Abstract
Background
Breast-conserving surgery (BCS) is the preferred surgical approach for the majority of patients with early-stage breast cancer. There are frequent issues regarding pathologic margin status, requiring margin re-excision, and, in the literature, there is significant variability in re-excision rates, suggesting this is a potential quality-of-care issue. Understanding the patient-, disease-, and physician-related factors influencing reoperation rates is of importance in an effort to minimize this occurrence.
Methods
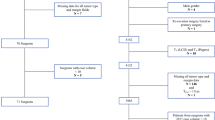
A retrospective analysis of all patients referred to our cancer center over a 3-year period (1 January 2011–31 December 2013) was performed. Surgeon volume, and patient- and tumor-related factors were assessed for their impact on re-excision rates. Multivariate logistic regression analysis was performed to identify variables of significance influencing reoperation rates after attempted BCS.
Results
Overall, 594 patients underwent initial BCS, with 159 (26.8%) patients requiring at least one re-excision to ensure negative pathologic margins. On multivariate analysis, low surgeon case volume, patient age (under 46 years of age), tumor size (>2 cm), and lobular carcinoma were associated with an increased re-excision rate.
Conclusion
Re-excisions are frequent after BCS and are influenced by surgeon volume, patient age, and tumor-related factors. These factors should be considered when counseling patients considering BCS, and also for quality assurance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Breast-conserving surgery (BCS) is the preferred surgical approach for the majority of patients with early breast cancer. While providing patients the benefit of maintaining their breast, a potential downside of this option is the high rate of reoperation required to obtain negative pathological margins. There is significant variability in the need for reoperation, with institutions reporting a range of 0–60 %,1 while larger population-based studies range from a low of 17 % to a high of 35 %.2 – 6
While re-excision is safe from an oncologic perspective,7 it has been shown to be associated with higher complication rates,8 negatively impacts cosmesis,9 , 10 and may influence patient satisfaction, as well as impact the costs associated with surgical management of breast cancer.11 Furthermore, re-excision after BCS has been suggested as one of several quality indicators related to breast cancer surgery.12 – 14
Both patient-2 , 3 , 5 and disease-related factors2 , 3 , 5 , 15 have been shown to influence the need for reoperation in patients undergoing BCS. In addition, low surgical case volumes on the part of both the surgeon2 , 16 and the institution2 , 17 have been shown to be associated with higher reoperation rates. We sought to determine what factors are predictive of the need for reoperation in our population.
Methods
Approval from the University of British Columbia Research Ethics Board was obtained for a retrospective chart review of patients referred to the British Columbia Cancer Agency Sindi Ahluwalia Hawkins Center for the Southern Interior (BCCA SAH-CSI) between 1 January 2010 and 31 December 2012. The BCCA is the only institution that provides radiation and medical oncology services within the province, with the BCCA SAH-CSI being one of six regional cancer centers within the province.
The BCCA SAH-CSI is the referral center for patients undergoing breast cancer surgical services within nine regional hospitals. In total, 36 general surgeons and 1 surgical oncologist provide these surgical services, each working in one facility. Each hospital has their own dedicated anatomic pathologists, each expected to comply with a regional standardized protocol. In complex cases, second opinions are obtained from either one of the higher-volume anatomic pathologists within the region, or referred to a core group of provincially based BCCA pathologists. Patients identified as having ductal carcinoma in situ, as well as invasive ductal carcinoma and lobular carcinoma were included for analysis. Cases with both in situ and invasive components were classified as malignant. During this time period, the BCCA recommended a pathologic margin of 2 mm in patients with in situ invasive breast cancer. To facilitate identification of patients undergoing BCS for early-stage breast cancer, we excluded patients who were identified as having any of the following:
-
Males
-
Age <18 years
-
Previous or recurrent breast cancer
-
T4 cancer on final histology
-
Initial mastectomy
-
Initial biopsy or surgical care outside the geographic catchment area of the BCCA SAH-CSI.
Data collected included patient age, as well as tumor-related factors [tumor size, grade, histology, pathologic margin status, estrogen receptor/progesterone receptor (ER/PR) status, human epidermal growth factor receptor 2 (HER2) status]. Pathologic margin status was divided into five categories: positive (tumor on ink), close but negative (no tumor on ink or <1 mm), 1–2 mm, negative (>2 mm), and indeterminate.
The method of biopsy required to obtain a pathologic cancer diagnosis was also collected, including image-guided core biopsies and surgical biopsies (wire localized, incisional, or excisional biopsies). Surgical biopsies performed for diagnostic purposes were not classified as BCS. Subsequent surgical procedures after a diagnostic surgical biopsy or image-guided biopsy were then considered to be the initial BCS in an attempt to minimize the influence of diagnostic biopsy method on the need for re-excision. Subsequent breast procedures performed after the initial BCS (re-excision or mastectomy) are considered a ‘re-excision’.
The influence of surgeon annual case volume was also considered, and classified as low (1–4 cases/year), intermediate (5–9 cases/year), high (10–24 cases/year), and very high volume (>25 cases/year). Information was also collected on the facility procedure volume, but, on exploratory analysis, no connection with the main outcome of interest (re-excision rate) was identified.
Data were collected on a Microsoft Excel spread sheet. Univariate analysis was performed on all variables described, and those values that had a low p-value of ≤0.2) were included in the multivariate model. Pathologic margin status was removed from multivariate analysis given its dominant effect on demographic-, tumor-, and surgeon-related factors predicting the need for re-excision.
Results
Over a 3-year period, 581 patients underwent attempted BCS. The majority of cases were ER-positive, intermediate-grade ductal carcinomas, and between 1 and 3 cm (Table 1). Overall, 23.8 % of patients were pathologically lymph node-positive; 13.8 % (80 of 581) required a surgical biopsy to establish a cancer diagnosis, and in 1 % of patients, the method of diagnosis was not available. Margins were pathologically positive in 17.4 % of patients after initial attempted BCS, with the remainder being ‘close but negative’ (13.3 %), 1–2 mm (18.1 %), and negative (49.8 %).
Overall, 25.1 % (146 of 581 patients) underwent reoperation to address concerns regarding the pathological status of surgical margins. Surgeons performing >25 cases per year had the lowest re-excision rate (17.8 %). As surgeon volumes decreased, re-excision rates increased in the high- (22.3 %), intermediate- (30.4 %), and low-volume surgeons (33.3 %) (Fig. 1). Less variability was noted in the reoperation rates within the very-high-volume surgeons (range 13–21 %) compared with the low-volume surgeons (range 14–71 %).
On univariate analysis, age <44 years, tumors >30 mm, lobular carcinoma, high-grade tumors, and low surgeon case volume were associated with a higher reoperation rate (Table 2). Pathologic margin status, including positive, close but negative, and 1–2 mm, were also highly associated with re-excision, but, due to their dominant effect on the need for re-excision, were excluded from the multivariate analysis.
On multivariate analysis, all factors from the univariate analysis maintained their significance on re-excision, except tumor grade (Table 3). Patients over 75 years of age (compared with those <45 years of age) were less likely to need re-excisions [odds ratio (OR) 0.24; p < 0.01], as were those operated on by very-high-volume surgeons (OR 0.41; p = 0.01). On multivariate analysis, the lowest threshold for tumor size decreased to 20 mm, with tumors between 20 and 29 mm having a higher probability of re-excision (OR 2.13; p = 0.04) compared with tumors <1.0 cm. Patients with lobular carcinoma were more likely to need re-excisions than those with ductal carcinoma (OR 2.50; p = 0.02).
Further exploratory analysis revealed that very-high-volume surgeons had a lower rate of pathologically positive margins (14.4 %) than those of the high- (16.8 %), intermediate- (17.6 %), and low-volume (23.8 %) surgeons (results not shown). On multivariate analysis, this only reached statistical significance (results not shown) on comparison of very-high-volume and low-volume surgeons (OR 0.54; p = 0.05).
In an attempt to elucidate management strategies between surgeons, we also analyzed re-excision rates in cases with pathologically positive and ‘negative’ surgical margins (including no tumor on ink, 1–2 mm, and to widely negative margins). There appeared to be a trend in the proportion of patients re-excised in each surgeon-volume category, for both pathologically positive and ‘negative’ margins (Fig. 1). In patients with pathologically positive margins, re-excisions were performed in fewer patients in the very-high-volume (82.4 %) and high-volume surgeons (71.8 %), than in the intermediate- (95.5 %) and low-volume surgeons (88.0 %). Similarly, in patients with ‘negative margins’, very-high-volume and high-volume surgeons reoperated less frequently than their respective intermediate- or low-volume surgeons (6.9, 12.3, 16.5, and 16.3 %, respectively). Furthermore, the rationale for re-excisions within each surgeon-volume category were similar, with the majority of re-excisions being undertaken for pathologically positive margins (66.7 vs. 53.9 vs. 53.3 vs. 62.9 %), with the remainder of re-excisions performed in those with various degrees of ‘negative’ margins (results not shown).
Discussion
Breast cancer represents the most common solid organ malignancy requiring surgical intervention in females,18 with BCS being the preferred initial surgical approach in the majority of patients with early breast cancer. In large population-based studies, approximately one-quarter of patients require reoperation to address the adequacy of pathological margins.2 – 6 Given the frequent need for surgical management of breast cancer, potential negative consequences of reoperation (patient anxiety, increased complications, worse cosmesis), and the attributable costs associated with reoperation,11 there is an increasing emphasis on minimizing the reoperation rate.13
There is no consensus on an acceptable rate of re-excision after attempted BCS; one group has suggested <20 %,13 while another specialist society have set 10 % as an achievable target.19 Despite the lack of consensus, it is apparent that there is the potential to significantly improve on the current status quo. While there is no single solution, a number of potential approaches have been suggested to maximize the chances of successful initial surgery, including both preoperative planning and intraoperative techniques.13 Potential intraoperative solutions include the use of frozen sections and touch preparations,1 cavity shaving,20 and novel margin assessment tools.21 Furthermore, the release of the Society of Surgical Oncology/American Society for Radiation Oncology (SSO/ASTRO) consensus guidelines, which suggest ‘no tumor on ink’ as an acceptable pathologic margin, has the potential to reduce the reoperation rate.22 Given the variability in patient and tumor characteristics, along with physician practice patterns, techniques, and expertise, this is likely to be an area of further investigation in the foreseeable future.
In order to address this issue, there is a need to understand what factors predict the need for re-excision after BCS. Fundamentally, these can be divided into patient-, disease-, and treatment-related factors, along with processes of care. The latter are outside the scope of this project, with the exception of the need to utilize a surgical biopsy to establish a cancer diagnosis. Given the association of surgical diagnostic biopsies with an increased re-excision rate,23 , 24 we considered a surgical biopsy a diagnostic procedure and the subsequent therapeutic surgical procedure as the initial BCS. This minimized the influence of access to, or local expertise in, image-guided biopsies on re-excision rates.
We have identified that patient age influences the need for re-excision, with patients <45 years of age undergoing more re-excisions than their older counterparts (70 years of age and older) (Table 2). This is consistent with other reports, which have found that patients <50 years of age more frequently undergo secondary operations.2 , 3 , 5
The association of tumor-related factors with the need for reoperation have been well documented. In our cohort of patients, tumors >2 cm were more likely to require re-excision, which is consistent with other reports.2 , 3 Pathologic subtype, in particular lobular carcinoma, was found to increase the need for re-excision.3 , 15 We did not identify ductal carcinoma in situ as a risk factor in multivariate analysis, as has been reported by others.3 , 5 , 15 Tumor grade,3 as well as the presence of lymphovascular invasion,25 have also previously been shown to influence the re-excision rate, which was not the case in our study. Tumor biomarkers have also been reported to impact the need for reoperation. In our cohort of patients, we did not find that ER/PR receptor status,3 nor HER2 status,2 influenced re-excision rates, as shown by others. Furthermore, we did not find that the presence of pathologically positive lymph nodes influenced re-excision rates.2 , 3 The discrepancy between our study and others, pertaining to the significance of tumor-related factors, likely relates to sample size.
There has been increasing interest in the role of surgeon- and hospital-related factors on patient outcomes in surgery.2 , 26 – 28 Most reports have focused on resource-intensive procedures with high perioperative complications, including cardiac procedures,28 and lung,29 esophageal,26 , 29 pancreatic,26 , 29 and gynecological surgery.29 With the exception of breast reconstruction,30 , 31 the perioperative complications associated with breast cancer surgery are unlikely to be a relevant quality measure. This has resulted in a focus on other quality measures of clinical significance, including patient satisfaction, cosmesis, and wait times for care, each of which fit within the six domains of quality healthcare as identified by the Institute of Medicine.32 Re-excision after BCS has the potential to influence each of these quality measures, which has resulted in re-excision rates being proposed as a potential quality measure in breast cancer surgery.33 Furthermore, escalating healthcare costs are a major concern,2 , 14, 34 – 36 with re-excision after BCS having significant projected economic costs.11
Several reports address the impact of surgeon volume on re-excision rates after BCS. A study based on an Irish cancer registry found that low-volume surgeons (<35 cases per year) had higher re-excision rates than high-volume surgeons (>70 cases per year), independent of patient- and tumor-related factors.2 Similar findings were noted utilizing a database from the American Society of Breast Surgeons program, in which they found that surgeons with low annual case volumes (<10 and 25 cases), had higher re-excision rates than those with >25 cases per year (38 vs. 26 vs. 15 %).16 There was no apparent improvement in re-excision rates once surgeon case volumes exceeded 25 cases per year. Contrary to these reports, McCahill et al. did not find that surgeon volume impacted re-excision rates; however, this was a single-institution study with a small number of surgeons, case conferences, and a low overall re-excision rate (17.5 %), with low intersurgeon variability (10.7–20.1 %).12 Finally, a regional report from Canada focused on both the requirement for re-excision and the presence of pathologic positive margins after initial attempts at BCS. Surgeon case volumes were divided into terciles, with ‘high-volume’ surgeons performing only 15 cases per year. This report documented a trend to lower positive pathologic margins, and a lower re-excision rate in the ‘high-volume’ surgeons compared with the medium- and low-volume surgical counterparts (17 vs. 32 vs. 30 %).25
Similar to the aforementioned studies, we found surgeons performing more than 25 cases per year had lower re-excision rates than low-volume surgeons (OR 0.41), with a trend to significance in our high-volume surgical cohort (10–24 cases per year). These results are consistent with two of the studies, with the exception of a higher threshold surgeon volume of 35 cases in one study.2 While the second study suggested similar findings, it was limited by a lack of relevant patient- and disease-related factors required to make any firm connection between surgeon case volume and re-excision rates.16 The two remaining studies did not find a statistical difference in re-excision rate, but definite trends were apparent.12, 25 These reports had notable limitations, including limited tumor-related information,12 a low number of surgeons,12 and very low case volumes in the ‘high-volume surgeons’,25 which may limit their conclusions. Our study adds to the current limited body, confirming a relationship between surgeon volume and re-excision rates independent of patient- and tumor-related variables in patients with breast cancer. Although open to interpretation, this collective information suggests that surgical case volumes between 25 and 35 cases per year are associated with lower re-excision rates after BCS.
Based on previous reports, it is unclear whether the disparities in re-excision rates attributed to surgeon volume are related to proficiency at obtaining a negative pathologic margin (i.e. no tumor on ink) or physician interpretation of what an adequate ‘negative’ margin is. To further elucidate the rationale for this disparity, re-excisions were subdivided into two different categories: (i) patients with positive pathologic margins (i.e. tumor on ink) after their initial BCS; and (ii) those with all other pathologically ‘negative margins’. Upon review, the majority of re-excisions performed were in patients with pathologically positive margins (range 53.3–66.7 %). Given that well over half of re-excisions were performed for pathologically positive margins suggests that the influence of surgeon case volume is reflective of planning and/or technical proficiency on the part of the surgeon. The controversy surrounding physician interpretation of an appropriate margin is also evident, with at least one-third of re-excisions performed in cases with ‘no tumor on ink’. The appropriateness of reoperation in this scenario cannot be commented on as our local guidelines at that time suggested a 2 mm pathological margin as ideal, and the SSO/ASTRO margin guideline for invasive breast cancer was not available at that time.22 Despite these limitations, there appears to be a trend in the management of margins based on surgeon case volume. Very-high-volume and high-volume surgeons re-excised pathologically negative margins less frequently (6.9 and 12.4 %) than intermediate- and low-volume surgeons (16.5 and 16.3 %) (Fig. 1). This trend also appeared in those with pathologically positive margins, with very-high-volume and high-volume surgeons re-excising margins less frequently (82.4 and 71.8 %) than intermediate- and low-volume surgeons (95.5 and 88 %). This suggests a potential connection between surgeon volume, training, and/or education on perception of an adequate margin, and the need for further surgery in those undergoing BCS.
It should be stated there are other structural- and process-related measures that have the potential to influence the re-excision rate. Hospital volume has been associated with improved outcomes within a number of disease sites, both within breast cancer2 and other areas of surgical oncology.29 Access to advanced breast imaging, radiologists, pathologists, and multidisciplinary rounds, and quality improvement initiatives, all have the potential to impact the rate of pathologically positive margins after BCS, as well as influencing the decision for further surgery in cases with ‘close but negative margins’ or other cases with ‘negative margins’. Finally, surgeon characteristics other than case volume have the potential to influence outcomes, including subspecialty training in breast surgery, which has been found to be associated with both higher breast-conservation rates37, 38 and lower reoperation rates after BCS.37 In exploratory analysis, we did not identify hospital volume as a meaningful independent predictor of the need for re-excision. The influence of the potential impact of other physician specialists (i.e. radiologists and pathologists) cannot be ruled out, but would collectively be included within hospital volume, which was investigated. Pathologic processing is unlikely to be a contributing factor given a standardized regional protocol. Surgeon subspecialization could not be investigated as there is only one regional surgical oncologist. Furthermore, although there are multidisciplinary rounds available at our regional cancer center, these are primarily focused on medical and radiation oncology decision support, and are not frequently attended by community surgeons, pathologists, or radiologists, making them unlikely to bias our results. Although not formally reviewed in this study, based on other previous work,39 no surgeons used advanced localizing intraoperative localizing techniques (i.e. radioactive seeds, intraoperative ultrasound), or detailed intraoperative specimen assessment (i.e. touch cytology, frozen sections, cavity shave) during the study time period. Although there are potentially other factors that could influence re-excision rates, collectively we believe that this unlikely.
The strength of this project is that it involves the work of multiple surgeons in highly variable communities and hospital settings. Although the case volumes may not be that of highly specialized breast centers, they are likely reflective of other community centers, where the bulk of breast cancer surgery is performed.40, 41 Furthermore, detailed tumor-related information was collected, which is not the case in most studies. In particular, the pathologic margin status provided a unique insight into the potential reasons for re-excision, which is not available in other studies. These results suggest that there are several potential avenues to address concerns regarding re-excision rates, including educating surgeons about the potential patient- and tumor-related factors influencing margin positivity, implementation of intraoperative methods to decrease margin positivity, and awareness of guidelines regarding the management of patients with questionable margins. Furthermore, multidisciplinary preoperative and postoperative breast rounds also have the potential to standardize patient management. Finally, regionalization of surgical breast cancer management could be considered given the apparent volume–outcome Association between surgeon volume and the need for re-excision.
Conclusions
Patients undergoing BCS frequently require revisional surgery to ensure adequate pathologic margin status. Re-excision rates are affected by surgeon case volume, patient age, and tumor-related factors. These factors should be considered in both surgical planning and counseling patients undergoing BCS, as well as in ongoing quality assurance.
References
Esbona K, Li Z, Wilke LG. Intraoperative imprint cytology and frozen section pathology for margin assessment in breast conservation surgery: a systematic review. Ann Surg Oncol. 2012;19:3236–3245.
de Camargo Cancela M, Comber H, Sharp L. Hospital and surgeon caseload are associated with risk of re-operation following breast-conserving surgery. Breast Cancer Res Treat. 2013;140:535–544.
Wilke LG, Czechura T, Wang C, Lapin B, Liederbach E, Winchester DP, et al. Repeat surgery after breast conservation for the treatment of stage 0 to II breast carcinoma: a report from the national cancer data base, 2004–2010. JAMA Surg. 2014;149:1296–1305.
Spilsbury K, Semmens JB, Saunders CM, Hall SE, Holman CD. Subsequent surgery after initial breast conserving surgery: a population based study. ANZ J Surg. 2005;75:260–264.
Jeevan R, Cromwell DA, Trivella M, Lawrence G, Kearins O, Pereira J, et al. Reoperation rates after breast conserving surgery for breast cancer among women in England: retrospective study of hospital episode statistics. BMJ. 2012;345:e4505.
Canadian Institute for Health Information. Breast cancer surgery in canada, 2007–2008 to 2009–2010. Ottawa: Canadian Institute for Health Information; 2012.
Vos EL, Jager A, Verhoef C, Voogd AC, Koppert LB. Overall survival in patients with a re-excision following breast conserving surgery compared to those without in a large population-based cohort. Eur J Cancer. 2015;51:282–291.
Xue DQ, Qian C, Yang L, Wang XF. Risk factors for surgical site infections after breast surgery: a systematic review and meta-analysis. Eur J Surg Oncol. 2012;38:375–381.
Parvez E, Cornacchi SD, Hodgson N, Thoma A, Kong I, Foster G, et al. A cosmesis outcome substudy in a prospective, randomized trial comparing radioguided seed localization with standard wire localization for nonpalpable, invasive, and in situ breast carcinomas. Am J Surg. 2014;208:711–718.
Wazer DE, DiPetrillo T, Schmidt-Ullrich R, Weld L, Smith TJ, Marchant DJ, et al. Factors influencing cosmetic outcome and complication risk after conservative surgery and radiotherapy for early-stage breast carcinoma. J Clin Oncol. 1992;10:356–363.
Pataky R, Baliski C. Reoperation costs in attempted breast conserving surgery: a decision analysis. Curr Oncol. 2016;23(10) (in press).
McCahill LE, Privette A, James T, Sheehey-Jones J, Ratliff J, Majercik D, et al. Quality measures for breast cancer surgery: initial validation of feasibility and assessment of variation among surgeons. Arch Surg. 2009;144:455–62; discussion 462-3.
Landercasper J, Attai D, Atisha D, Beitsch P, Bosserman L, Boughey J, et al. Toolbox to reduce lumpectomy reoperations and improve cosmetic outcome in breast cancer patients: the American Society of Breast Surgeons Consensus Conference. Ann Surg Oncol. 2015;22:3174–3183.
Landercasper J, Tafra L. The relationship between quality and cost during the perioperative breast cancer episode of care. Breast. 2010;19:289–296.
Kryh CG, Pietersen CA, Rahr HB, Christensen RD, Wamberg P, Lautrup MD. Re-resection rates and risk characteristics following breast conserving surgery for breast cancer and carcinoma in situ: a single-centre study of 1575 consecutive cases. Breast. 2014;23:784–789.
Landercasper J, Whitacre E, Degnim AC, Al-Hamadani M. Reasons for re-excision after lumpectomy for breast cancer: insight from the american society of breast surgeons mastery (SM) database. Ann Surg Oncol. 2014;21:3185–3191.
McCahill LE, Single RM, Aiello Bowles EJ, Feigelson HS, James TA, Barney T, et al. Variability in reexcision following breast conservation surgery. JAMA. 2012;307:467–475.
Canadian cancer statistics. Canadian cancer statistics 2015; 25 Mar 2016.
Del Turco MR, Ponti A, Bick U, Biganzoli L, Cserni G, Cutuli B, et al. Quality indicators in breast cancer care. Eur J Cancer. 2010;46:2344–2356.
Chagpar AB, Killelea BK, Tsangaris TN, Butler M, Stavris K, Li F, et al. A randomized, controlled trial of cavity shave margins in breast cancer. N Engl J Med. 2015;373:503–510.
Schnabel F, Boolbol SK, Gittleman M, Karni T, Tafra L, Feldman S, et al. A randomized prospective study of lumpectomy margin assessment with use of MarginProbe in patients with nonpalpable breast malignancies. Ann Surg Oncol. 2014;21:1589–1595.
Moran MS, Schnitt SJ, Giuliano AE, Harris JR, Khan SA, Horton J, et al.; Society of Surgical Oncology, American Society for Radiation Oncology. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol. 2014;32:1507–1515.
Eberth JM, Xu Y, Smith GL, Shen Y, Jiang J, Buchholz TA, et al. Surgeon influence on use of needle biopsy in patients with breast cancer: a national medicare study. J Clin Oncol. 2014;32:2206–2216.
Smitt MC, Horst K. Association of clinical and pathologic variables with lumpectomy surgical margin status after preoperative diagnosis or excisional biopsy of invasive breast cancer. Ann Surg Oncol. 2007;14:1040–1044.
Lovrics PJ, Cornacchi SD, Farrokhyar F, Garnett A, Chen V, Franic S, et al. Technical factors, surgeon case volume and positive margin rates after breast conservation surgery for early-stage breast cancer. Can J Surg. 2010;53:305–312.
Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777–783.
Kulkarni GS, Urbach DR, Austin PC, Fleshner NE, Laupacis A. Higher surgeon and hospital volume improves long-term survival after radical cystectomy. Cancer. 2013;119(19):3546–54.
O’Connor GT, Plume SK, Olmstead EM, Coffin LH, Morton JR, Maloney CT, et al. A regional prospective study of in-hospital mortality associated with coronary artery bypass grafting. The Northern New England Cardiovascular Disease Study Group. JAMA. 1991;266:803–809.
Finley CJ, Schneider L, Shakeel S. Approaches to high risk, resource intensive surgical care in Canada. Canadian Partnership Against Cancer; 2015.
Albornoz CR, Cordeiro PG, Hishon L, Mehrara BJ, Pusic AL, McCarthy CM, et al. A nationwide analysis of the relationship between hospital volume and outcome for autologous breast reconstruction. Plast Reconstr Surg. 2013;132:192e–200e.
Tanna N, Clayton JL, Roostaeian J, Perry AD, Crisera CA. The volume-outcome relationship for immediate breast reconstruction. Plast Reconstr Surg. 2012;129:19–24.
Agency for Healthcare Research and Quality. The 6 domains of health care quality. Available at: http://www.ahrq.gov/professionals/quality-patient-safety/talkingquality/create/sixdomains.html.
Schwartz T, Degnim AC, Landercasper J. Should re-excision lumpectomy rates be a quality measure in breast-conserving surgery? Ann Surg Oncol. 2013;20:3180–3183.
Birkmeyer JD, Gust C, Dimick JB, Birkmeyer NJ, Skinner JS. Hospital quality and the cost of inpatient surgery in the United States. Ann Surg. 2012;255:1–5.
Camp MS, Greenup RA, Taghian A, Coopey SB, Specht M, Gadd M, et al. Application of ACOSOG Z0011 criteria reduces perioperative costs. Ann Surg Oncol. 2013;20:836–841.
Fischer JP, Fox JP, Nelson JA, Kovach SJ, Serletti JM. A longitudinal assessment of outcomes and healthcare resource utilization after immediate breast reconstruction-comparing implant- and autologous-based breast reconstruction. Ann Surg. 2015;262:692–699.
Zork NM, Komenaka IK, Pennington RE Jr, Bowling MW, Norton LE, Clare SE, et al. The effect of dedicated breast surgeons on the short-term outcomes in breast cancer. Ann Surg. 2008;248:280–285.
Blair SL, O’Shea KE, Orr RK. Surgeon variability in treating nonpalpable breast cancer: Surgical oncology as a value-added specialty. Ann Surg Oncol. 1998;5:28–32.
Eng J, Baliski C. Uptake and impact of synoptic reporting on breast cancer operative reports in a community care setting. Can J Surg. 2015;48:S238.
Lovrics PJ, Gordon M, Cornacchi SD, Farrokhyar F, Ramsaroop A, Hodgson N, et al. Practice patterns and perceptions of margin status for breast conserving surgery for breast carcinoma: national survey of Canadian general surgeons. Breast. 2012;21:730–734.
Porter GA, McMulkin H, Lovrics PJ. Sentinel lymph node biopsy in breast cancer: Canadian practice patterns. Ann Surg Oncol. 2003;10:255–260.
Acknowledgements
We would like to thank the UBC Faculty of Medicine Summer Research Program (Interior Health Authority, Florence and George Heighway Endowement Fund) and the Anita Cochrane Endowment Fund for their financial support of Ms. Hughes
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hughes, L., Hamm, J., McGahan, C. et al. Surgeon Volume, Patient Age, and Tumor-Related Factors Influence the Need for Re-Excision After Breast-Conserving Surgery. Ann Surg Oncol 23 (Suppl 5), 656–664 (2016). https://doi.org/10.1245/s10434-016-5602-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5602-8