Abstract
Background
Robotic total mesorectal excision (R-TME), a novel way for minimally invasive treatment of rectal cancer, was shown in previous studies to be safe and effective. However, comparison with laparoscopic total mesorectal excision (L-TME) has drawn contradictory disputes, especially concerning operative high-risk patients. The aim of this study was to compare R-TME and L-TME on the rectal technical approach.
Methods
Between October 2009 and March 2013, a total of 120 consecutive rectal carcinomas, operated for sphincter-saving procedure, were enrolled. The patient population included the last 60 laparoscopic procedures and the first 60 robotic surgeries (six hybrid approaches, then 54 full robotic surgeries). There were no exclusions.
Results
Patients’ baseline characteristics were similar in both the R-TME and L-TME groups. Outcomes were equivalent for blood loss (200 vs. 100 mL), postoperative hospital stay (12 vs. 11 days), conversion rate (3.2 vs. 4.8 %), lymph nodes yield (15 vs. 19), no positive distal margin (0 %), positive radial margin (6.4 vs. 9.3 %), diverting ileostomy (73 vs. 58 %) and severe morbidity (28 vs. 20 %). Significant differences were found for median operative time (274 vs. 228 min; p = 0.003) and proctectomy performed via transanal approach (1.7 vs. 16.7 %; p = 0.004). The R-TME operative time curve stabilized to 245 min after the first 25 procedures.
Conclusions
For rectal cancer, R-TME may be as feasible and safe as L-TME in terms of technique. In our practice and for difficult cases, R-TME allows complete rectal dissection by an abdominal approach, while L-TME requires a transanal approach.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Classically, the laparoscopic approach for total mesorectal excision (L-TME) improved short-term outcomes and provided a clearer intraoperative view compared with the open approach in a deep and narrow pelvis.1,2 Results from the COLOR II trial confirmed improved patient recovery and similar safety, same resection margins, and completeness of resection using L-TME compared with the results of open surgery.2 Long-term results from the CLASICC and COLOR II trials supported the use of laparoscopic surgery for colorectal cancer and showed no difference between L-TME and conventional open resection on overall survival, disease-free survival, and local recurrence.1,3 Nevertheless, two recent trials questioned these issues, especially the non-inferiority of laparoscopic resections according to the rate of successful resections.4,5 The laparoscopic resection of rectal cancer, especially in a deep and narrow pelvis, is technically challenging and demands a long learning curve. Technical limitations include restricted mobility of straight laparoscopic instruments and associated loss of dexterity, unstable camera view, and compromised ergonomics for the surgeon. To avoid this drawback, for patients with high-risk of conversion we have described the transanal TME (TaTME) approach performed with the TEO device (Karl Storz Gmbh & Co., Tuttlingen, Germany).6 A randomized clinical trial comparing TaTME and traditional L-TME for rectal cancer is due to start (COLOR III trial).
Robotic technology was developed to reduce these limitations, and offers the advantages of intuitive manipulation of laparoscopic instruments with wristed articulation, a three-dimensional field of view, a stable camera platform with zoom magnification, dexterity enhancement, and an ergonomic operating environment. A major advantage of this approach is the surgeon’s simultaneous control of the camera and of the two or three additional instruments, which facilitates traction and counter-traction. The technological advantages of robotic surgery should also allow a finer dissection in a narrow pelvic cavity.7,8 However, total robotic surgery for rectal cancer is still technically challenging and involves two operative fields (splenic flexure and rectum), potential collision of the robotic arms, and lack of tactile feedback.
Robotic and laparoscopic rectal cancer surgery showed similar intraoperative results and morbidity, postoperative recovery, and short-term oncologic outcomes;7–12 however, longer operation times were described as a disadvantage of the robotic system. On the other hand, all meta-analyses comparing robotic-assisted total mesorectal excision (R-TME) and L-TME concluded in the reduction of the conversion rate.13–19 The first results of the ROLARR trial, whose primary endpoint was the conversion rate, were reported by D. Jayne during the 2015 American Society of Colon and Rectal Surgeons (ASCRS) annual meeting. Robotic surgery does not seem to show any statistically significant evidence of superiority compared with laparoscopic surgery.20
R-TME clinical advantages are thus still discussed, especially regarding the cost of the device.21 The aim of the present study was to compare the outcomes of the two techniques, and analyze the impact of R-TME on the TaTME rectal approach for difficult patients.
Patients and Methods
Patients
Between October 2009 and March 2013, a total of 120 consecutive patients underwent TME for non-metastatic rectal carcinoma at the Montpellier Cancer Institute, France. Sixty patients underwent L-TME before February 2012, followed by 60 patients who underwent R-TME. Prior to this study, we had performed more than 300 L-TME procedures; however, we had no prior experience with R-TME. No patient undergoing a sphincter-saving procedure was excluded from either group during the study period. All relevant data were registered in a prospectively maintained database. The study was approved by the local Ethics Committee and was conducted in accordance with the Declaration of Helsinki principles.
Preoperative Staging and Treatments
Preoperative staging was standardized: colonoscopy with biopsy; chest, abdominal and pelvic imaging by computed tomography (CT); endoscopic ultrasound, magnetic resonance imaging (MRI) or both, and carcinoembryonic antigen (CEA).
Patients with locally advanced disease were administered neoadjuvant chemotherapy (capecitabine 25 mg/m2 twice daily) and radiotherapy (45 Gy in 25 fractions over 5 weeks with a 5 Gy boost). Follow-up was performed every 3 months for 3 years, then every 6 months until the fifth year.
Surgical Technique
A single surgeon (PR) performed all procedures, in both the L-TME and R-TME series. Patients were consecutive, and the indications for TaTME were homogeneous, and were linked to the operative technical difficulties.
We used the zero-degree camera for both approaches. The patient was placed in the lithotomy-Trendelenburg position and tilted to the right. The splenic flexure was completely mobilized after ligation of the inferior mesenteric artery and vein with Hem-o-lok® clips (Weck Closure System, Research Triangle Park, NC, USA), followed by medial-to-lateral dissection of the omentum and the transverse mesocolon.
The TME procedure was standardized in both groups, beginning with posterior dissection in the avascular plane, followed by anterior and lateral dissections. Care was always taken to preserve the sexual nerves. In case of a low rectal anastomosis, resection of the rectum was performed with Echelon Flex Endopath Stapler (Ethicon Endo-Surgery, Inc.). The colorectal anastomosis was always side-to-end, performed with a Proximate ILS stapler (Ethicon Endo-Surgery, Inc.). In case of coloanal anastomoses, the endoanal dissection was performed with partial or complete inter-sphincteric resection. A defunctioning ileostomy could generally be performed, depending on the surgeon’s discretion.
As with the laparoscopic approach, four trocars were used in the robotic procedures [da Vinci Si-e surgical system (Intuitive Surgical, Sunnyvale, CA, USA)]. The patient cart was positioned obliquely at a 60° angle and at the left-lower quadrant of the abdomen. The robotic arms were then docked to the three trocars (Fig. 1).
Port placement. Positioning of the camera port, two robotic arms R1–R2, constant assistant port (12 mm), and inconstant port (5 mm). R1 robotic arm 1, R2 robotic arm 2, C camera port, 12 mm AP assistant port, 5 mm AP assistant port (only in difficult cases), MCL median clavicular line, SUL spine umbilicus line
The first six cases (10 %) of the R-TMEs involved a hybrid approach, while the remaining 54 cases (90 %) were performed using a standardized, single-docking, fully robotic approach. The position of the arms was determined to avoid collisions. Robotic anastomoses involved a double-stapling technique with a circular stapler, followed by methylene blue. Protective ileostomy was performed at the surgeon’s discretion.
In both groups, in case of predictive, difficult rectal dissection (male patient, high body mass index [BMI], narrow pelvis, residual anterior bulky tumor), a TaTME was decided in order to avoid conversion and maintain good radial margin. The decision for this TaTME was made either preoperatively or perioperatively according to the immediate preoperative examination or during a difficult pelvic dissection. A transanal endoscopic operation (TEO) device (Karl Storz Gmbh & Co) was used.
Statistical Analysis
Categorical and ordinal variables were analyzed by means of frequency and percentages using the Pearson Chi square or Fisher’s exact tests. Continuous variables were presented using medians, means and range values, and compared using the non-parametric Kruskal–Wallis test. Overall survival was measured from the date of surgery to death from any cause, and relapse-free survival was measured from the date of surgery to the observation of local recurrence. Both rates were estimated using the Kaplan–Meier method. Survival curves were compared using the log-rank test.
All reported p values were two-sided and were considered significant at the 5 % level.
Statistical analysis was performed using the STATA 13 software (StataCorp LP, College Station, TX, USA).
Results
Sixty patients were included in each group. Patients’ baseline characteristics were similar in both groups, including age, sex, BMI, American Society of Anesthesiologists’ grade, tumor stage, tumor location, and preoperative radiochemotherapy (Table 1). With regard to intraoperative outcomes (Table 2), the type of operation was similar in the two groups either for low rectal or coloanal anastomoses. For low rectal tumors, the transanal dissection was mostly performed with a partial inter-sphincteric resection. A lateral coloanal anastomosis was sometimes performed in the R-TME group, while most of the L-TME group had a J-pouch after resection. The rate of conversion was similar in both groups (3.2 vs. 4.8 %; p = 0.661). Converted patients showed a fatty mesenterium with rectal exposure inability (n = 2), or a complete peritoneal adhesion after previous abdominal surgery (n = 3). In difficult cases with a high-risk of conversion, a TaTME was performed. Only one patient (1.7 %) in the R-TME group underwent a TaTME, whereas this procedure was performed in 16.7 % (n = 10) of the L-TME patients (p = 0.004). For the L-TME group, the reasons were mostly multiple, such as narrow pelvis and a bad tumor response for initially advanced tumors (Table 3). The opposite was observed for the R-TME group, where the only TaTME made was for an ultra-low tumor, with a first transanal approach conducted to confirm the possibility of a sphincter-saving surgery.
Protective ileostomies were performed in 44 (73 %) R-TME patients and 35 (58 %) L-TME patients (p = 0.08). Median estimated blood loss (EBL) was similar between the two groups. Median operative times were significantly different—274 min (range 125–437) for the R-TME group, including setting-up and docking of the robot, versus 228 min (range 127–431) for the L-TME group (p = 0.003).
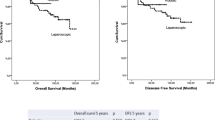
Postoperative outcomes and pathological findings are presented in Table 4. The median hospital stay was similar in both groups: 12 days (range 6–27) in the R-TME group versus 11 days (range 6–60) in the L-TME group. Severe morbidity (Dindo classification 3–4) was similar between the groups. Pathological findings reflected the quality of resected specimen and were not significantly different. The median distal resection margins ranged from 2.0 to 7.3 cm in the R-TME group, versus 0.3–8.0 cm in the L-TME group. Circumferential resection margins were <1.0 mm in three (6.4 %) and four (9.3 %) patients in the R-TME and L-TME groups, respectively (p = 0.66). The median follow-up was 24 months, 17 months (95 % CI 15–19), and 32 months (95 % CI 30–35) for R-TME and L-TME patients. Despite a short follow-up, overall survival at 18 months was similar in the two groups (95 vs. 96.7 %; p = 0.414), as was relapse-free survival (92.1 vs. 96 %; p = 0.595) (electronic supplementary Fig. 1).
Discussion
Robotic surgery addresses most of the limitations of laparoscopic surgery because it offers a three-dimensional stable view with a camera controlled by the surgeon, and a wristed instrumentation allowing a better range of motions for precise dissection. Given the narrowness of the pelvis, rectal cancer dissection should be the indication of choice for the use of robotic surgery. Nevertheless, the clinical benefits of robotic surgery in low rectal lesions have not yet been defined. Our preliminary experience confirms similar morbidity and oncologic results for the two techniques, as shown in previous studies. It underlines the need for a dedicated robotic learning curve, the standardization of the technique, and the ability of the robot to perform a complete abdominal approach in difficult cases when laparoscopy requires a transanal approach.
Learning Curve and Operative Time
During our study, the operative time evolved in the R-TME group from 352 min for the first six patients to 245 min for the last 35 patients compared with 228 min for the L-TME group, with no significant difference (electronic supplementary Fig. 2).
Park et al. demonstrated that 90 laparoscopic procedures were required to overcome the learning curve versus only 20 robotic surgeries to reach the first plateau of 220 min.9 D’Annibale et al. showed a significant decrease in the mean operative time from 312 min for the 25 first procedures to 238 min for the 10 last procedures.7
Recently, Kuo et al. used a seventh-order moving average method for the construction of a R-TME learning curve.22 The first plateau was observed after 19 patients, with a mean operative time of 519.5 min, reduced significantly to 448.2 min for the following 17 patients (p = 0.02).
These reports regarding the operative time reflect the importance of the learning curve in robotic rectal cancer surgery. This was recently illustrated by the ROLARR trial, one major critic being the surgeons’ short baseline experience, and thus the reasons for conversion (47.4 % for pelvic dissection in the robotic arm). In our experience, most of the extraoperative time in R-TME was seen in the initial series before we came to standardizing the technique.
Standardization of the Technique
Correct port placement is the principal consideration in this procedure. Despite the rapid acceptance of robots in prostate cancer surgery, this application in rectal cancer surgery seems relatively delayed. One reason is that the range of the operative field is not limited to the pelvis but is expanded to the splenic flexure. Such a wide range may frequently lead to external collision of the robotic arms during surgery. Consequently, surgeons seem reluctant to adopt it. Some authors described a hybrid procedure mobilizing the splenic flexure and ligating the inferior mesenteric vessels via laparoscopic approach.10,23,24 Other authors published a multi-docking procedure with cart repositioning or arm replacement,7,8 or showed that both the patient cart and the arms could not be modified during the entire procedure.25 Our technique uses a single-docking and a fully robotic approach without port re-docking. Two steps are crucial, the tailoring of the port placement after induction of pneumoperitoneum, and the testing of arms-free movement before insertion of the instruments. They allow the adjustment of the positioning of the arm elbows and facilitate their movement.
Risk of Conversion and Effects for the Rectal Approach
L-TME raises the question of conversion rate and curability, assessed through the radial margin, especially for difficult patients. Seven meta-analyses13–19 compared R-TME with L-TME, and they all concluded that R-TME was associated with a lower conversion rate, whereas other variables were often statistically similar. The largest series of R-TME (965 patients), stemmed from the US national cancer database, confirmed a 9.5 % conversion rate compared with a 16.4 % conversion rate with L-TME (p < 0.001).26
Jayne20 who conducted the phase III ROLARR trial, recently presented results on the conversion rate in 471 randomized patients. They showed that it was not significantly different (odds ratio [OR] 0.61) between the R-TME and the L-TME groups. Nevertheless, possible benefits in males, low anterior resection, and obese patients seem conceivable (OR 0.46, 0.49 and 0.58, respectively).
The conversion rate to open surgery in the laparoscopic groups varied between 1 and 34 %. This rate was high in the CLASICC trial (34 %), remained at 17 % in the COLOR II trial, fell to 1 % in the Korean trial, and stabilized between 9 and 11 % in the ALaCaRT and ACOSOG trials.4,5 Reasons for conversion were mostly adhesions, fibrosis, and a too-narrow pelvis.
A new way for rectal dissection may solve the problem: the TaTME. To date, 200 patients who underwent TaTME were reported in the literature in the last 3 years. The results are homogeneous and promising, especially for the curability rate (electronic supplementary Table 1), which varied from 87 to 100 % according to the indication of the TaTME. If its feasibility is proved, a longer follow-up is needed to assess the functional and oncological results.
In our series, the conversion rate was equivalent between the two techniques with the same R0 resection rate. This rate was only 2 % in the laparoscopic group, due to our bottom–up rectal resection with the TEO device (TaTME). The TaTME rate was 16.7 % in the L-TME versus 1.7 % in the R-TME group (p = 0.004). These data implicitly confirmed the ability to perform a safe dissection with the robotic approach in a limited space as the narrow pelvis, and more specifically the retro-prostatic space for the anterior middle-third rectal tumor.
The two recent phase III trials comparing the open laparotomy and laparoscopy techniques have renewed the debate on the best surgical procedure. Our primary choice for a high-risk rectal patient is the robotic approach due to the ease, even in a narrow pelvis, of a deep rectal dissection. We used the TaTME approach for low tumors in case of an uncertain sphincter-saving procedure.
Conclusions
For rectal cancer, R-TME may be as feasible and safe as L-TME not only in terms of morbidity and short-term overall survival but also in terms of technical feasibility. In our practice, R-TME allows complete rectal dissection by an abdominal approach, while L-TME requires a transanal approach in order to avoid conversion in difficult cases.
Several ongoing prospective trials aim to randomize L-TME to Ta-TME. Our paper allows extending this question to R-TME.
References
Green BL, Marshall HC, Collinson F, et al. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg. 2013;100:75–82.
van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–8.
Bonjer HJ, Deijen CL, Abis GA, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372:1324–32.
Fleshman J, Branda M, Sargent DJ, et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA. 2015;314:1346–55.
Stevenson ARL, Solomon MJ, Lumley JW, et al. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: The ALaCaRT Randomized Clinical Trial. JAMA. 2015;314:1356–63.
Rouanet PMD, Mourregot AMD, Azar CCMD, et al. Transanal endoscopic proctectomy: an innovative procedure for difficult resection of rectal tumors in men with narrow pelvis. Dis Colon Rectum. 2013;56:408–15.
D’Annibale A, Pernazza G, Monsellato I, et al. Total mesorectal excision: a comparison of oncological and functional outcomes between robotic and laparoscopic surgery for rectal cancer. Surg Endosc. 2013;27:1887–95.
Kwak JM, Kim SH, Kim J, Son DN, Baek SJ, Cho JS. Robotic vs laparoscopic resection of rectal cancer: short-term outcomes of a case-control study. Dis Colon Rectum. 2011;54:151–6.
Park JS, Choi G-S, Lim KH, Jang YS, Jun SH. Robotic-assisted versus laparoscopic surgery for low rectal cancer: case-matched analysis of short-term outcomes. Ann Surg Oncol. 2010;17:3195–202.
Patel CB, Ragupathi M, Ramos-Valadez DI, Haas EM. A three-arm (laparoscopic, hand-assisted, and robotic) matched-case analysis of intraoperative and postoperative outcomes in minimally invasive colorectal surgery. Dis Colon Rectum. 2011;54:144–50.
Bianchi PP, Ceriani C, Locatelli A, et al. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a comparative analysis of oncological safety and short-term outcomes. Surg Endosc. 2010;24:2888–94.
Popescu I, Vasilescu C, Tomulescu V, Vasile S, Sgarbura O. The minimally invasive approach, laparoscopic and robotic, in rectal resection for cancer. A single center experience. Acta Chir Iugosl. 2010;57:29–35.
Lin S, Jiang H-G, Chen Z-H, Zhou S-Y, Liu X-S, Yu J-R. Meta-analysis of robotic and laparoscopic surgery for treatment of rectal cancer. World J Gastroenterol. 2011;17:5214–20.
Trastulli S, Farinella E, Cirocchi R, et al. Robotic resection compared with laparoscopic rectal resection for cancer: systematic review and meta-analysis of short-term outcome. Colorectal Dis. 2012;14:e134–56.
Memon S, Heriot AG, Murphy DG, Bressel M, Lynch AC. Robotic versus laparoscopic proctectomy for rectal cancer: a meta-analysis. Ann Surg Oncol. 2012;19:2095–101.
Yang Y, Wang F, Zhang P, et al. Robot-assisted versus conventional laparoscopic surgery for colorectal disease, focusing on rectal cancer: a meta-analysis. Ann Surg Oncol. 2012;19:3727–36.
Gonzálezfernández AM, Mascareñasgonzález JF. Total laparoscopic mesorectal excision versus robot-assisted in the treatment of rectal cancer: a meta-analysis [in Spanish]. Cir Esp. 2012;90:348–54.
Ortiz-Oshiro E, Sánchez-Egido I, Moreno-Sierra J, Pérez CF, Díaz JS, Fernández-Represa JÁ. Robotic assistance may reduce conversion to open in rectal carcinoma laparoscopic surgery: systematic review and meta-analysis. Int J Med Robot. 2012;8:360–70.
Xiong B, Ma L, Zhang C, Cheng Y. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a meta-analysis. J Surg Res. 2014;188:404–14.
Jayne D. The evidence, where are we? Robotic versus laparoscopic resection for rectal cancer trial results. Oral communication. ASCRS Annual Meeting, 1 Jun 2015.
Park EJ, Cho MS, Baek SJ, et al. Long-term oncologic outcomes of robotic low anterior resection for rectal cancer: a comparative study with laparoscopic surgery. Ann Surg. 2015;261:129–37.
Kuo L-J, Lin Y-K, Chang C-C, Tai C-J, Chiou J-F, Chang Y-J. Clinical outcomes of robot-assisted intersphincteric resection for low rectal cancer: comparison with conventional laparoscopy and multifactorial analysis of the learning curve for robotic surgery. Int J Colorectal Dis. 2014;29:555–62.
Kim HJ, Choi G-S, Park JS, Park SY. Multidimensional analysis of the learning curve for robotic total mesorectal excision for rectal cancer: lessons from a single surgeon’s experience. Dis Colon Rectum. 2014;57:1066–74.
Baek J-H, Pastor C, Pigazzi A. Robotic and laparoscopic total mesorectal excision for rectal cancer: a case-matched study. Surg Endosc. 2010;25:521–5.
Park YA, Kim JM, Kim SA, et al. Totally robotic surgery for rectal cancer: from splenic flexure to pelvic floor in one setup. Surg Endosc. 2010;24:715–20.
Speicher PJ, Englum BR, Ganapathi AM, Nussbaum DP, Mantyh CR, Migaly J. Robotic low anterior resection for rectal cancer: a national perspective on short-term oncologic outcomes. Ann Surg. 2015;262(6):1040–5.
Acknowledgments
The authors would like to thank Dr. Hélène de Forges for her writing assistance and editorial help. They also thank Nadia Bensmail for her significant work as clinical research associate, and the data managers from the Biostatistics Unit of the Montpellier Cancer Institute (ICM), France; this unit is a certified DataCenter for Cancer Clinical Trials (CTD, INCa).
Disclosures
P. Rouanet is proctor for Intuitive and Ethicon. F. Quénet is proctor for Ethicon.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Colombo, PE., Bertrand, M.M., Alline, M. et al. Robotic Versus Laparoscopic Total Mesorectal Excision (TME) for Sphincter-Saving Surgery: Is There Any Difference in the Transanal TME Rectal Approach?. Ann Surg Oncol 23, 1594–1600 (2016). https://doi.org/10.1245/s10434-015-5048-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-5048-4