Abstract
Background
In February 2014 , the Society of Surgical Oncology and the American Society for Radiation Oncology released guidelines standardizing a negative margin after breast-conserving surgery (BCS) as “no ink on tumor” in patients with early-stage invasive cancer. We sought to determine whether reexcision rates after initial BCS decreased after guideline publication, using the ASBrS MasterySM of Breast Surgery Program.
Methods
Between January 2013 and June 2015, data from the ASBrS MasterySM database was analyzed to determine reexcision rates pre and post guideline publication. Reasons for reexcision were evaluated as were the associations with patient and provider characteristics. Chi square test, Fisher’s exact test, Student’s t test, ANOVA, and multivariable logistic regression were used as appropriate. All analyses were performed using Microsoft Excel and SPSS, with p value <0.05 as significant.
Results
Among 252 providers, the overall reexcision rate after initial BCS decreased by 3.7 % from 20.2 to 16.5 % (p < 0.001). Notable was a 13.8 % decrease (p < 0.001) in reexcisions being done for close margins. Of the analyzed physician and patient characteristics the majority of subgroups showed decreases between the two time periods; however, only “Percent Breast Surgery in Practice” was significant. On adjusted analysis, there were no specific patient factors associated with a reduction in reexcision rates.
Conclusions
Following the SSO-ASTRO “no ink on tumor” guideline publication, a reduction in overall reexcision rates and reexcision rates for close margins after initial BCS was observed in the ASBrS MasterySM database. More widespread implementation outside this group of early adopters is anticipated with ongoing dissemination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Breast-conserving surgery (BCS) has long been established as an alternative to mastectomy for the surgical treatment of early stage breast cancer. Multiple prior randomized trials have found that this less aggressive surgery carries a small increased risk of ipsilateral breast tumor recurrence (IBTR), but equivalent long-term survival to mastectomy when combined with postoperative radiation therapy.1 – 3 Breast conservation is predicated on the ability to get adequate margins while preserving an acceptable cosmetic outcome.4 – 7 However, there has been a lack of consensus as to what constitutes an adequate margin.8 – 11 At least in part due to this lack of consensus over the past 20 years, there has been considerable variability in surgical practice, with 1 in 4 patients undergoing reexcision surgery after initial BCS.12 – 14 These additional procedures can be detrimental to the patient’s cosmetic outcome, add increased anxiety and distress, increase the cost of cancer care, and increase the risk of surgical complications.15
In February 2014, a joint panel of members from the Society for Surgical Oncology (SSO) and the American Society for Radiation Oncology (ASTRO) released a consensus guideline on margins for breast-conserving surgery with whole-breast irradiation for patients with stages I and II invasive breast cancer. From a meta-analysis of margin width and IBTR in 33 studies, they found that margin reexcision in patients with close and/or negative margins did not significantly decrease risk of IBTR.12 Thus, a reexcision should ideally only occur in the event of positive margins (defined as ink on invasive cancer or ductal carcinoma in situ (DCIS)). This new guideline endorses “no ink on tumor” as the standard for a negative margin, and as such, it has the potential to decrease the rate of reexcision without an increase in IBTR.16
We hypothesized that the rate of reexcision following initial BCS overall and by indication (positive margins or close margins) would decrease following publication of the SSO/ASTRO guideline in a select group of providers who utilize the ASBrS MasterySM database.
Methods
Data Source
The ASBrS Mastery of Breast SurgerySM database was developed by the American Society of Breast Surgeons in 2006 as a platform for physicians to document their breast procedures and patient care, with the goal of assessing clinical performance and allowing for peer-performance comparison and quality measurements.17 The database includes de-identified, Health Insurance Portability and Accountability Act–compliant information about patients, patient procedures, and the documenting providers. When the data was acquired for this study in June 2015, the MasterySM database held information on 1549 physicians and 296,632 patients.
Cohort Identification
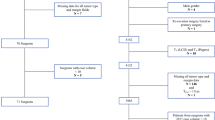
The MasterySM database was queried to identify patients undergoing BCS from January 2013 to June 2015. As the SSO-ASTRO guidelines apply only to patients with invasive disease, all surgeries performed for diagnosis (excisional biopsy), pure DCIS, benign, or high-risk lesions were excluded. Each documented procedure was assigned a time period: either “pre” (January 2013–2014) or “post” (June 2014–2015) guideline. Cases entered between February 2014 and May 2014 were not included in the analysis to allow for adoption of the published guidelines. All physicians who performed less than ten initial lumpectomies in either time period or who did not record information for both time periods were excluded from the analysis.
Primary and Secondary Outcomes
Reexcision lumpectomy rates (RELR) were defined as the number of patients undergoing a second procedure of any type for oncologic control (BCS vs. mastectomy) divided by the total number of initial BCS procedures. RELR was calculated per physician per time period. The reason for the RELR (positive margins, close margins, or other) also was extracted (Table 1). In the Mastery, a close margin is defined as cancer ≤2 mm from the tissue edge, a negative margin is cancer >2 mm from tissue edge, and positive margin is defined as cancer involvement at tissue edge. Reexcisions with no reason given were excluded from these calculations. We then calculated a benchmark of care for reexcision rates using the Centers for Medicaid and Medicare Services (CMS) recommended methodology.18,19 The benchmark-setters are identified by summing the number of cases of each surgeon, starting with the highest performer, and successively including the next highest performer, until the sum of patients includes 10 % of the total cases. The benchmark is then calculated by dividing the sum of adverse events of the benchmark-setters by the total patient number .
Covariates
A number of patient and provider factors were extracted for analysis. Patient factors included race, age, and primary insurer. Physician variables included years in practice, practice type, geographic practice location, provider practice setting, the portion of breast surgery in their practice, whether the providers perform ultrasounds or stereotactic guided procedures, and whether providers were enrolled in the PQRS quality program or any quality program (Table 2).
Statistical Analysis
The overall reexcision rate before and after the guidelines were compared using Student’s t test. Group comparisons were performed using Student’s t test and ANOVA for continuous variables and Chi square test and Fisher’s test for categorical variables, as appropriate. Multivariable logistic regression models were fit to examine physician and patient characteristics associated with a change in reexcision rate following guideline publication. Statistical significance was defined as p < 0.05. Analyses were performed using Microsoft Excel 2010 and SPSS, version 21.0.
Compliance
All data received from MasterySM database was de-identified. This study therefore was exempt as not human subjects by the Gunderson Lutheran Institutional Review Board in collaboration with providers at the University of Wisconsin, Department of Surgery.
Results
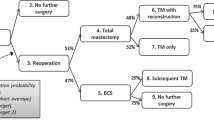
We identified 252 surgeons who actively participate in Mastery with greater than ten cases documented per year; this accounts for 16 % of all the surgeons who participate in the Mastery program. The overall reexcision rate after initial BCS was 20.2 % (2482/13,297) in the pre-period and 16.5 % (1851/12,805) in the post-period—a decrease of 3.8 %, which was statistically significant (p < 0.005; Fig. 1). The indications for procedure also changed significantly from the pre-period to the post-period. There was a reduction in the indication of close margins from 37.4 % in the pre-period to 25.8 % in the post-period (p < 0.001) with an increase in indication of positive margins, from 56.8 % pre- to 70.5 % in the post-period (p < 0.005). No significant change in reexcision rates were observed for any other reason recorded (Fig. 2). The missing data for reasons for reexcision included 32 % (797/2482) from the pre-guideline time period and 13 % (240/1851) from the post-guideline time period. The percentage calculation for the change in reexcisions due to positive versus close margins in Fig. 2 excludes this missing data (Table 1).
In a univariate analysis of physician factors (including years in practice, practice type, geographic practice location, the portion of breast surgery, whether the providers perform ultrasounds or stereotactic guided procedures, and whether providers were enrolled in the PQRS quality program or any quality program) a decrease in overall reexcision rates and in those due to close margins was seen for the majority of subgroups. Significant differences were primarily noted in the groups where the largest number of surgeons provided data to the MasterySM. In a multivariate model, only 100 % breast surgery practice was a significant variable associated with a decrease in overall reexcision rates between the two time periods. No single physician factor was associated in multivariate analyses with a decrease in reexcision due to close margins (Table 2). The pre- and post-guideline intersurgeon variability in reexcision rates decreased from 0–100 to 0–54 % among 251 surgeons (Fig. 3). One surgeon was an outlier and removed from the data with a 150 % reexcision rate due to more reported reexcisions than primary BCS surgery.
In a multivariable logistic regression of patient factors and reexcision rates due to any reason, a patient was significantly more likely to undergo a reexcision after initial BCS before the “no ink on tumor” guidelines (p < 0.001). However, while it was demonstrated that race and age are separate but significant influencing factors in whether a patient will undergo a reexcision procedure following BCS, the overall decrease in reexcision rates from pre- to post- did not appear to be dependent on any one patient characteristic (data not shown).
To determine a benchmark reexcision rate, we utilized the CMS methodology described for calculating a benchmark of care, and our calculated benchmark for reexcision rate was 2 %, based on 18 surgeons. These Mastery participants performed a total of 1409 initial lumpectomies post-SSO/ASTRO guideline release and 31 reexcisions. The majority of these providers were 100 % breast surgeons (13/18) with 12 of the 18 participating in PQRS. The average number of breast conservation surgeries performed by these 18 surgeons annually was 78, whereas the average number of annual BCS for the remaining cohort of surgeons was 25.
Discussion
In February 2014, SSO-ASTRO released and endorsed a guideline using “no ink on tumor” as the standard for a negative margin after BCS for early-stage invasive cancer.16 These guidelines have been endorsed by several groups, including the American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), and the ASBrS.20,21 These guidelines were published with the goal of reducing the rate of reexcision surgery without compromising the risk of IBTR. To determine if dedicated breast physicians were adopting these new guidelines, we extracted patient and provider information from the ASBS MasterySM of breast surgery database to calculate overall and per provider reexcision BCS rates both before and after guideline release with a 4-month interval window to allow for guideline adoption.
Whereas multiple studies have looked at trends and rates for repeat breast surgery, this study is one of the first to use a large multi-institutional/society database of physician reported data rather than single institution or survey data, to determine if surgical behavior was affected following a specific guideline release22–25 Chung et al. from Cedars Sinai utilized their institutional database to show a 6 % reduction in reexcision surgeries following release of the SSO/ASTRO guidelines.26 Merrill et al. reported a potential 50 % decrease in reexcisions from 36 to 18 % if the guidelines had been applied to 437 BCS cases.27 Our data from 252 surgeons participating in the ASBrS MasterySM program demonstrates that reexcisions after initial BCS for invasive breast cancer decreased following the SSO-ASTRO guideline publication. Furthermore, we were able to verify that this overall decrease in re-excision rates can be attributed to a reduction in re-excisions for close margins.
In multivariable analyses, the only physician or patient factor that was associated with a reduction in reexcision rates was the cohort of surgeons who report that 100 % of their practice is dedicated to breast surgery. The impact of specialization and volume has been mixed in the literature to date. In a single institution analysis, Zork and colleagues noted a reduction in reexcision rates for surgeries performed by dedicated breast surgeons (from 39 to 21 %).28 McCahill et al., however, noted significant intersurgeon variability in reexcision rates (0–70 %) in a multi-institutional study that was not associated with surgeon volume.14 Similar to the McCahill study, our study confirmed significant variability in reexcision rates by surgeon with a range of 0–100 % prior to publication of the SSO/ASTRO guidelines, with post-guideline variability decreasing to 0–54 %.
Despite our evidence of margin guideline adoption, the marked variability in surgeon reexcision rates persisting in our members suggests a gap in the quality of care for patients undergoing BCS. The variability we identified also invites the question of “what is the benchmark for reexcision rates”? The European Union of Breast Cancer Specialists has endorsed a target goal for “one step surgical success” for breast cancer operations, but to our knowledge, no organization has yet endorsed a target goal for lumpectomy reexcision rates.29 CMS recently endorsed a methodology for defining an achievable benchmark of care (ABC) for performance measures.18,29 After application of their specific formula for benchmarking, the reexcision result was 2 %. In our opinion, such a low level of reexcision rate is neither achievable nor safe. This likely reflects the highly specialized practices of the surgeons under investigation in this study. Evaluation of the practice of these 18 surgeons, who reflect a group of “higher-volume” breast surgeons with greater than 70 breast conservation surgeries per year, may be warranted to determine best practices for BCS. Reexcision rates less than 10 % have been reported, albeit uncommonly, but a target goal of less than 5 % could result in unintended and adverse consequences, such as high mastectomy rates from “risk aversion” to lumpectomy by surgeons concerned about meeting an unachievable benchmark30–32 The ASBrS convened a consensus conference in 2015 to develop a “Toolbox” for providers to help reduce their reexcision rates.33 The conference participants had uniform agreement to set a 5-year target goal for reducing the national reexcision rate, but there was lack of uniformity for the actual level of the quality target. Two-thirds of participants recommended a goal of less than 20 %. In the study herein, members of the ASBrS have already achieved this target, but lower rates are achievable because significant variability of performance remains. Through use of the 11 tools provided and through increased dissemination programs to a larger variety of surgeons, it is anticipated that the national reexcision rate will continue to decrease for patients with invasive breast cancer. With broader dissemination, opportunities will arise for calculation of a more generalizable, achievable benchmark of care.
Despite the strong correlation between re-excision rate drop and the SSO-ASTRO “no ink on tumor” guideline release, several limitations of the MasterySM database must be considered. Our sample population is biased in that the 252 physicians evaluated had to have performed at least ten initial lumpectomies in both time periods. Because many general surgeons perform less than ten breast surgeries per year, the group of physicians analyzed in this study is not reflective of the overall population of general surgeons who participate in the care of patients with breast. Moreover, a large number of reexcision surgeries were logged without a reason given, and it is unknown whether these procedures were done for close margins. As a result, we may be underestimating these rates. Additionally, if this information was missing nonrandomly (i.e., not listing if done for close margin given guidelines), this could bias our results. Additionally missing from the MasterySM database is patient-specific information that could impact the decision for reexcision regardless of SSO-ASTRO guidelines: receptor status, presence of genetic mutations, and whether the patient underwent neoadjuvant chemotherapy.17 Without information on these confounders, we do not know if they were unequally distributed between our pre- and post-margin guideline periods. A survey of 3,057 ASBrS members suggests that although no evidence was found on the meta-analysis, many surgeons still consider biology in the decision to reexcise.35 However, this would only bias the results if the cohorts varied before and after the guidelines were introduced. Given that other characteristics were stable, we have no reason to believe they would. We also are limited in our analysis of the MasterySM database in that we are unable to determine which initial BCS surgeries in the post time period resulted in close margins but were not reexcised. We therefore do not know which physicians were actually adhering to the guidelines.
The strengths of the ASBrS Mastery database include its ability to capture a large number of “reasons for reexcision,” entered by surgeon providers at point of care. Knowing the actual “reasons” is critical to ascertain whether the margin guideline is being adopted. Another strength is the rapidity with which performance data is available to researchers compared with most other national datasets, in which the patient user files may not be accessible for years after new guidelines are developed.
Conclusions
In this analysis of the ASBS MasterySM of Breast Surgery database, we found that adoption of the SSO-ASTRO “no ink on tumor” guidelines reduced overall rates of reexcision and the proportion of reexcisions attributable to close margins. These findings support ongoing efforts to increase clinical uptake of these published guidelines beyond this group of “early adopters” who participate in the Mastery of Breast Surgery program.
References
Gomez SL, Glaser SL, McClure LA, et al. The California neighborhoods data system: a new resource for examining the impact of neighborhood characteristics on cancer incidence and outcomes in populations. Cancer Causes Control. 2011;22(4):631-47.
Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233-41.
Lazovich D, Solomon CC, Thomas DB, Moe RE, White E. Breast conservation therapy in the United States following the 1990 National Institutes of Health Consensus Development Conference on the treatment of patients with early-stage invasive breast carcinoma. Cancer. 1999;86(4):628-37.
King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011;29(16):2158-64.
Smitt MC, Nowels KW, Zdeblick MJ, et al. The importance of the lumpectomy surgical margin status in long-term results of breast conservation. Cancer. 1995;76(2):259-67.
Tartter PI, Kaplan J, Bleiweiss I, et al. Lumpectomy margins, reexcision, and local recurrence of breast cancer. Am J Surg. 2000;179(2):81-5.
Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24(13):2028-37.
Amato B, Rispoli C, Iannone L, Testa S, Compagna R, Rocco N. Surgical margins of resection for breast cancer: current evidence. Minerva Chir. 2012;67(5):445-52.
Dillon MF, Hill AD, Quinn CM, McDermott EW, O’Higgins N. A pathologic assessment of adequate margin status in breast-conserving therapy. Ann Surg Oncol. 2006;13(3):333-9.
Coopey S, Smith BL, Hanson S, et al. The safety of multiple re-excisions after lumpectomy for breast cancer. Ann Surg Oncol. 2011;18(13):3797-801.
Revesz E, Khan SA. What are safe margins of resection for invasive and in situ breast cancer? Oncology (Williston Park). 2011;25(10):890-5.
Houssami N, Macaskill P, Marinovich ML, Morrow M. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann Surg Oncol. 2014;21(3):717-30.
Jagsi R, Smith BD, Sabel M, Pierce L. Individualized, patient-centered application of consensus guidelines to improve the quality of breast cancer care. Int J Radiat Oncol Biol Phys. 2014;88(3):535-6.
McCahill LE, Single RM, Aiello Bowles EJ, et al. Variability in reexcision following breast conservation surgery. JAMA. 2012;307(5):467-75.
Pleijhuis RG, Graafland M, de Vries J, Bart J, de Jong JS, van Dam GM. Obtaining adequate surgical margins in breast-conserving therapy for patients with early-stage breast cancer: current modalities and future directions. Ann Surg Oncol. 2009;16(10):2717-30.
Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann Surg Oncol. 2014;21(3):704-16.
American Society of Breast Surgeons MasterySM Program. http://masterybreastsurgeons.org/. Accessed 5 Apr 2014.
Kiefe CI, Weissman NW, Allison JJ, Farmer R, Weaver M, Williams OD. Identifying achievable benchmarks of care: concepts and methodology. Int J Qual Health Care. 1998;10(5):443-7.
Hatfield MD, Ashton CM, Bass BL, Shirkey BA. Surgeon-specific reports in general surgery: establishing benchmarks for peer comparison within a single hospital. J Am Coll Surg. 2016;222(2):113-21.
Buchholz TA, Somerfield MR, Griggs JJ, et al. Margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: American Society of Clinical Oncology endorsement of the Society of Surgical Oncology/American Society for Radiation Oncology consensus guideline. J Clin Oncol. 2014;32(14):1502-6.
National Comprehensive Cancer Network: Endorsement of SSO-ASTRO Margin Status Guidelines. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
Wilke LG, Czechura T, Wang C, et al. Repeat surgery after breast conservation for the treatment of stage 0 to II breast carcinoma: a report from the National Cancer Data Base, 2004-2010. JAMA Surg. 2014;149(12):1296-305.
Landercasper J, Whitacre E, Degnim AC, Al-Hamadani M. Reasons for re-excision after lumpectomy for breast cancer: insight from the American Society of Breast Surgeons Mastery(SM) database. Ann Surg Oncol. 2014;21(10):3185-91.
Gupta A, Subhas G, Dubay L, et al. Review of re-excision for narrow or positive margins of invasive and intraductal carcinoma. Am Surg. 2010;76(7):731-4.
Waljee JF, Hu ES, Newman LA, Alderman AK. Predictors of re-excision among women undergoing breast-conserving surgery for cancer. Ann Surg Oncol. 2008;15(5):1297-303.
Chung A, Gangi A, Amersi F, Bose S, Zhang X, Giuliano A. Impact of consensus guidelines by the Society of Surgical Oncology and the American Society for Radiation Oncology on margins for breast-conserving surgery in stages 1 and 2 invasive breast cancer. Ann Surg Oncol. 2015;22 Suppl 3:422-7.
Merrill AL, Coopey SB, Tang R, et al. Implications of new lumpectomy margin guidelines for breast-conserving surgery: changes in reexcision rates and predicted rates of residual tumor. Ann Surg Oncol. 2016;23(3):729-34.
Zork NM, Komenaka IK, Pennington RE, et al. The effect of dedicated breast surgeons on the short-term outcomes in breast cancer. Ann Surg. 2008;248(2):280-5.
Del Turco MR, Ponti A, Bick U, et al. Quality indicators in breast cancer care. Eur J Cancer. 2010;46(13):2344-56.
Osborn JB, Keeney GL, Jakub JW, Degnim AC, Boughey JC. Cost-effectiveness analysis of routine frozen-section analysis of breast margins compared with reoperation for positive margins. Ann Surg Oncol. 2011;18(11):3204-9.
Schwartz T, Degnim AC, Landercasper J. Should re-excision lumpectomy rates be a quality measure in breast-conserving surgery? Ann Surg Oncol. 2013;20(10):3180-3.
Boughey JC, Hieken TJ, Jakub JW, et al. Impact of analysis of frozen-section margin on reoperation rates in women undergoing lumpectomy for breast cancer: evaluation of the National Surgical Quality Improvement Program data. Surgery. 2014;156(1):190-7.
Landercasper J, Attai D, Atisha D, et al. Toolbox to reduce lumpectomy reoperations and improve cosmetic outcome in breast cancer patients: the American Society of Breast Surgeons Consensus Conference. Ann Surg Oncol. 2015;22(10):3174-83.
Pass HA, Klimberg SV, Copeland EM. Are “breast-focused” surgeons more competent? Ann Surg Oncol. 2008;15(4):953-5.
DeSnyder SM, Hunt KK, Smith BD, Moran MS, Klimberg S, Lucci A. Assessment of Practice Patterns Following Publication of the SSO-ASTRO Consensus guideline on margins for breast-conserving therapy in stage I and II invasive breast cancer. Ann Surg Oncol. 2015;22(10):3250-6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schulman, A.M., Mirrielees, J.A., Leverson, G. et al. Reexcision Surgery for Breast Cancer: An Analysis of the American Society of Breast Surgeons (ASBrS) MasterySM Database Following the SSO-ASTRO “No Ink on Tumor” Guidelines. Ann Surg Oncol 24, 52–58 (2017). https://doi.org/10.1245/s10434-016-5516-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5516-5