Abstract
Background
Solid-pseudopapillary neoplasms are rare pancreatic neoplasms with low malignant potential that predominantly arise in young women. We sought to characterize this population and the evolving trend at our institution towards laparoscopic management.
Methods
We identified all patients at our institution that were surgically treated for solid-pseudopapillary neoplasm from 2008-2015. Demographic and clinical information were queried from the medical record, and descriptive statistics were performed. Student’s t test and chi-square analysis were used for comparison where appropriate.
Results
We identified 11 women and 1 man (average age 26 years; range 14–48 years) who were surgically treated for solid-pseudopapillary neoplasms; 5 with distal pancreatectomy (4 open, 1 laparoscopic), 6 with pancreaticoduodenectomy (3 open, 3 laparoscopic), and 1 open enucleation. From 2008 to 2013, seven of eight (87 %) procedures were performed open. Since 2014, three of four (75 %) procedures have successfully been completed laparoscopically (see video clips). Length of stay was similar for patients who had open versus laparoscopic procedures (8 vs. 9 days, p = 0.61). Two-thirds of patients (5/8) who had open procedures experienced postoperative complications compared with half (2/4) of patients who had laparoscopic procedures (p = 0.28). There have been no recurrences.
Conclusions
Minimally invasive surgical management of solid-pseudopapillary neoplasms is becoming more popular, can be performed safely, and appears to have comparable outcomes to an open approach. Quality of life is an important metric for this relatively young population and may be improved with a laparoscopic approach, which warrants further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Solid-pseudopapillary neoplasm (SPN) are rare pancreatic tumors that predominantly arise in young women. Several eponyms are used for them in the literature, including Frantz tumors, Gruber-Frantz tumors, and Hamoudi tumors. SPN have low malignant potential but often are treated surgically to alleviate symptoms and eliminate the possibility of malignant transformation.1 During the past two decades, laparoscopic pancreatic surgery has transitioned from a rare technique to becoming accepted within the standard of care, for procedures ranging from enucleation of pancreatic masses to distal pancreatectomy, and more recently, pancreaticoduodenectomy. SPN lend themselves to minimally invasive management, because they tend to be less invasive to surrounding structures and afflict a younger population. Most reports of minimally invasive pancreatic surgery remain small; thus, few focus on specific neoplasms but rather combine patients who received similar surgeries for a variety of indications. We sought to examine our experience with SPN, specifically focusing on the changing management trend in laparoscopic pancreas surgery. We also present our technique on laparoscopic pancreaticoduodenctomy for SPN.
Methods
After institutional review board approval, we identified all patients at the University of Colorado Hospital and at Children’s Hospital Colorado who were surgically treated for SPT from 2008 to 2015. Demographic and surgery specific information were collected from the medical charts. Patients who were enrolled prospectively were given a quality of life survey preoperatively, and again at 2 weeks, 2 months, 6 months, and 1 year postoperatively. Statistical analysis was performed using Student’s t test and chi-square analysis where appropriate. Averages are presented as the mean ± SD.
Results
Patient Characteristics
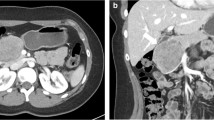
We identified 12 patients who were surgically treated for SPN during the study period. Most patients were female (11/12, 92 %), slender (average body mass index [BMI] 25.7 ± 5.4), and tended to be young adults (average age 26 years; range 14–48 years). Nine of 12 patients had a preoperative endoscopic ultrasound (EUS) with fine-needle aspiration (FNA) to facilitate diagnosis. One patient came from an outside hospital with a CT-guided FNA. All FNA biopsies were diagnostic. Average tumor size was 5.3 ± 3.8 cm in maximum diameter. A variety of surgical approaches were applied depending on the size and location of each patient’s tumor. Five patients were treated with distal pancreatectomy (4 open, 1 laparoscopic and spleen sparing), six patients with pancreaticoduodenectomy (3 open, 3 laparoscopic), and one patient treated with an open tumor enucleation. From 2008 to 2013, prior to the expansion of our laparoscopic pancreatic surgical program, seven of eight (87 %) procedures were performed open. Since 2014, three of four (75 %) procedures have successfully been completed laparoscopically.
Surgical Outcomes
Average length of stay was similar for patients who had open surgery (8 ± 3 days) and laparoscopic surgery (9 ± 1 days, p = 0.61). Two-thirds of patients (5/8) experienced postoperative complications after open procedures, including three pancreatic fistulas, one postoperative abscess, one chyle leak, and one ventral hernia requiring repair. Two patients who had laparoscopic procedures (2/4, p = 0.28) developed a postoperative complication, both pancreatic fistulas. Operative time and estimated blood loss (EBL) were available for nine patients in our series and were similar between patients who had laparoscopic (average time 5:13, average EBL 88 mL) and open procedures (average time 4:11, average EBL 245 mL, p = 0.40, 0.26 respectively). There have been no recurrences, with an average follow-up of 1.9 years (range 2 months–3.5 years, 2 patients excluded who did not follow-up).
Since starting our pancreatic multidisciplinary conference, we have been administering quality of life surveys to patients pre- and postoperatively. One patient in this series who had a laparoscopic pancreaticoduodenectomy completed these surveys and demonstrated rapid recovery of physical and emotional wellbeing.
The steps of the laparoscopic approach are different than the classic open pancreaticoduodenectomy and have been included below with video clips to facilitate understanding of this procedure and the laparoscopic approach for the treatment of SPN.
Laparoscopic Pancreaticoduodenectomy for SPN
We place the patient supine and then insert five laparoscopic ports into the abdomen. Upon entering the abdomen and ruling out metastatic disease, the lesser sac is then entered, identifying the gastroduodenal artery, common hepatic artery, and proper hepatic artery. The gastroduodenal artery is test occluded with a laparoscopic vascular clamp to confirm the pulse in the proper hepatic artery. The gastroduodenal artery is then ligated. Next, the portal vein is identified posteriorly, allowing us to estimate the location of the superior mesenteric vein. Dissection along the lower edge of the pancreas permits the creation of a tunnel underneath the pancreas neck. At this point, the pancreas neck is transected, using endosonic shears to seal the pancreaticoduodenal vessels inferiorly and superiorly, and using hook cautery through the middle of the gland to identify the main pancreatic duct (video clip 1).
Next, the pylorus of the stomach is identified and transected just proximal to the pylorus. The gallbladder is removed, a bulldog is placed on the proximal common bile duct, and the common bile duct is divided. A Kocher maneuver is then performed to mobilize the first and second portion of the duodenum and the head of the pancreas. This is followed by mobilization of the proximal jejunum to the extent that it can rest adjacent to the body of the stomach without tension once pulled up through the ligament of Trietz. The duodenal-jejunal junction is then transected with another endo GIA stapler. The remaining attachments of the uncinate process are taken down with direct visualization of the superior mesenteric artery, and the specimen is removed from the body through an enlarged umbilical port to 20 mm.
Reconstruction is performed in a similar fashion to the open pancreaticoduodenectomy. The pancreatic edge is first approximated to the jejunum using absorbable barbed locking suture. A pediatric feeding tube is then inserted to stent open the pancreatic duct, and a duct to mucosa pancreaticojejunostomy is created using intra-corporeal hand sewn interrupted sutures. A front row of barbed locking suture is added to complete the pancreaticojejunostomy (video clip 2). Interrupted sutures are also used to create the hepaticojejunostomy. Another pediatric feeding tube is inserted to stent open this anastomosis prior to the last few anterior stitches (video clip 3). A stapled antecolic gastrojejunostomy is performed, and the entrance for the stapler is closed with interrupted stitches, completing the operation. Our practice is to leave two drains at the end of the operation adjacent to the pancreatic and hepatic duct anastomoses.
Discussion
Minimizing invasiveness is becoming more prevalent in nearly all aspects of surgery, and pancreas surgery is no exception.2,3 SPNs are ideal for minimally invasive management, because they typically lack invasiveness to surrounding organs and can be easily visualized laparoscopically. Furthermore, patients with SPN are frequently good candidates because of their young age, low BMI, few comorbidities, and high functional status. We presented a series of 12 patients surgically treated for SPN—4 who had their tumors resected laparoscopically. This series of SPN patients is the largest single institutional series presented in the United States. Thus far, patients treated with a minimally invasive approach appear to have similar outcomes to those treated with an open operation, including length of surgery, rate of complications, and length of hospital stay. Another series of SPN out of China comparing the laparoscopic to open approach specifically for distal pancreatectomy also showed noninferiority in the laparoscopic group, with some short-term benefits.4 EBL tended to be higher in the open group, which is similar to our series, but given the retrospective nature of these studies, this is likely is a reflection of selection bias rather than surgical technique.
During the past decade, the frequency of SPN reports has increased significantly.1 In a recent review of all reported SPN in the literature before 2012, only 39 of 2,744 (1.4 %) patients had a laparoscopic resection of their tumor.1 This number, however, has increased since then, with a number of reports focusing on minimally invasive techniques in recent years.4–8 As our description and video clips demonstrate, there are some aspects of laparoscopic pancreatic surgery that are different from classic open procedures. However, the overall oncologic principles remain the same. While most studies have aimed just to show equivalence in early outcomes, there are some additional theoretical benefits to a laparoscopic approach for SPN that have yet to be well studied. Improved postoperative functional status, minimizing wound/hernia complications and issues related to adhesions are important potential advantages of minimally invasive surgery, especially for a disease that primarily affects the young with many years of life ahead of them. One study comparing laparoscopic hand-assisted to open pancreaticoduodenectomy showed improved functional status up to 6 months postoperatively.9 These factors are difficult to quantify, however, especially in a retrospective fashion. We have begun prospectively measuring quality of life metrics in patients scheduled for pancreatic surgery to allow objective comparisons of the two surgical approaches in the future. Another advantage is the hypothetical reduction in future small-bowel obstructions that are associated with all abdominal procedures.10 This again may be of greater importance for patients who are young and are expected to live several more decades, because survival after resection of SPN is high. Some of these tumors are ultimately considered malignant, with a 5–17 % recurrence rate, but the mean time to recurrence is 50 months and only 1.5 % of patients succumb to their disease.1,11,12 We recommend annual surveillance with MRI to limit lifelong ionizing radiation.
One significant drawback for laparoscopic pancreatic surgery is the technical expertise that is required to perform it safely. Like all progress in surgical techniques, there is a learning curve for surgeons. Operative times, especially initially, can be quite long, but this declines with increasing experience.13,14
References
Law JK, Ahmed A, Singh VK, et al. A systematic review of solid-pseudopapillary neoplasms: are these rare lesions?. Pancreas. 2014;43:331–7.
Paniccia A, Schulick RD, Edil BH. Total laparoscopic pancreaticoduodenectomy: a single-institutional experience. Ann Surg Oncol. 2015;22:4380–1.
Stewart CL, Raeburn C, Edil BH. Laparoscopic distal pancreatectomy for an ACTH secreting pancreatic neuroendocrine tumor. VideoEndocrinology. 2015;2:4.
Zhang RC, Yan JF, Xu XW, Chen K, Ajoodhea H, Mou YP. Laparoscopic vs. open distal pancreatectomy for solid pseudopapillary tumor of the pancreas. World J Gastroenterol. 2013;19:6272–277.
Kang CM, Choi SH, Kim SC, Lee WJ, Choi DW, Kim SW, Korean Pancreatic Surgery Club. Predicting recurrence of pancreatic solid pseudopapillary tumors after surgical resection: a multicenter analysis in Korea. Ann Surg. 2014;260:348–55.
Afridi SA, Kazaryan AM, Marangos IP, Rozok BI, Fretland AA, Yaqub S, Edwin B. Laparoscopic surgery for solid pseudopapillary tumor of the pancreas. JSLS. 2014;18:236–42.
Nakamura Y, Matsushita A, Katsuno A, Yamahatsu K, Sumiyoshi H, Mizuguchi Y, Uchida E. Clinical outcomes for 14 consecutive patients with solid pseudopapillary neoplasms who underwent laparoscopic distal pancreatectomy. Asian J Endosc Surg. 10.1111/ases.12256.
Senthilnathan P, Patel N, Nalankilli VP, Palanivelu C, Parthasarthi R, Praveenraj P. Laparoscopic pylorus preserving pancreaticoduodenectomy in paediatric age for solid pseudopapillary neoplasm of head of the pancreas—case report. Pancreatology. 2014;14:550–2.
Langan RC, Graham JA, Chin AB, et al. Laparoscopic-assisted versus open pancreaticoduodenectomy: Early favorable physical quality-of-life measures. Surgery. 2014;156:379–84.
Angenete E, Jacobsson A, Gellerstedt M, Haglind E. Effect of laparoscopy on the risk of small-bowel obstruction: a population-based register study. Arch Surg. 2012;147:359–65.
Hwang J, Kim DY, Kim SC, Namgoong JM, Hong SM. Solid-pseudopapillary neoplasm of the pancreas in children: can we predict malignancy? J Pediatr Surg. 2014;49:1730–3.
Manuballa V, Amin M, Cappell MS. Clinical presentation and comparison of surgical outcome for segmental resection vs. Whipple’s procedure for solid pseudopapillary tumor: Report of six new cases & literature review of 321 cases. Pancreatology. 2014;14:71–80.
Kim SC, Song KB, Jung YS, et al. Short-term clinical outcomes for 100 consecutive cases of laparoscopic pylorus-preserving pancreatoduodenectomy: improvement with surgical experience. Surg Endosc. 2013;27:95–103.
Speicher PJ, Nussbaum DP, White RR, et al. Defining the learning curve for team-based laparoscopic pancreaticoduodenectomy. Ann Surg Oncol. 2014;21:4014–9.
Disclosure
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MP4 90274 kb)
Supplementary material 2 (MP4 94525 kb)
Supplementary material 3 (MP4 70109 kb)
Rights and permissions
About this article
Cite this article
Stewart, C.L., Meguid, C., Chapman, B. et al. Evolving Trends Towards Minimally Invasive Surgery for Solid-Pseudopapillary Neoplasms. Ann Surg Oncol 23, 4165–4168 (2016). https://doi.org/10.1245/s10434-016-5491-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5491-x