Abstract
Background
This study aimed to evaluate the short- and long-term outcomes of hepatectomy for hepatocellular carcinoma (HCC) with bile duct tumor thrombus (BDTT), including cases with obstructive jaundice.
Methods
The study reviewed 42 HCC patients with BDTT, including six patients who needed preoperative biliary drainage due to obstructive jaundice, and 732 HCC patients without BDTT. The authors analyzed the impact of BDTT on the surgical outcomes and assessed the outcomes of hepatectomy for patients presenting with obstructive jaundice.
Results
The HCC patients with BDTT, almost all with stage 3 or 4 disease, had increased alpha-fetoprotein expression, larger tumors, and more portal vein invasion status. The survival of the HCC patients with BDTT was significantly inferior to that of the patients without BDTT (p = 0.0003). Survival did not differ significantly between the HCC patients with BDTT and those without BDTT when the two groups were matched by stage (p = 0.3366). The HCC patients with BDTT who presented with obstructive jaundice demonstrated outcomes similar to those for the HCC patients with BDTT who did not present with obstructive jaundice in terms of the overall survival rate (p = 0.5469). The perioperative outcomes for the HCC patients with BDTT did not depend on the presence or absence of preoperative jaundice. No patients in either BDTT group demonstrated 90-day mortality in this study.
Conclusions
Hepatectomy should be considered for HCC patients with BDTT, even for patients with obstructive jaundice, because the surgical outcomes equivalent to those for HCC without BDTT can be achieved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hepatocellular carcinoma (HCC) with bile duct tumor thrombus (BDTT) is rare relative to HCC with portal vein thrombus.1 Frequently, HCC with portal vein thrombus is recognized, and its prognosis is poor, but the prognosis of HCC with BDTT is not well recognized due to its rarity. In particular, HCC with BDTT showing obstructive jaundice is called “icteric hepatoma,” and this type of HCC is known to present difficult problems in the differential diagnosis of conditions such as advanced liver cirrhosis and biliary tract cancer.2 HCC with BDTT showing obstructive jaundice leads to severe symptoms such as cholangitis and hemobilia and can cause hepatic failure.3–5 However, it is difficult to determine the surgical indications for patients with obstructive jaundice because jaundice due to advanced liver cirrhosis is a contraindication for hepatectomy.
Several studies have investigated HCC with BDTT, and it is clear that a surgical approach for HCC with BDTT is important.6–8 However, it currently is unclear whether surgical treatment is valid for HCC with BDTT that presents with obstructive jaundice because the short- and long-term outcomes in such cases are unknown. In addition, the optimal surgical approach for HCC patients with BDTT, whether to preserve the bile duct or not, also is unclear.
In this study, we reviewed HCC patients with and without BDTT and analyzed the clinicopathologic features and surgical outcomes. Furthermore, we reviewed the surgical management of patients with BDTT that present with obstructive jaundice and evaluated the validity of the surgical approaches for this type of HCC.
Patients and Methods
Between January 1996 and May 2015, 774 HCC patients underwent several types of hepatectomy in the Department of Gastroenterological Surgery 1 at Hokkaido University Hospital. Of these 774 patients, 42 (5.4 %) had HCC with BDTT that was diagnosed histopathologically. The study defined BDTT-positive patients as those who had BDTT in the biliary tree or the intrahepatic bile duct histopathologically and BDTT-negative patients as those who had no BDTT in the biliary tree or the intrahepatic bile duct. Microscopic BDTT was defined as BDTT that developed in more than only the second branch of the intrahepatic bile duct. Macroscopic BDTT was defined as BDTT existing in the second or first branch of the biliary tree or the common bile duct.
The study included 21 microscopic BDTT cases and 21 macroscopic BDTT cases. Of the 42 BDTT cases, 6 HCC patients with BDTT demonstrated obstructive jaundice, which required preoperative biliary drainage. Macroscopic portal vein tumor thrombus (PVTT) was defined as PVTT involving the first or second branches or the main trunk of the portal vein. This study had 20 macroscopic PVTT cases among the HCC patients with BDTT and 82 macroscopic PVTT cases among the HCC patients without BDTT. The median follow-up period for these patients was 45.4 months (range 0.2–233.2 months).
The study was approved by the institutional review board of Hokkaido University Hospital (approval number 015-0251). All analyses in this study were performed in accordance with the ethical guidelines of Hokkaido University Hospital.
Preoperative Management
Preoperative management was performed according to our previous report.9 Our algorithm, which incorporates the indocyanine green retention rate at 15 min (ICGR15) and remnant liver volume, was used to determine the operative procedure, as previously described.9 If the ICGR15 is <15 % and the resected liver volume is <60 %, hemihepatectomy or extended hemihepatectomy can be performed. However, if the ICGR15 is <15 % and the resected liver volume is >60 %, then percutaneous transhepatic portal embolization is performed before surgery. For patients with an ICGR15 of 15–20 %, sectionectomy can be performed, whereas for patients with an ICGR15 of 20–25 %, segmentectomy can be performed, and for patients with an ICGR15 of 25–40 %, a limited resection can be performed. If the ICGR15 is >40 %, hepatectomy is contraindicated.
For the patients in this study with BDTT who presented with obstructive jaundice, biliary drainage was performed first, and hepatic functional reserve was evaluated after the serum bilirubin level was lower than 2 mg/dl. A liver resection then was performed next when possible according to our algorithm.
Surgical Methods
The surgical methods used for liver resection were previously described.9 Anatomic resection was defined as a resection in which the lesion or lesions are anatomically removed completely based on Couinaud’s classification (segmentectomy, sectionectomy and hemihepatectomy, or extended hemihepatectomy). We preserved the bile duct in all HCC patients with BDTT.
Our surgical procedure is similar to bile duct–preserving surgery reported by Yamamoto et al.10 We cut the bile duct, peeled off the tumor thrombus, closed the bile duct incision site by running sutures with 5–0 or 6–0 absorbable monofilament thread, and inserted a C-tube into the cystic duct to decompress the bile duct. We performed cholangiography to verify the absence of the tumor thrombus.
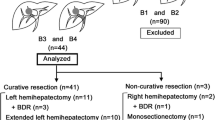
Hepatectomy for HCC with BDTT in this study included two right trisectionectomies, one left trisectionectomy, five extended right hepatectomies, four extended left hepatectomies, 13 right hepatectomies, eight left hepatectomies, one central bisectionectomy, two right anterior sectionectomies, two right posterior sectionectomies, two left lateral sectionectomies, and two partial resections.
Statistical Analysis
The correlation between BDTT and the clinicopathologic features was evaluated using Fisher’s exact test for categorical variables and the Mann–Whitney U test for continuous variables. The overall survival rates and the time to recurrence were calculated using the Kaplan–Meier method and compared between groups using the log-rank test. Potential prognostic factors were identified by univariate analysis using the log-rank test. Independent prognostic factors were evaluated using a Cox proportional hazards regression model. In this study, a p value lower than 0.05 was considered significant. Statistical analyses were performed using JMP (version 12 for Windows; SAS Institute, Cary, NC, USA).
Results
Differences in Clinicopathologic Features and Surgical Outcomes According to the Presence or Absence of BDTT
The clinicopathologic features of the HCC patients with and without BDTT are presented in Table 1. The median age, sex, proportion of hepatitis B surface antigen, proportion of hepatitis C virus antibody, Child-Pugh classification, ICGR15, and proportion of liver cirrhosis in the BDTT-negative and BDTT-positive groups were similar. On the other hand, the HCC patients with BDTT relative to those without BDTT demonstrated significantly different alpha-fetoprotein expression, tumor-node-metastasis (TNM) stage, tumor size, portal vein invasion status, and proportion of anatomic resection. In terms of histologic differentiation, the two groups did not differ significantly, but the BDTT-positive group showed a tendency toward poorer differentiation (p = 0.061) (Table 1). The HCC patients with BDTT showed an overall survival rate of 75.1 % at 1 year, 44.9 % at 3 years, and 36.6 % at 5 years compared respectively with 89.0, 72.4, and 61.9 % for the HCC patients without BDTT (p = 0.0003) (Fig. 1a). The median survival times (MST) were 2.46 for the HCC patients with BDTT and 7.62 years for the HCC patients without BDTT. Almost all the HCC patients with BDTT (41 of 42 patients) had stage 3 or 4 disease. Therefore, when the study was limited to stage 3 or 4 patients, the survival rate did not differ significantly between the HCC patients with BDTT and those without BDTT (p = 0.3366) (Fig. 1b).
a Correlation between bile duct tumor thrombus (BDTT) and clinical outcomes for hepatocellular carcinoma (HCC) patients after surgery. The HCC patients with BDTT demonstrated poorer overall survival than the patients without BDTT. b Correlation between BDTT and clinical outcomes for HCC patients after surgery when the patients are limited to those with stage 3 or 4 disease. The survival rate did not differ significantly between the stage 3 or 4 HCC patients with BDTT and those without BDTT. c Correlation between micro- and macroscopic BDTT and clinical outcomes for HCC patients after surgery. The survival rate did not differ significantly between the HCC patients with microscopic BDTT and those with macroscopic BDTT. d Correlation between preoperative jaundice and clinical outcomes of HCC patients with BDTT after surgery. The survival rate did not differ significantly between the patients with and those without obstructive jaundice
Risk Factors for Survival and Recurrence in HCC Patients with BDTT
For the HCC patients with BDTT, the univariate analysis showed that tumor size, differentiation, and portal vein invasion were significant prognostic factors for survival and that tumor size was a significant prognostic factor for recurrence (Table 2). The multivariate analysis showed that tumor size, differentiation, and portal vein invasion were independent prognostic factors for survival and that tumor size was prognostic for recurrence (Table 2).
In terms of BDTT extent, the survival rate did not differ significantly between the HCC patients with microscopic BDTT and the HCC patients with macroscopic BDTT (p = 0.4796), and the MST values were respectively 2.43 and 2.46 years (Table 2; Fig. 1c). The median time to recurrence was 0.48 years for the microscopic BDTT patients and 1.24 years for the macroscopic BDTT patients, but this difference also was not significant (p = 0.3331) (Table 2).
Among the HCC patients with BDTT, the survival rate did not differ significantly between the patients with macroscopic PVTT and those without macroscopic PVTT (p = 0.0820). But among the HCC patients without BDTT, the survival rate differed significantly between those with macroscopic PVTT and those without macroscopic PVTT (p < 0.0001).
Recurrence Sites and Treatments After Hepatectomy for HCC with BDTT
The sites of first recurrence after hepatectomy and treatments are listed in Table 3. The most frequent site of recurrence was the liver (15 of 24 patients; 62.5 %), and intrahepatic recurrence was most commonly treated using transcatheter arterial chemoembolization (TACE) (10 of 24 patients; 41.6 %). The current study had one case of recurrence, which developed in the form of BDTT. As a relapse treatment, this BDTT was peeled off, and the bile duct was preserved. Since this relapse treatment, the patient has been without recurrence for 19 months.
Perioperative Surgical Outcome for Patients with BDTT Who Present with Obstructive Jaundice
Six patients in the current study series needed preoperative biliary drainage due to jaundice (Table 4). Four patients needed endoscopic nasobiliary drainage, and two patients needed percutaneous transhepatic biliary drainage. The maximum serum bilirubin level before biliary drainage ranged from 3.4 to 22.7 mg/dl, with a median concentration of 6.6 mg/dl.
Liver resection for the cases with obstructive jaundice included five right hepatectomies and one right anterior sectionectomy. The overall survival rate did not differ significantly among the HCC patients with BDTT in terms of the presence or absence of preoperative jaundice (p = 0.5469) (Table 2; Fig. 1d).
The perioperative outcomes for HCC with BDTT are presented in Table 4. The two groups did not differ significantly depending on the presence or absence of preoperative jaundice in terms of operation time, blood loss, anatomic resection, postoperative complications, or postoperative hospital stays. In addition, no 90-day mortalities occurred in either study group.
Discussion
HCC with BDTT is a rare phenomenon that occurs in about 2.5–3.4 % of patients with HCC.6–8 Yeh et al.11 reported the relationship between the pathogenesis of HCC with BDTT and the microRNA-200 family. As reported, HCC with BDTT demonstrates pathologic features such as a higher incidence of vascular invasion and less histologic differentiation.6,11,12 The current results (Table 1) are consistent with these earlier findings.
The 5-year survival rate of HCC patients with BDTT is reportedly about 30 %.7,8,12 In the current series, the 5-year survival rate of HCC patients with BDTT was 36.6 %, and survival did not differ significantly between the HCC patients with BDTT and those without BDTT when the two groups were matched by stage. Wong et al.7 also reported similar survival rates between HCC patients with BDTT and those without BDTT using their matching criteria. Macrovascular invasion, including portal vein invasion and hepatic vein invasion, is correlated with a poor prognosis for HCC patients.13–17
In contrast, regarding BDTT, Esaki et al.8 reported that macroscopic bile duct invasion demonstrates a favorable impact on outcomes for HCC patients with BDTT. In the current series, the survival rate and the time to recurrence did not differ significantly between the HCC patients with microscopic BDTT and those with macroscopic BDTT. The data indicated that BDTT has a lower malignant potential than vascular invasion and that macroscopic BDTT is not a contraindication for hepatectomy.
Our results also indicate that tumor size, differentiation, and portal vein invasion are independent prognostic factors for survival and that tumor size is an independent factor for recurrence. Kasai et al.18 reported previously that major vascular invasion is a negative prognostic indicator for HCC patients with BDTT. These results suggest that BDTT alone does not affect prognosis and does so only in conjunction with other prognostic factors such as vascular invasion, poor differentiation, and large size.
Regarding a hepatectomy for HCC with BDTT, there is an issue about whether to preserve the bile duct or not. Wong et al.7 proposed extrahepatic bile duct resection for HCC with BDTT to minimize bile duct recurrence. However, according to several reports, routine bile duct resection is not recommended unless direct invasion of the BDTT into the bile duct is suspected.8,12,18 Shiomi et al.19 reported that the survival of their bile duct–preserved group was similar to that of their bile duct–resected group.
Recently, Yamamoto et al.10 reported using bile duct–preserving surgery for HCC with BDTT, called the “peeling off technique,” which is similar to our surgical approach. Preserving the bile duct is important for two reasons. First, BDTT demonstrates expansive growth and does not usually adhere to the bile duct wall.10 Second, treatments for recurrence, such as TACE or radiofrequency ablation (RFA), are restricted after resection of the common bile duct because liver abscess formation after TACE and RFA is relatively common when an underlying bilioenteric anastomosis is present.20,21
As indicated in Table 3, intrahepatic recurrence is the most common recurrence after liver resection for HCC, and TACE can be performed in this circumstance after preservation of the bile duct. Thus, by preserving the bile duct, the peeling-off technique is suitable for HCC with BDTT. However, careful observations must be made to check for BDTT-type recurrence. One patient with BDTT in the current series presented with recurrence in the bile duct, and a salvage operation was performed to peel off the BDTT. No recurrence was noted thereafter in this case. Bile duct–preserving hepatectomy for HCC with BDTT may cause this type of recurrence due to direct invasion of the BDTT into the bile duct, with the BDTT remaining in the small branches of the bile ducts with intraoperative seeding along them. Thus, thee selection criteria for preserving or resecting the bile duct still is unclear, and further studies are needed.
Jaundice affects the functional liver reserve, and patients with obstructive jaundice often present with cholangitis and hemobilia that can cause hepatic failure.3–5 Therefore, biliary drainage is mandatory before hepatectomy for patients with BDTT who present with obstructive jaundice. We perform preoperative biliary drainage at our hospital in accordance with the preoperative management of biliary tract cancer.22 Biliary drainage of the remnant liver is performed until the serum bilirubin level is lower than 2 mg/dl because a higher level is a contraindication for hepatectomy according to Makuuchi’s criteria, and hepatectomy is performed if the functional liver reserve is sufficient.23
In the current study, perioperative outcomes were similar between the groups depending on the presence or absence of preoperative jaundice. The postoperative complications according to the Clavien–Dindo classification24 and the periods of the postoperative hospital stays also were similar between our two study groups, and no 90-day mortalities occurred in either group. These data suggest that hepatectomy for patients with BDTT who present with obstructive jaundice can be performed safely if sufficient functional liver reserve remains after biliary drainage.
In conclusion, a hepatectomy should be considered for HCC patients with BDTT, including patients with obstructive jaundice, because surgical outcomes equivalent to those for HCC without BDTT can be achieved if their functional liver reserve is sufficient.
References
Ikai I, Arii S, Kojiro M, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004;101:796–802.
Kojiro M, Kawabata K, Kawano Y, Shirai F, Takemoto N, Nakashima T. Hepatocellular carcinoma presenting as intrabile duct tumor growth: a clinicopathologic study of 24 cases. Cancer. 1982;49:2144–7.
Suh YG, Kim do Y, Han KH, Seong J. Effective biliary drainage and proper treatment improve outcomes of hepatocellular carcinoma with obstructive jaundice. Gut Liver. 2014;8:526–35.
Minami Y, Kudo M. Hepatocellular carcinoma with obstructive jaundice: endoscopic and percutaneous biliary drainage. Dig Dis. 2012;30:592–7.
Sugiyama G, Okabe Y, Ishida Y, et al. Evaluation of endoscopic biliary stenting for obstructive jaundice caused by hepatocellular carcinoma. World J Gastroenterol. 2014;20:6968–73.
Satoh S, Ikai I, Honda G, et al. Clinicopathologic evaluation of hepatocellular carcinoma with bile duct thrombi. Surgery. 2000;128:779–83.
Wong TC, Cheung TT, Chok KS, et al. Outcomes of hepatectomy for hepatocellular carcinoma with bile duct tumour thrombus. HPB Oxf. 2015;17:401–8.
Esaki M, Shimada K, Sano T, Sakamoto Y, Kosuge T, Ojima H. Surgical results for hepatocellular carcinoma with bile duct invasion: a clinicopathologic comparison between macroscopic and microscopic tumor thrombus. J Surg Oncol. 2005;90:226–32.
Kamiyama T, Nakanishi K, Yokoo H, et al. Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg. 2010;211:443–9.
Yamamoto S, Hasegawa K, Inoue Y, et al. Bile duct-preserving surgery for hepatocellular carcinoma with bile duct tumor thrombus. Ann Surg. 2015; 261:e123–5.
Yeh TS, Wang F, Chen TC, Yeh CN, Yu MC, Jan YY, Chen MF. Expression profile of microRNA-200 family in hepatocellular carcinoma with bile duct tumor thrombus. Ann Surg. 2014;259:346–54.
Moon DB, Hwang S, Wang HJ, et al. Surgical outcomes of hepatocellular carcinoma with bile duct tumor thrombus: a Korean multicenter study. World J Surg. 2013;37:443–51.
Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36.
Minagawa M, Makuuchi M, Takayama T, Ohtomo K. Selection criteria for hepatectomy in patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg. 2001;233:379–84.
Kamiyama T, Nakanishi K, Yokoo H, et al. Efficacy of preoperative radiotherapy to portal vein tumor thrombus in the main trunk or first branch in patients with hepatocellular carcinoma. Int J Clin Oncol. 2007;12:363–8.
Inoue Y, Hasegawa K, Ishizawa T, et al. Is there any difference in survival according to the portal tumor thrombectomy method in patients with hepatocellular carcinoma? Surgery. 2009;145:9–19.
Kokudo T, Hasegawa K, Yamamoto S, et al. Surgical treatment of hepatocellular carcinoma associated with hepatic vein tumor thrombosis. J Hepatol. 2014;61:583–8.
Kasai Y, Hatano E, Seo S, Taura K, Yasuchika K, Uemoto S. Hepatocellular carcinoma with bile duct tumor thrombus: surgical outcomes and the prognostic impact of concomitant major vascular invasion. World J Surg. 2015;39:1485–93.
Shiomi M, Kamiya J, Nagino M, et al. Hepatocellular carcinoma with biliary tumor thrombi: aggressive operative approach after appropriate preoperative management. Surgery. 2001;129:692–8.
Woo S, Chung JW, Hur S, Joo SM, Kim HC, Jae HJ, Park JH. Liver abscess after transarterial chemoembolization in patients with bilioenteric anastomosis: frequency and risk factors. AJR Am J Roentgenol. 2013;200:1370–7.
Iida H, Aihara T, Ikuta S, Yamanaka N. Risk of abscess formation after liver tumor radiofrequency ablation: a review of 8 cases wtih a history of enterobiliary anastomosis. Hepatogastroenterology. 2014;61:1867–70.
Miyazaki M, Yoshitomi H, Miyakawa S, et al. Clinical practice guidelines for the management of biliary tract cancers 2015. 2nd English edition. J Hepatobiliary Pancreat Sci. 2015;22:249–73.
Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, Kawasaki S. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298–304.
Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96.
Conflict of interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orimo, T., Kamiyama, T., Yokoo, H. et al. Hepatectomy for Hepatocellular Carcinoma with Bile Duct Tumor Thrombus, Including Cases with Obstructive Jaundice. Ann Surg Oncol 23, 2627–2634 (2016). https://doi.org/10.1245/s10434-016-5174-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5174-7