Abstract
Background
Assessing prognostic and predictive factors like the Ki67 labelling index (Ki67-LI) in breast cancer core needle biopsies (CNB) may be hampered by undersampling. Our aim was to arrive at a representative assessment of Ki67-LI in CNB of luminal breast cancers by defining optimal cutoffs and establishing the minimum CNB volume needed for highest concordance of Ki67-LI between CNB and subsequent surgical excision biopsy (SEB).
Methods
We assessed the Ki67-LI in CNB and subsequent SEB of 170 luminal breast cancers according to two counting methods recommended by the International Ki67 in Breast Cancer Working Group and applied the cutoffs to distinguish low and high proliferation given by the St Gallen 2013 and 2015 consensus, respectively. We then compared CNB volume characteristics for cases with concordant and discordant Ki67-LI between CNB versus SEB.
Results
Highest concordance (75%, κ = 0.44) between CNB and SEB was achieved using the method that assesses the average tumor Ki67-LI and a cutoff of 20%. No significant differences were found between cases with concordant and discordant Ki67-LI in CNB versus SEB for number of biopsy cores, total core length, tumor tissue length, or total CNB or tumor tissue area size in the CNB for two various cutoffs.
Conclusions
A concordance of 75% between CNB and SEB can be achieved for the Ki67-LI using a method assessing average Ki67-LI at the threshold of 20%. Increasing CNB volume did not result in improved agreement rates, indicating that reliability of Ki67 levels in CNB of luminal breast cancers is unaffected by CNB volume.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Sampling error in presurgical breast cancer diagnostics occurs when the core needle biopsy (CNB) does not contain the most representative or aggressive part of a tumor due to intratumoral heterogeneity.1–3 Recommendations for the handling of some predictive and prognostic factors prone to sampling error in CNB, like hormone and human epidermal growth factor receptor 2 (HER2) receptor status, have already been established that prescribe to repeat and reassess immunohistochemical stains in the surgical excision biopsy (SEB) for optimal tumor characterization.4–6 For Ki67, a widely used immunohistochemical biomarker to measure proliferative activity, standards for assessment, statistical approach and clinical use are currently debated.7–13 Still, the Ki67 labelling index (Ki67-LI) has been considered a measure of tumor proliferation for therapeutic decisions in estrogen receptor (ER) positive and HER2 negative (‘luminal’) breast cancer: while most patients with triple negative or HER2 positive tumors require cytotoxic treatment, patients with luminal disease are less likely to benefit from chemotherapy and can be spared an aggressive treatment in early stages if tumor proliferation is low.10,14,15
However, Ki67 levels may vary between CNB and subsequent SEB, and in case of neoadjuvant chemotherapy or heat ablation therapy, the CNB is the only source of material to assess Ki67 as a prognostic and predictive tool.2,16–21 Previous studies using varying Ki67 assessment methods and cutoff definitions report concordance rates of 53 to 82% between CNB and SEB for the Ki67-LI.2,16–21 However, information on factors that could potentially reduce or compensate sampling error and improve CNB reliability, like the volume of biopsy sample material or cutoffs adjusted to the CNB setting, are sparse2.
Our aim was therefore to study concordance rates of Ki67-LI between CNB and subsequent SEB of luminal breast cancers using two different Ki67 assessment methods recommended by the International Ki67 in Breast Cancer Working Group and the two cutoffs to distinguish high and low proliferation given by the St Gallen consensus panels 2015 and 2013.7,10,14 We investigated whether concordance rates were improved by CNB-specific cutoff definitions or higher CNB volume.
Methods
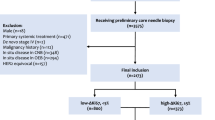
This study was approved by the Research Ethics Commission of the University Greifswald, Germany (protocol BB 143/15). One hundred seventy female breast cancer patients were selected out of 694 patients who had undergone surgery in the Dietrich Bonhoeffer Medical Centre and its associated hospitals from 2010 to 2013. Study inclusion criteria were: unifocal, unilateral invasive breast cancers either international union against cancer stage I or II; pathologic tumor size >5 to <50 mm; no neoadjuvant treatment; available presurgical ultrasound-guided CNB and subsequent surgical excision; ER positivity, HER2 negativity, and a progesterone receptor (PgR) status ≥20%; and either invasive lobular or cancers of no special type. Other special type cancers were excluded from the analysis due to their characteristic clinical behavior and outcome which do not necessarily require Ki67 assessment according to the St Gallen consensus recommendations.14,15
Fourteen (8.2%) patients had pT1b, 79 (46.5%) pT1c, and 77 (45.3%) had pT2 disease, respectively. 129 (76%) patients were pN0, 35 (21%) had one to three positive lymph nodes, and 6 (3%) had micro metastases only in the sentinel node. 37 (22%) patients had grade 1, 78 (45%) grade 2, and 56 (33%) grade 3 disease in the SEB. 140 tumors were breast cancers of no special type (82%) and 30 were invasive lobular cancers (18%). 50 cancers were screen detected (30%), and 120 cancers were non–screen detected (70%).
All SEB had been sampled within 30 min after excision by a specialized breast pathologist according to the Berlin-Buch protocol and the European guidelines for quality assurance in breast cancer screening and diagnosis for optimal tumor tissue fixation and minimal cold ischemia time.22,23
Immunohistochemistry
For immunohistochemical staining of ER, PgR, HER2 and Ki67, 4 µm thin tissue sections were dewaxed in xylene, acetone and Tris-buffered saline, followed by heat-induced epitope retrieval in pH 6.0 in a microwave oven (750 W). Sections were subsequently stained using Ki67 (clone MIB1, Dako, Glostrup, Denmark, 1:200), ERα (clone 1D5, Dako, 1:50), PgR (clone 636, Dako, 1:50) and HER2 (clone CB-11, Zytomed Systems, Berlin, Germany, 1:100) monoclonal antibodies. The sections were incubated with the primary antibody for 1 h using the Dako REAL-detection system based on the labelled streptavidin–biotin method (Dako), autostained (LINK48, Dako), and visualized with 3-amino-9-ethylcarbazole, giving a brown staining in a hematoxylin blue counterstaining. Normal breast tissue within the sections was used as internal positive and/or negative control.
ER, PgR and HER2 status were assessed on CNB according to the American Society of Clinical Oncology 2007 and 2010 recommendations.4,5 All tumors that were HER2 negative and grade 3 or had a PgR status <20% in the CNB were retested for the respective receptors in the SEB and results were adjusted accordingly.
Ki67-LI assessment
All cores of the CNB and the paraffin tissue block of the SEB with the highest mitotic activity in hematoxylin and eosin staining were used for Ki67-labelling. Slides were digitized at 40× with a high resolution slide scanner (SCN400, Leica Biosystems, Wetzlar, Germany) at the department of pathology of the Dietrich Bonhoeffer Medical Centre, and images were analyzed manually at 40× on a PC monitor by one breast pathologist (CMF) trained in Ki67 measurement and virtual microscopy. Two different protocols suggested by the International Ki67 in Breast Cancer Working Group were then applied for this study (Fig. 1)7. First is Ki67–510per; a total of 510 cancer cells was counted in hot spot (spot of highest proliferation) and two spots at the tumor periphery (170 cells each). Second is Ki67–510av; a total of 510 cancer cells was counted in 3 high-power field (170 cells each) including hot spot, cold spot (spot of lowest proliferation) and an area of intermediate proliferation.
First, the spot of 170 cells was outlined, touching each cell with the mouse cursor, starting from the left upper corner, moving to the right and back in a meandering fashion. Then, all positive cells within the spot were counted. Any nuclear staining of an invasive tumor cells was considered positive. The number of positive cells in the three spots was used to calculate the Ki67-LI of the tumor. When a field did contain <170 cells, it was expanded by counting additional tumor cells of a neighboring field. This occurred almost exclusively in invasive lobular cancers due to low tumor cell concentration. Areas of necrosis, inflammation, micro-invasion, poor section quality, insufficient fixation and ductal carcinoma-in situ were excluded from the analysis.
Assessment of Core Biopsy Volume Characteristics
Number of cores, total core length in mm, tumor tissue length in mm, CNB total area size (mm2) and tumor area size of the biopsy sample (mm2) were measured by the same observer on the digitized Ki67 stained slides using the Slidepath Digital Image Hub software version 4.0.4 (Leica Biosystems). Tumor tissue was defined as invasive epithelial cells and accompanying tumor stroma because both components correlate with the mass lesion seen on ultrasound.
Statistical Analysis
As Ki67 levels may vary among labs, the St Gallen consensus panel 2015 recommended to use the lab’s individual median Ki67-LI of luminal breast cancers as cutoff to discriminate tumors with low and high proliferation. To compare Ki67 levels in CNB and SEB, we used the respective assessment method-specific median Ki67-LI of the SEB as cutoff to distinguish between luminal A and luminal B (HER2 negative) cancers. Additionally, we applied the formerly recommended cutoff of 20% for both methods.14 To investigate whether concordance rates can be improved when the CNB cutoff is adjusted or assessment methods are mixed, we also used the CNB specific median Ki67-LI for CNB and the SEB specific median for SEB, and combined Ki67–510per in CNB with Ki67–510av in SEB.
We calculated Cohen’s κ and agreement between Ki67-LI of CNB and related SEB for each method as well as percentage of cases with over- and underestimated Ki67-LI in CNB.
To investigate intraobserver reproducibility for both Ki67 assessment methods, 30 randomly selected cases (15 CNB, 15 SEB) were reassessed 3 months after first analysis. The ratings were categorized according to the SEB specific median Ki67-LI of the respective assessment method into low and high proliferation, and Cohen’s κ and intrarater agreement were calculated.
We then tested the null hypothesis that there is no difference between median Ki67-LI of CNB and SEB for the Ki67–510p and the Ki67–510a method with a Mann–Whitney test. With additional Mann–Whitney tests we investigated whether CNB volume features and pathologic tumor size differed between concordant and discordant cases. p < 0.05 was assumed to indicate significant differences.
All statistical tests were performed with SPSS Statistics 23 (IBM SPSS, Chicago, IL, USA).
Results
Median Ki67-LI and standard deviation for the 170 luminal breast cancers were: 11.66 ± 10.8% for Ki67–510av in CNB, 15.69 ± 14.67% for Ki67–510av in SEB, 20.68 ± 17.2% for Ki67–510per in CNB, and 25.19 ± 16.69% for Ki67–510per in SEB, respectively. The Ki67-LI was substantially higher in SEB than in CNB for both assessment methods (Ki67–510av: p < 0.0001; Ki67–510per: p = 0.002).
Agreement rates between CNB and SEB, κ values, and percentage of over- and underestimated cases for both Ki67 assessment methods and different cutoff definitions are shown in Table 1. Highest concordance between CNB and SEB Ki67-LI was found using the Ki67–510av method with cutoffs of 20% (75% concordance, κ = 0.44). The combination of Ki67–510per in the CNB and Ki67–510av in the SEB showed lowest concordance and κ values, regardless of the applied cutoff.
Also the percentage of Ki67-LI overestimated cases in CNB was lowest using Ki67–510av with a cutoff of 20%. Lowest percentage of underestimated cases in CNB was found using Ki67–510av with the median Ki67-LI as threshold.
CNB volume characteristics for concordant and discordant cases for both assessment methods and respective cutoffs cases are shown in Table 2. CNB area size was borderline significantly higher in concordant than in discordant cases using the Ki67–510av method with a cutoff of 20% for both CNB and SEB (p = 0.048), but not for other cutoff definitions or for Ki67–510per. No substantial differences between concordant and discordant cases were found for number of cores, total core length, tumor tissue length in CNB, tumor area size in CNB, or pathologic tumor size.
Intraobserver reproducibility was good for both Ki67 assessment methods with concordance between first and second rating in 90% of cases (κ = 0.80) for Ki67–510av, and 93% (κ = 0.87) for Ki67–510per.
Discussion
The reliability of prognostic information in CNB has been subject of several studies on breast cancer, showing high diagnostic accuracy for detection of malignancy as well as for morphologic tumor characteristics and immunohistochemical biomarkers. However, important prognostic factors like histologic grade and mitotic count are known to be underestimated in the CNB in 20 to 30% of cases due to undersampling of the most proliferative tumor areas3. Some authors have suggested to overcome this issue by using the Ki67-LI while others found Ki67-LI underestimation rates in CNB similar to those of grade, indicating that assessment on SEB should be preferred.17,19,24 In neoadjuvant or experimental settings (e.g., heat ablation therapy), however, the CNB may be the only source of tumor material available, and reliability of prognostic information derived from the CNB is then of the essence.
As described by other authors, Ki67 levels were substantially lower in CNB than in SEB in our study.17,19 Applying the current recommendation of the International Ki67 in Breast Cancer Working Group, highest concordance (75%) between CNB and SEB of luminal breast cancers was found for Ki67–510av when 20% or the median Ki67-LI of the SEB was used as a cutoff, comparable to previously published rates.2,7,16–21
While underestimation of proliferation in CNB is also described to be a common sampling error for mitotic activity and histologic grade, the interpretation of ‘overestimated’ Ki67-LI in CNB is more complex.25–27 To date, there is no consensus whether a higher Ki67-LI in the CNB should replace a lower final Ki67 rate of the SEB or should rather be considered a sampling error corrected by the true Ki67-LI of the SEB. Published rates of higher Ki67-LI in CNB vary substantially from 6 to 20%, suggesting that overestimation rates could be affected by Ki67 assessment method selection and cutoff definition.18,19 Though the highest Ki67-LI of a tumor may indeed be found in the CNB by chance, this seems unlikely because overestimation of other proliferation-related prognostic factors like histologic grade is infrequent in CNB, occurring in <10%.26,27 In our study, the Ki67 assessment method that resulted in lowest overestimation rates showed at the same time highest overall concordance.
Although both over- and underestimation of Ki67-LI in the CNB can be assumed a sampling error, we did not find clear evidence for an association between improved CNB and SEB agreement and the volume of biopsy material available for Ki67 assessment. In our hands, there are four possible reasons why sampling error was not reduced with increasing sample size. First, the tumor periphery is underrepresented in CNB. In the periphery, Ki67 levels are usually higher than in the center, and hot spots are found most frequently 1–2 mm peripheral from the border between tumor and benign tissue.28 Assuming a spherical tumor configuration and respective different relative volume fractions of the outer shell and center, the CNB procedure which takes a cylindrical tissue sample along an axis perpendicular to the tumor border will contain exponentially more tissue of the center than of the periphery. Second, the amount of material could still be too low to make a significant impact. The median number of four cores per biopsy set in our series is comparable to two previous studies showing moderate to high concordance of 73 to 82% between CNB and SEB.2,16 Lower concordance (65%; κ = 0.29) was found in one study with a median number of two cores taken per patient.17 Other authors, however, did not report CNB characteristics.18–20 However, our finding that Ki67-LI concordance between CNB and SEB seems not to be influenced by CNB volume is supported by Greer et al., who described only slightly improved agreement rates for cases with one to five compared to more than six cores.2 Third, we decided to include the stromal component in the definition of tumor tissue because both epithelial and stromal components correlate with the mass seen and targeted on ultrasound, but Ki67-LI was assessed only in the epithelial component. Fourth, the finding that concordance rates did not improve neither with increased CNB volume nor different cutoff definitions could also indicate the limitations of the specific Ki67-LI assessment methods to adequately represent intratumoral heterogeneity.
Efforts to optimize presurgical diagnostics by adjusting prognostic cutoffs specifically to the biopsy setting have been made for histologic grade but showed only slight or no improvement of overall agreement between CNB and SEB.29,30 As Ki67-LI were significantly lower in CNB than in the SEB in our study, we lowered the CNB cutoff to the specific CNB median Ki67-LI and combined hot spot Ki67 assessment in CNB with the average Ki67 assessment method in SEB to reduce rates of underestimated proliferation in the biopsy sample. Our approach did result in a decrease of underestimated cases in CNB, actually, but did not improve overall concordance and increased the rate of overestimated cases in CNB instead. It should be noted, though, that unlike mitotic scores of histologic grade, the Ki67-LI cutoffs used in our study are not based on prospective prognostic studies but rather on current expert consensus recommendations and that the Ki67 levels described herein are specific for our lab in a subset of luminal tumors.10,14,31
Although low biopsy volume did not affect the reliability of Ki67 assessment in the CNB and the number of cancer cells per tumor actually counted was only 510, we cannot provide a specific minimum total number of tumor cells that should be contained in the biopsy sample for reliable Ki67 assessment based on our data. In this context it is of note that the selection of regions to be counted is based on the relative differences of Ki67 expression in the defined spots. The selected spots, however, were almost never neighboring directly, indicating that a total amount of tumor cells higher than 510 is needed for adequate spot selection.
In conclusion, concordance rates between CNB and SEB of luminal breast cancers of 75% can be achieved using a straightforward method assessing average CNB Ki67-LI at the usual threshold of 20%. Neither CNB-specific cutoffs nor increased CNB volume resulted in improved CNB versus SEB agreement rates, indicating that reliability of Ki67 levels in CNB of luminal breast cancers is unaffected by CNB volume.
References
Nassar A, Radhakrishnan A, Cabrero IA, et al. Intratumoral heterogeneity of immunohistochemical marker expression in breast carcinoma: a tissue microarray–based study. Appl Immunohistochem Mol Morphol. 2010;18:433–41.
Greer LT, Rosman M, Mylander WC, et al. Does breast tumor heterogeneity necessitate further immunohistochemical staining on surgical specimens? J Am Coll Surg. 2013;216:239–51.
Rakha EA, Ellis IO. An overview of assessment of prognostic and predictive factors in breast cancer needle core biopsy specimens. J Clin Pathol. 2007;60:1300–6.
Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. 2010;134:e48–72.
5. Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43.
Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013.
Dowsett M, Nielsen TO, A’hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst. 2011;103:1656–64.
Denkert C, Budczies J, von Minckwitz G, Wienert S, Loibl S, Klauschen F. Strategies for developing Ki67 as a useful biomarker in breast cancer. Breast. 2015:24:S67–S72
Luporsi E, Andre F, Spyratos F, et al. Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat. 2012;132:895–915.
Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies—improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015. doi:10.1093/annonc/mdv221.
Hayes DF. From genome to bedside: are we lost in translation? Breast. 2013;22(Suppl 2):S22–6.
12. Hayes DF. Biomarker validation and testing. Mol Oncol. 2015;9:960–6.
Guiu S, Michiels S, Andre F, et al. Molecular subclasses of breast cancer: how do we define them? The IMPAKT 2012 Working Group statement. Ann Oncol. 2012;23:2997–3006.
Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24:2206–23.
Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22:1736–47.
Ricci MD, Calvano Filho CM, Oliveira Filho HR, et al. Analysis of the concordance rates between core needle biopsy and surgical excision in patients with breast cancer. Rev Assoc Med Bras. 2012;58:532–6.
Knutsvik G, Stefansson IM, Aziz S, et al. Evaluation of Ki67 expression across distinct categories of breast cancer specimens: a population-based study of matched surgical specimens, core needle biopsies and tissue microarrays. PLoS ONE. 2014;9:e112121.
Romero Q, Bendahl PO, Klintman M, et al. Ki67 proliferation in core biopsies versus surgical samples—a model for neo-adjuvant breast cancer studies. BMC Cancer. 2011;11:341.
Chen X, Sun L, Mao Y, et al. Preoperative core needle biopsy is accurate in determining molecular subtypes in invasive breast cancer. BMC Cancer. 2013;13:390.
Connor CS, Tawfik OW, Joyce AJ, et al. A comparison of prognostic tumor markers obtained on image-guided breast biopsies and final surgical specimens. Am J Surg. 2002;184:322–4.
Ough M, Velasco J, Hieken TJ. A comparative analysis of core needle biopsy and final excision for breast cancer: histology and marker expression. Am J Surg. 2011;201:692–4.
22. Decker T, Ruhnke M, Schneider W. [Standardized pathologic examination of breast excision specimen. Relevance within an interdisciplinary practice protocol for quality management of breast saving therapy]. Pathologe. 1997;18:53–9.
Perry N, Broeders M, de WC, et al. European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition—summary document. Ann Oncol. 2008;19:614–22.
Kwok TC, Rakha EA, Lee AH, et al. Histological grading of breast cancer on needle core biopsy: the role of immunohistochemical assessment of proliferation. Histopathology. 2010;57:212–9.
Harris GC, Denley HE, Pinder SE, et al. Correlation of histologic prognostic factors in core biopsies and therapeutic excisions of invasive breast carcinoma. Am J Surg Pathol. 2003;27:11–5.
26. Daveau C, Baulies S, Lalloum M, et al. Histological grade concordance between diagnostic core biopsy and corresponding surgical specimen in HR-positive/HER2-negative breast carcinoma. Br J Cancer. 2014;110:2195–200.
27. Zheng J, Alsaadi T, Blaichman J, et al. Invasive ductal carcinoma of the breast: correlation between tumor grade determined by ultrasound-guided core biopsy and surgical pathology. AJR Am J Roentgenol. 2013;200:W71–4.
Gudlaugsson E, Skaland I, Janssen EA, et al. Comparison of the effect of different techniques for measurement of Ki67 proliferation on reproducibility and prognosis prediction accuracy in breast cancer. Histopathology. 2012;61:1134–44.
O’Shea AM, Rakha EA, Hodi Z, et al. Histological grade of invasive carcinoma of the breast assessed on needle core biopsy—modifications to mitotic count assessment to improve agreement with surgical specimens. Histopathology. 2011;59:543–8.
30. Dhaliwal CA, Graham C, Loane J. Grading of breast cancer on needle core biopsy: does a reduction in mitotic count threshold improve agreement with grade on excised specimens? J Clin Pathol. 2014;67:1106–8.
Rakha EA, El-Sayed ME, Lee AH, et al. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol. 2008;26:3153–8.
Disclosure
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Focke, C.M., Decker, T. & van Diest, P.J. Reliability of the Ki67-Labelling Index in Core Needle Biopsies of Luminal Breast Cancers is Unaffected by Biopsy Volume. Ann Surg Oncol 24, 1251–1257 (2017). https://doi.org/10.1245/s10434-016-5730-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5730-1