nodes on MRI. Of several criteria for the diagnosis of negative lymph nodes on MRI, the diameter of the short axis of the lymph nodes was the only generally accepted criterion. The size criteria for negative lymph nodes on MRI, ranging between 4 and 15 mm, have been evaluated in previous studies, with the 1 cm criterion being the most common criterion for the upper limit of negative lymph nodes on MRI.18,19,20,21,22,23 In the study by Choi et al., a 9 mm and 1 cm criteria for the short axis of the lymph nodes on MRI showed the best performance in the diagnosis of negative lymph nodes in uterine cancer compared with a smaller size criteria.20 Many clinical trials on gynecologic cancers also adopted the criterion of a 1 cm short-axis diameter of lymph nodes in discrimination of negative lymph nodes. We therefore used the 1 cm criterion of the short-axis diameter of lymph nodes for the negative lymph nodes on MRI.
For the evaluation of lymph node status in endometrial cancer, lymph node dissection is still the gold standard. Further studies are required to replace lymph node dissection with a non-invasive evaluation method. The use of sentinel lymph node mapping with indocyanine green and near-infrared fluorescence imaging technology in the management of endometrial cancer is currently being evaluated.24 A recent multicenter, prospective, cohort trial (FIRES) showed the safety and efficacy of sentinel lymph node mapping in endometrial cancer and suggested this can safely replace lymph node dissection in the staging of endometrial cancer.25 PET/MR and new radionuclide isotopes are also being studied for the management of endometrial cancer.15,26 However, only preliminary experiences regarding PET/MRI are available in the literature. In a retrospective study, PET/MRI showed better accuracy in the staging of endometrial cancer than PET/CT.27 Further evaluation is needed to evaluate the role of PET/MRI in endometrial cancer.
Conclusion
Preoperative FDG/PET/CT has low value in detecting lymph node metastasis in endometrial cancer patients with negative lymph nodes on MRI. Measured use of FDG-PET/CT is needed and further studies are required to determine the clinical indications and cost effectiveness of FDG-PET/CT.
References
Green CS, Kattner F, Siegel MH, et al. Differences in perceptual learning transfer as a function of training task. J Vis 2015; 15:5.
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136:E359–86.
Jung KW, Won YJ, Oh CM, et al. Prediction of cancer incidence and mortality in Korea, 2015. Cancer Res Treat 2015; 47:142–8.
Huang CY, Chen CA, Chen YL, et al. Nationwide surveillance in uterine cancer: survival analysis and the importance of birth cohort: 30-year population-based registry in Taiwan. PLoS One 2012; 7:e51372.
Ushijima K. Current status of gynecologic cancer in Japan. J Gynecol Oncol 2009; 20:67–71.
Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet 2016;387(10023):1094–1108.
ASTEC Study Group, Kitchener H, Swart AM, et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet 2009; 373:125–36.
Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst 2008; 100:1707–16.
May K, Bryant A, Dickinson HO, et al. Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst Rev 2010;(1):CD007585.
Choi HJ, Roh JW, Seo SS, et al. Comparison of the accuracy of magnetic resonance imaging and positron emission tomography/computed tomography in the presurgical detection of lymph node metastases in patients with uterine cervical carcinoma: a prospective study. Cancer 2006; 106:914–22.
Manfredi R, Mirk P, Maresca G, et al. Local-regional staging of endometrial carcinoma: role of MR imaging in surgical planning. Radiology 2004; 231:372–8.
Chang MC, Chen JH, Liang JA, et al. 18F-FDG PET or PET/CT for detection of metastatic lymph nodes in patients with endometrial cancer: a systematic review and meta-analysis. Eur J Radiol 2012; 81:3511–7.
Park JY, Kim EN, Kim DY, et al. Comparison of the validity of magnetic resonance imaging and positron emission tomography/computed tomography in the preoperative evaluation of patients with uterine corpus cancer. Gynecol Oncol 2008; 108:486–92.
Kim HJ, Cho A, Yun M, et al. Comparison of FDG PET/CT and MRI in lymph node staging of endometrial cancer. Ann Nucl Med 2016; 30:104–13.
Lee SI, Catalano OA, Dehdashti F. Evaluation of gynecologic cancer with MR imaging, 18F-FDG PET/CT, and PET/MR imaging. J Nucl Med 2015; 56:436–43.
Bollineni VR, Ytre-Hauge S, Bollineni-Balabay O, et al. High diagnostic value of FDG-PET/CT in endometrial cancer: Systematic review and meta-analysis of the literature. J Nucl Med 2016;57(6):879–85.
Chou HH, Chang TC, Yen TC, et al. Low value of [18F]-fluoro-2-deoxy-d-glucose positron emission tomography in primary staging of early-stage cervical cancer before radical hysterectomy. J Clin Oncol 2006; 24:123–8.
Outwater E, Kressel HY. Evaluation of gynecologic malignancy by magnetic resonance imaging. Radiol Clin North Am 1992; 30:789–806.
Kim SH, Kim SC, Choi BI, et al. Uterine cervical carcinoma: evaluation of pelvic lymph node metastasis with MR imaging. Radiology 1994; 190:807–11.
Choi HJ, Kim SH, Seo SS, et al. MRI for pretreatment lymph node staging in uterine cervical cancer. AJR Am J Roentgenol 2006; 187:W538–43.
Togashi K, Nishimura K, Sagoh T, et al. Carcinoma of the cervix: staging with MR imaging. Radiology 1989; 171:245–51.
Yu KK, Hricak H, Subak LL, et al. Preoperative staging of cervical carcinoma: phased array coil fast spin-echo versus body coil spin-echo T2-weighted MR imaging. AJR Am J Roentgenol 1998; 171:707–11.
Yang WT, Lam WW, Yu MY, et al. Comparison of dynamic helical CT and dynamic MR imaging in the evaluation of pelvic lymph nodes in cervical carcinoma. AJR Am J Roentgenol 2000; 175:759–66.
Paley PJ, Veljovich DS, Press J, et al. A prospective investigation of fluorescence imaging to detect sentinel lymph nodes at robotic-assisted endometrial cancer staging. Am J Obstet Gynecol 2016;215(1):117.e1–7.
Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol 2017; 18:384–92.
Queiroz MA, Kubik-Huch RA, Hauser N, et al. PET/MRI and PET/CT in advanced gynaecological tumours: initial experience and comparison. Eur Radiol 2015; 25:2222–30.
Kitajima K, Suenaga Y, Ueno Y, et al. Value of fusion of PET and MRI for staging of endometrial cancer: comparison with (1)(8)F-FDG contrast-enhanced PET/CT and dynamic contrast-enhanced pelvic MRI. Eur J Radiol 2013; 82:1672–6.
Disclosures
Jeong-Yeol Park, Jong Jin Lee, Hyuck Jae Choi, In Hye Song, Chang Ohk Sung, Hye Ok Kim, Sun-Young Chae, Young-Tak Kim, and Joo-Hyun Nam declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Park, JY., Lee, J.J., Choi, H.J. et al. The Value of Preoperative Positron Emission Tomography/Computed Tomography in Node-Negative Endometrial Cancer on Magnetic Resonance Imaging. Ann Surg Oncol 24, 2303–2310 (2017). https://doi.org/10.1245/s10434-017-5901-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-5901-8