Abstract
Background
High morbidity, increased mortality, and impaired long-term oncologic outcome have been reported after deep surgical site infection (SSI) in rectal cancer surgery. The rate, risk factors and consequences of deep SSI after (chemo)radiotherapy [(C)RT], and surgery for locally advanced rectal cancer (LARC) in a tertiary university hospital single centre cohort of 540 patients are presented.
Methods
Patients with LARC, operated between January 1, 2007 and December 31, 2015, were identified in the institutional prospective database. All patients had tumours threatening the mesorectal fascia or invading adjacent organs, with a high rate of T4 tumours (60 %), and all received (C)RT. Risk factors for deep SSI were calculated by multivariable logistic regression analysis. Morbidity data were assessed. Overall survival (OS) and disease-free survival (DFS) between patients with or without deep SSI were estimated.
Results
Of 540 patients, 104 (19 %) experienced a deep SSI, with the highest rate in the abdominoperineal resection (APR) group with 25 %. APR, good response to (C)RT (low tumour regression grade), age, and operative blood loss were identified as significant (P < 0.05) risk factors for deep SSI in multivariable analysis. No difference was found in OS (P = 0.995) or DFS (P = 0.568). Hospital stay increased with 5 days (P < 0.001), and complete wound healing at the 3-month follow-up decreased from 86 to 45 % (P < 0.001) after deep SSI.
Conclusions
Deep SSI is a frequent and major complication after rectal surgery for LARC, with high morbidity, increased hospital stay and protracted wound healing. Interestingly, deep SSI did not influence long-term oncologic outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Locally advanced rectal cancer (LARC) includes tumours threatening the mesorectal fascia or invading adjacent organs and structures.1 Preoperative (chemo)radiotherapy [(C)RT] for the treatment of LARC have increased survival and improved local control, presumably by shrinking or down-staging tumours to improve the rate of complete surgical removal to reduce the incidence of local recurrence.2,3 After the broad introduction of (C)RT in the mid 2000s, reports have emerged on a concomitant rise in deep surgical site infection (SSI), including anastomotic leak (AL) and wound dehiscence.4 Factors, such as fibrosis, inflammation, and compromised microcirculation, in the irradiated pelvic cavity have been suggested as causative.5 – 7 Deep SSI is a great challenge both for the patient and the health care system, as for instance time to heal after perineal wound dehiscence can exceed 100 days.8
In the present study, the frequency of deep SSI within 30 days after primary surgery is presented from a 9-year, single-centre cohort of LARC patients, all receiving preoperative (C)RT. Risk factors and consequences for short-term outcome and long-term oncologic outcome are assessed and discussed.
Methods
Study Cohort and Treatment
Between January 1, 2007 and December 31, 2015, 574 patients underwent surgery for LARC (≤15 cm from the anal verge by rigid proctoscopy). Our institution is a tertiary university hospital referral centre that provides multimodal treatment of LARC. The patients are highly selected, all with tumours or tumour deposits threatening the mesorectal fascia or invading adjacent organs or structures and with a particularly high rate of T4 cancers (60 %). The rate of abdominoperineal resections (APR) is also high, as advanced tumours located in the lower rectum are overrepresented. Patients received neoadjuvant RT given as short- (5 Gray (Gy) × 5) (n = 49 patients) or long-course (2 Gy × 25) (n = 491 patients) regimens. Thirty-four patients did not receive an RT dose of at least 25 Gy and were excluded from analysis. The resulting study population thus included 540 patients. The mean time from end radiation to surgery was 8.5 weeks for both short- and long-course RT. Only five patients had surgery within 4 weeks of short-course RT. Concomitant fluoropyrimidine-based chemotherapy was given to patients receiving long-course RT (determined by the treating oncologist). The patients were operated with either of three open surgical methods: low anterior resection (LAR), rectal resection with end colostomy (Hartmann’s operation), or APR.
Pretreatment workup comprised chest and abdominal computed tomography (CT), pelvic magnetic resonance imaging (MRI), and proctoscopy. Radiological assessment was scheduled at baseline and at 4 weeks after completion of neoadjuvant (C)RT. Adjuvant chemotherapy was not routinely given, in accordance with national guidelines for treatment of rectal cancer in Norway.
The study cohort was identified from the prospective institutional colorectal database. Missing data and preoperative comorbidity data were retrospectively collected from patients’ records. Survival data were obtained from the National Registry of Norway on April 11, 2016, and patients alive at this date were censored.
Definition of Deep SSI
Deep SSI was defined as a characteristic collection of fluid in the pelvic cavity on CT with concurrent clinical or biochemical signs of infection. Such signs included fever, increased heart and respiratory rates, deterioration of general condition, ileus, nausea, vomiting and abdominal pain, or biochemical signs, such as rise in C-reactive protein and/or white blood cell count. In addition, clinically or radiologically confirmed anastomotic leak and rupture of the closed rectum after Hartmann’s procedure were defined as deep SSI. Deep SSI within the first 30 days after surgery was included in this study.
Definition of Wound Healing
In the APR group, wound healing was defined as complete healing of the perineal incision. Rectal closure without defect, as assessed clinically by digital rectal exploration signified wound healing in the Hartmann group. An intact colorectal anastomosis after reversal of the temporary loop ileostomy signified wound healing in the LAR group.
Pathology
Surgical specimens were classified according to the TNM system. “N+” included both N1 and N2 stage. Tumour regression grade (TRG) was classified according to Bouzourene et al. to assess histological tumour response to preoperative RT.9 A circumferential resection margin (CRM) >1 mm signified complete resection or R0, a margin ≤1 mm or microscopically involved margins were classified as R1, and macroscopic residual cancer, R2.
Statistical Analyses
Associations between patient, tumour, and operative characteristics and the presence of deep SSI were calculated by Fisher’s exact test for categorical variables and independent samples t test for continuous variables. Factors significant in each association analysis in addition to age and gender were further examined using multivariable logistic regression analysis. Receiver operating characteristics (ROC) analyses were performed for continuous variables that were associated with deep SSI to investigate clinically relevant cutoffs. The Kaplan–Meier method was used to estimate OS with or without deep SSI, defined from the time of rectal surgery to death or the censoring date April 11, 2016, and DFS from rectal surgery to the time of local recurrence, distant metastases, last follow-up, death, or censoring date. Only patients with localised disease at time of definitive surgery were included in the survival analyses. Patients had follow-up for 5 years postoperatively. Survival curves were compared using log rank test. P values <0.05 were considered statistically significant. Statistical analyses were conducted using SPSS software (version 21.0, IBM SPSS, Chicago, IL).
Ethics
The project was approved by the Regional Ethics Committee of South-East Norway (2014/1188), and written informed consent was obtained.
Results
Patient and Tumour Characteristics
Patient characteristics are presented in Table 1. Of the total cohort, 249 patients (46 %) underwent APR, 170 (32 %) underwent LAR, and 121 (22 %) had Hartmann’s resection. Median age was 64 (range, 31–88) years; 207 patients (38 %) were female and 333 (62 %) were male. The American Society of Anesthesiologists physical status classification system (ASA) score was 1–2 in most patients (421 patients, 79 %) and 3–4 in 114 (21 %) patients. Median operating time was 265 (range, 114–835) min, and median operative blood loss was 7.5 (range, 1.0–130) dL. Multivisceral resections were performed in 319 patients (60 %).
In Table 2, tumour characteristics are presented. Six patients with cT2 (1 %) and 214 patients with cT3 tumours (40 %) were given neoadjuvant (C)RT as the primary tumour, tumour deposits, or lymph node metastases threatened or invaded the mesorectal fascia. The remaining majority of 320 patients had cT4 tumours (59 %). Local lymph node metastases were suspected in 449 patients (84 %). Distant metastases (cM+) were diagnosed in 93 patients (18 %). Thirty-seven patients (7 %) were labelled cMx.
Upon histopathological examination, 145 of the surgical specimens (27 %) were classified as ypT0-2 and 395 (73 %) were ypT3-4. Fifty-five cases (10 %) were diagnosed with poorly differentiated tumours, 366 (68 %) were moderately differentiated and 22 (4 %) were well-differentiated. In 97 cases (18 %), tumour differentiation could not be determined, reflecting the state of the tissue after response to (C)RT. Metastatic local lymph nodes were found in 198 of the cases (37 %). The circumferential resection margin (CRM) was free in 464 specimens (86 %), whereas 74 cases (14 %) had a resection margin ≤1 mm (R1). Only two patients had macroscopically remaining tumour tissue (R2). TRG assessment showed 249 tumours (48 %) with TRG 1–2 and 268 (52 %) with TRG 3–5.
Deep SSI, Microbiology, Treatment and Wound Healing
Of the 540 patients, 104 (19 %) had a deep pelvic SSI (Table 1). The rates in the three surgical groups were 62 (25 %) in the APR group, 21 (12 %) in the LAR group, and 21 (17 %) in the Hartmann’s resection group. Most patients needed an invasive procedure to treat the infection, as 58 (56 %) were treated with insertion of a radiological-guided pelvic drain and 44 (42 %) with operative drainage (Table 1).
Microbiological culture was performed in 86 of the patients (83 %) and revealed a range of microbes (Table S1, Supporting Information), the most frequent of which were Escherichia coli with growth in 31 cases (36 %). No anaerobic bacteria were cultured, and Candida albicans was cultured in only three patients (4 %).
Complete wound healing was registered at clinical follow-up after 3 months in 391 cases (72 %), in 108 cases (20 %) healing was not complete, and in 41 cases data were missing (8 %; Table 1). In patients with deep SSI, healing was complete in only 44 cases (45 %) compared with 347 (86 %) in patients without deep SSI.
Risk Factors for Development of Deep SSI
Gender (P = 0.004), type of surgical procedure (P = 0.005), body mass index (P = 0.033), perioperative blood loss (P = 0.001), operating time (P = 0.001), and TRG (P = 0.010) were identified as risk factors for development of deep SSI and were included in the multivariable analysis, in addition to age at the time of surgery (Tables 3 and 4). APR (odds ratio [OR] 2.25; 1.14–4.43; P = 0.019), low TRG (OR 2.12, 1.25–3.61; P = 0.006), age at the time of surgery (OR 0.97, 0.94–0.99; P = 0.005), and operative blood loss (OR 1.02, 1.00–1.04; P = 0.038) were identified as risk factors for deep SSI in the multivariable analysis
A range of other potential patient, tumour, and operation variables, including diabetes, COPD/asthma, smoking, immunosuppressive drugs, ASA, multivisceral resection, preoperative T and N stage (cTN), postoperative T and N stage (ypTN), tumour differentiation grade, and CRM were not associated with deep SSI (Tables 3 and 4). Body mass index, operating time, and operative blood loss were included in ROC calculations, but no clinically relevant cutoff was found for either of these variables (data not shown).
Survival Analyses
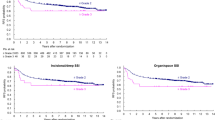
The 5-year OS was estimated to 71 % in the patients without deep SSI and 75 % in the patients with deep SSI (Fig. 1a; P = 0.995). The 5-year DFS was estimated to 57 % in the patients without deep SSI and 65 % in the patients with deep SSI (Fig. 1b; P = 0.568).
Hospital Stay and Wound Healing
Patients with deep SSI had prolonged postoperative hospital stays with a median of 15 (range, 4–72) days compared with patients without SSI with a median of 10 days (range, 3–54 days; P < 0.001).
Incomplete wound healing after 3 months was registered in 53 patients (55 %) with deep SSI compared with 55 patients (14 %) without deep SSI (P < 0.001). In the APR group, 32 (54 %) perineal wounds were not healed after 3 months in the deep SSI group compared with 43 cases (25 %) without deep SSI (P < 0.001).
Discussion
The frequency of deep SSI seemed to be in line with previously published studies. As RT has become standard treatment in LARC to improve local control, the incidence of deep SSI and wound complications have increased.3,10 – 13 However, the frequency of deep SSI, including AL after rectal surgery, varies considerably in the literature, as some have reported no infections, whereas others report up to 40 %.4,14 Definition of deep SSI, heterogeneous cohorts, and low number of cases in the studies may partly explain the variable results. Two recent studies, including only LARC patients receiving preoperative RT, reported deep SSI rates of 10 and 30 % in the LAR group and 10 and 40 % in the APR group respectively.4,15
The particularly high risk of deep SSI after APR is noteworthy, and APR was found to be an independent risk factor in our cohort, which is in line with previous reports.16 Several factors may explain this finding. A large dead space created in the pelvis after removal of the entire rectum and anus will accumulate fluid that may promote bacterial growth.17 Also, injury to the peritoneum will evoke inflammation, leading to activation of innate cascade systems and a procoagulant state, which in turn will predispose for infection.7 Finally, impaired microcirculation and thereby reduced oxygen supply, a well-known effect of RT, may be a particular challenge to the healing process in the remnants of the pelvic floor.5
Interestingly, patients with tumours that responded well to RT (TRG 1–2) had a significantly increased risk of deep SSI. To our knowledge, this has not been reported previously. Good responders may have increased local inflammation surrounding the tumour predisposing for SSI, as inflammatory products increase the risk of infection.7 The local tissue toxicity and systemic effects of (C)RT contributes to impaired wound healing, but whether this effect is more pronounced in good responders and thereby could explain the observed difference is unknown.18 However, the finding is interesting and further studies on biology of the irradiated pelvis using, e.g., microdialysis to retrieve metabolites and inflammatory mediators are warranted.19,20
Operative blood loss is known to be a risk factor for deep SSI, which also was found in our study.15 Previous studies have defined specific cutoffs with respect to blood loss that predict the risk off SSI; however, cutoffs have varied 40- to 50-fold between studies.15,21 In our cohort, ROC calculations did not reveal a clinically relevant threshold. Younger age was identified as a risk factor for deep SSI. Age correlation varies between studies; the majority found no correlation, whereas others have found correlation with both younger and older age.15,21 – 24
Microbiological culture revealed a range of bacteria. No anaerobic bacterial species were detected, and only three patients had growth of C. albicans. All patients received preoperative metronidazole and doxycycline as standard infection prophylaxis. In Norway, there is little metronidazole resistance among anaerobes, which seems to be reflected in our results, and fungal infection was rare and not a clinical problem in these patients. There is not much data in Norway regarding resistance to doxycycline against many of the bacteria found in these bacterial cultures (Specialist in Microbiology, Gorm Hansen, MD, personal communication). Thus, it seems that the anaerobic prophylaxis was adequate, while it is difficult to know if the patients would have benefitted from improved aerobic prophylaxis.
No associations were found between the occurrence of deep SSI and long-term outcome (DFS and OS). Interestingly, previous publications vary widely in this respect. Some have presented increased risk of local recurrence or impaired DFS, particularly after AL, whereas others have found no correlation with outcome neither after AL, nor after deep SSI after APR.12,25 – 28 Exfoliated cancer cells that are implanted in the pelvic cavity, in addition to a potential proinflammatory drive to boost tumour growth have been suggested as explanation for local recurrence after AL, but without clear evidence.25 As with DFS, OS has been reported to be reduced after deep SSI in both APR and LAR, whereas others have found no difference.8,12,27
The median hospital stay increased with 5 days after deep SSI, already at this time point signifying an adverse outcome for these patients. In 55 % of the patients with deep SSI, wound healing was not complete at 3-month follow-up, in contrast to 14 % in the patients without. Wound healing after deep SSI is a major challenge; in a study of wound dehiscence in APR patients, the mean healing time was 117 days with a range of up to 1096 days.8 Delayed wound healing up to 1 year is common, and some patients will have to live with chronic perineal wounds.11
Conclusions
Deep SSI was a frequent and severe complication, but no associations with long-term oncologic outcome were detected. However, the morbidity was high with increased hospital admission time and reduced wound healing. Further studies, including basal studies on the biology of the pelvic cavity after resection, are warranted to understand the mechanisms promoting this frequent complication.
References
Berardi R, Maccaroni E, Onofri A, et al. Locally advanced rectal cancer: the importance of a multidisciplinary approach. World J Gastroenterol. 2014;20(46):17279–87.
Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40.
Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–33.
Schiffmann L, Wedermann N, Gock M, et al. Intensified neoadjuvant radiochemotherapy for rectal cancer enhances surgical complications. BMC Surg. 2013;13:43.
de Lussanet QG, Backes WH, Griffioen AW, et al. Dynamic contrast-enhanced magnetic resonance imaging of radiation therapy-induced microcirculation changes in rectal cancer. Int J Radiat Oncol Biol Phys. 2005;63(5):1309–15.
Krol R, Hopman WP, Smeenk RJ, Van Lin EN. Increased rectal wall stiffness after prostate radiotherapy: relation with fecal urgency. Neurogastroenterol Motil. 2012;24(4):339-e166.
Sammour T, Kahokehr A, Soop M, Hill AG. Peritoneal damage: the inflammatory response and clinical implications of the neuro-immuno-humoral axis. World J Surg. 2010;34(4):704–20.
Hawkins AT, Berger DL, Shellito PC, Sylla P, Bordeianou L. Wound dehiscence after abdominoperineal resection for low rectal cancer is associated with decreased survival. Dis Colon Rectum. 2014;57(2):143–50.
Bouzourene H, Bosman FT, Seelentag W, Matter M, Coucke P. Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer. 2002;94(4):1121–30.
Bullard KM, Trudel JL, Baxter NN, Rothenberger DA. Primary perineal wound closure after preoperative radiotherapy and abdominoperineal resection has a high incidence of wound failure. Dis Colon Rectum. 2005;48(3):438–43.
Artioukh DY, Smith RA, Gokul K. Risk factors for impaired healing of the perineal wound after abdominoperineal resection of rectum for carcinoma. Colorectal Dis. 2007;9(4):362–7.
Musters GD, Sloothaak DA, Roodbeen S, van Geloven AA, Bemelman WA, Tanis PJ. Perineal wound healing after abdominoperineal resection for rectal cancer: a two-centre experience in the era of intensified oncological treatment. Int J Colorectal Dis. 2014; 29(9):1151–7.
van der Vaart MG, van der Zwet WC, Arends JW, Eeftinck Schattenkerk M, Eddes EH. Rectal carcinoma treated with short-term preoperative radiotherapy followed by abdominoperineal resection. Significantly more presacral abscesses with absence of local recurrence. Dig Surg. 2006;23(3):173–7 (Discussion 177–8).
Sato T, Ozawa H, Hatate K, et al. A Phase II trial of neoadjuvant preoperative chemoradiotherapy with S-1 plus irinotecan and radiation in patients with locally advanced rectal cancer: clinical feasibility and response rate. Int J Radiat Oncol Biol Phys. 2011;79(3):677–83.
Vermeer TA, Orsini RG, Daams F, Nieuwenhuijzen GA, Rutten HJ. Anastomotic leakage and presacral abscess formation after locally advanced rectal cancer surgery: incidence, risk factors and treatment. Eur J Surg Oncol. 2014;40(11):1502–9.
Kamrava A, Mahmoud NN. Prevention and management of nonhealing perineal wounds. Clin Colon Rectal Surg. 2013;26(2):106–11.
Pang J, Broyles JM, Berli J, et al. Abdominal- versus thigh-based reconstruction of perineal defects in patients with cancer. Dis Colon Rectum. 2014;57(6):725–32.
Baur M, Horvath M, Stattner S, et al. Chemoradiotherapy with 5-fluorouracil/leucovorin, surgery and adjuvant chemotherapy for locally advanced rectal cancer. Oncol Lett. 2010;1(1):189–94.
Waelgaard L, Pharo A, Tonnessen TI, Mollnes TE. Microdialysis for monitoring inflammation: efficient recovery of cytokines and anaphylotoxins provided optimal catheter pore size and fluid velocity conditions. Scand J Immunol. 2006;64(3):345–52.
Matthiessen P, Strand I, Jansson K, et al. Is early detection of anastomotic leakage possible by intraperitoneal microdialysis and intraperitoneal cytokines after anterior resection of the rectum for cancer? Dis Colon Retcum. 2007;50(11):1918–27.
Telem DA, Chin EH, Nguyen SQ, Divino CM. Risk factors for anastomotic leak following colorectal surgery: a case-control study. Arch Surg. 2010;145(4):371–6 (Discussion 376).
Pommergaard HC, Gessler B, Burcharth J, Angenete E, Haglind E, Rosenberg J. Preoperative risk factors for anastomotic leakage after resection for colorectal cancer: a systematic review and meta-analysis. Colorectal Dis. 2014;16(9):662–71.
Althumairi AA, Canner JK, Gearhart SL, Safar B, Sacks J, Efron JE. Predictors of perineal wound complications and prolonged time to perineal wound healing after abdominoperineal resection. World J Surg. 2016;40(7):1755–62.
Althumairi AA, Canner JK, Gearhart SL, et al. Risk factors for wound complications after abdominoperinal resection: analysis of the ACS NSQIP database. Colorectal Dis. 2016;18(7):O260-6.
Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011;253(5):890–9.
Ptok H, Marusch F, Meyer F, et al. Impact of anastomotic leakage on oncological outcome after rectal cancer resection. Br J Surg. 2007;94(12):1548–54.
den Dulk M, Marijnen CA, Collette L, et al. Multicentre analysis of oncological and survival outcomes following anastomotic leakage after rectal cancer surgery. Br J Surg. 2009;96(9):1066–75.
Eriksen MT, Wibe A, Norstein J, Haffner J, Wiig JN, Norwegian Rectal Cancer G. Anastomotic leakage following routine mesorectal excision for rectal cancer in a national cohort of patients. Colorectal Dis. 2005;7(1):51–7.
Acknowledgment
The authors thank the staff at the Department of Surgery at the Norwegian Radium Hospital Oslo University Hospital for their daily efforts and dedication for the patients. A special thanks goes to Veronica M. Alexandersen for her invaluable help with sorting and plotting of patient data.
Disclosure
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. G. Larsen and K. Flatmark have contributed equally to the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thorgersen, E.B., Goscinski, M.A., Spasojevic, M. et al. Deep Pelvic Surgical Site Infection After Radiotherapy and Surgery for Locally Advanced Rectal Cancer. Ann Surg Oncol 24, 721–728 (2017). https://doi.org/10.1245/s10434-016-5621-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5621-5