Abstract
Background
Microvascular invasion (MiVI) is a major risk factor of survival outcomes after curative resection for patients with hepatocellular carcinoma (HCC). This study aimed to investigate the impact of postoperative adjuvant transcatheter arterial chemoembolization (PA-TACE) on HCC patients with MiVI.
Methods
From January 2004 to June 2013, HCC patients with histologically confirmed MiVI and well-tolerated liver function who underwent PA-TACE after R0 hepatectomy (RH) or RH alone were studied retrospectively. In the PA-TACE group, PA-TACE was given 4 weeks after RH. Uni- and multivariate analyses were used to identify the prognostic significance of PA-TACE.
Results
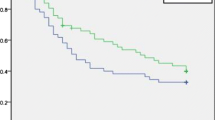
Of the 322 HCC patients with MiVI included in the analysis, 185 entered into the RH group and 137 entered into the PA-TACE group. The baseline characteristics of the two groups were similar except for alanine aminotransferase (ALT) level (p = 0.037). The 1-, 2-, 3-, and 5-year recurrence-free survival (RFS) rates were respectively 69.3, 55.5, 46.7, and 35.0 % for the PA-TACE group and 47.0, 36.2, 34.1, and 30.3 % for the RH group (log-rank, χ2 = 6.309; p = 0.012). The 1-, 2-, 3-, and 5-year overall survival (OS) rates were respectively 94.2, 78.8, 71.5, and 54.0 % for the PA-TACE group and 78.9, 62.2, 54.1, and 43.2 % for the RH group (log-rank, χ2 = 7.537; p = 0.006). Multivariate Cox proportional hazards regression analysis showed PA-TACE to be an independent risk factor of postoperative RFS and OS.
Conclusions
This study showed that PA-TACE may be beneficial for HCC patients with MiVI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hepatocellular carcinoma (HCC) is a common cancer with a dismal prognosis. Although R0 hepatectomy (RH) is the most widely practiced therapy aimed at curing HCC, it has a 70 % rate of tumor recurrence 5 years after operation.1 Early spread of cancer cells via the bloodstream, especially in HCC with microvascular invasion (MiVI), is a key mechanism for intrahepatic metastasis and early tumor recurrence.2 – 4 In addition, MiVI shows aggressive tumor behavior and is closely linked to a larger tumor burden.5 Unfortunately, there is still no recommended postoperative treatment strategy for HCC patients with MiVI, which renders the postoperative management of these patients a major challenge.
Transcatheter arterial chemoembolization (TACE) is the therapy most commonly used to treat intermediate-stage HCC. By arterial injection of chemotherapeutic drugs and embolizing agents, TACE decreases blood flow to the tumor and induces tumor ischemic necrosis.6
The effectiveness of TACE as an adjuvant therapy for HCC has been documented in clinical studies.7 , 8 A randomized controlled study (RCT) showed postoperative adjuvant (PA) TACE to be beneficial for patients with HCC larger than 5 cm in diameter, multiple nodules, or macroscopic vascular invasion.9 Similar results were obtained for patients with macroscopic portal vein tumor thrombus (m-PVTT).10 – 12 Although Ke-Wei et al.13 reported that PA-TACE improves trends in both recurrence-free survival (RFS) and overall survival (OS), the small sample size prevented their results from achieving statistical significance. Thus, the effectiveness of PA-TACE for HCC patients with MiVI needs further investigation.
This study was designed to evaluate the effectiveness of PA-TACE for HCC patients with MiVI. The RFS and OS of these patients were analyzed.
Materials and Methods
Patients and Design
We consecutively reviewed the demographic, clinical, and pathologic data of HCC patients with MiVI who underwent RH alone or PA-TACE from January 2004 to June 2013 from our team at the Eastern Hepatobiliary Surgery Hospital. In this study, MiVI was defined as microscopic tumor invasion identified in the portal and hepatic veins of the surrounding liver tissue contiguous with the tumor edge and confirmed histologically in the resected specimens by three senior pathologists from the Pathology Department of our hospital.
All the patients were reexamined in our hospital 4 weeks after RH. If no recurrence was found, the PA-TACE treatment strategy was recommended by the attending physician (e.g., S.Q.C. or J.J.S.) due to the pathologic MiVI. Whether the patients followed the physician’s recommendation or not depended on their socioeconomic status or compliance with the attending physician. If they followed the recommendation, PA-TACE was administered. Patients who refused PA-TACE underwent RH only.
Intraoperative liver ultrasound was routinely performed for all patients at the time of operation. If the patients were found to have multiple tumors or a single tumor during the first evaluation 4 weeks after RH, they were considered to have tumor recurrence and excluded from this study. The diagnosis of recurrence was based on computed tomography (CT) scan and/or magnetic resonance imaging (MRI) and raised serum alpha-fetoprotein (AFP) level.
The study enrolled patients who met the following criteria: (1) no previous treatment for HCC; (2) no other malignancies; (3) curative resection (R0) performed previously, defined as complete macroscopic removal of the tumor with negative histologic resection margins for the tumor14; (4) no macrovascular invasion, such as m-PVTT or macroscopic hepatic vein tumor thrombus (m-HVTT); (5) functional liver status of Child-Pugh A-B, with a serum bilirubin level 1.5 times the upper limit of normal or less and alanine aminotransferase and aspartate aminotransferase 2 times the upper limit of normal or less; and (6) hemoglobin level 90 g/L or higher, white blood cell level 3000 cells/mm3 or higher, and platelet level 80.000 cells/mm3 or higher.15
When recurrent HCC was diagnosed during the study period, the patients were actively treated with radiofrequency ablation, percutaneous ethanol injection, transarterial chemoembolization, or repeat liver resection, according to the general condition of the patient, the underlying functional status of the liver, and the number and location of HCC recurrence. The tumor stage was determined according to the Barcelona Clinic Liver Cancer (BCLC) staging system.16
The patients recruited for this study were well informed about the hazard of MiVI and the potential benefit of PA-TACE. Written informed consent for the patients’ data to be used for research purposes was obtained from all the patients. The study protocol was approved by the Institutional Ethics Committee (IEC) and the Institution Review Board (IRB) of the Eastern Hepatobiliary Hospital.
PA-TACE
At 4 weeks after RH, when the liver function of the patient had recovered, a hepatic arterial catheter was placed into the proper hepatic artery through the femoral artery using the Seldinger technique, and TACE was performed for the entire remnant liver. Hepatic angiography, CT angiography, or both were performed to detect any obvious tumor stains in the remnant liver.
An emulsion of doxorubicin hydrochloride (10 mg), pirarubicin (THP) or pharmorubicin (20–40 mg), and lipiodol (2–10 ml) (Lipiodol Ultrafluide, Guerbet, Aulnay-Sous-Bois, France) then was infused through the catheter. The dosage of lipiodol and doxorubicin was determined by body surface area and underlying liver function.11 After 1 month of follow-up evaluation, a CT scan was performed to determine the effects of TACE.
Surgical Procedures
All surgical procedures were performed in accordance with our previous study.17 Only patients with normal or Child-Pugh class A or selected B liver function were offered major hepatic resection (≥3 Couinaud’s segments) or minor hepatic resection (≤2 Couinaud’s segments).
During surgery, we carefully searched the abdominal cavity to determine the extent of local disease, extrahepatic metastases, and peritoneal seeding. After mobilization of the liver, intraoperative ultrasound was performed to assess the number and size of the lesions, as well as the relation of the tumor to vascular structures. The Pringle maneuver was applied to occlude the blood inflow of the liver. Liver resection was performed by a clamp-crushing method.
Follow-Up Evaluation and Survival Analyses
Postoperative patient surveillance and management were performed in accordance with our previous study.18 All HCC patients with MiVI were first evaluated for recurrence 4 weeks after RH by CT scan and a measurement of the serum AFP level. The patients then were reevaluated every 3–4 months by measurement of their serum AFP level and by CT or MRI until death or dropout from the follow-up program. The reported recurrence rates are based on radiologic findings.
Recurrence-free survival was defined as the interval (in months) between RH and the diagnosis of HCC recurrence using either intrahepatic recurrence or extrahepatic metastasis as end points. Overall survival was defined as the interval (in months) from the date of RH to the date of death. This study was censored on 31 August 2014.
Statistical Analysis
Survival was analyzed by the Kaplan–Meier method, and survival curves were compared by the log-rank test. Uni- and multivariate analyses were based on the Cox proportional analysis. Continuous variables are expressed as mean ± standard deviation or median (range) as appropriate. Categorical variables were compared using the Chi square test or Fisher’s exact test, and continuous variables were compared using Student’s t test. A p value lower than 0.05 was considered to be statistically significant. The analysis was performed using SPSS for Windows 20.
Results
Patient Characteristics
From January 2004 to June 2013, the study enrolled 370 HCC patients with MiVI who received RH alone or PA-TACE. Patients were excluded from the final analysis if they had missing data (6 RH patients and 2 PA-TACE patients), had other malignancies (2 RH patients), or were lost to follow-up evaluation (25 RH patients and 13 PA-TACE patients). Notably, nine patients were found to have recurrent HCC by hepatic angiography when PA-TACE was given (RFS < 4 weeks) and were not excluded from the study because the hepatic angiography was not given to the RH group. Finally, 322 patients (185 RH patients and 137 PA-TACE patients) were enrolled in the analysis. A flow chart is shown in Fig. 1.
The characteristics of the patients in the RH and PA-TACE groups are shown in Table 1. The mean sex of the patients and their ages (49.9 ± 0.7 years for RH and 48.9 ± 0.9 years for PA-TACE) were well matched between the two groups. The tumor characteristics, such as the largest tumor diameter (7.0 ± 0.3 for RH and 6.5 ± 0.3 for PA-TACE), number of tumors, BCLC stages, AFP level, tumor capsule, microsatellites, and Edmonson grade also were similar between the two groups. The alanine aminotransferase (ALT) levels differed significantly between the two groups (p = 0.037). There was no significant difference in hepatitis B antigen positivity, total bilirubin, albumin, ALT level, or Child-Pugh classification.
The Operative Outcomes
The operative outcomes are shown in Table 2. There were no significant differences in operative time, operative blood loss, perioperative blood transfusion, or type of surgery between the two groups. The surgical morbidity rates were similar between the two groups (6.5 %, n = 12 vs 6.6 %, n = 9; p = 0.976). According to the Clavien-Dindo classification, no grade 5 morbidity effects related to surgery were observed. The RH group had more tumor-related deaths (112 [60.5 %] vs 73[39.5 %]) than the PA-TACE group (62 [45.3 %] vs 75 [54.7 %]) (p = 0.01).
Adverse Reactions to PA-TACE
The adverse reactions to PA-TACE are shown in supplemental Table 1. There were no grade 4 or 5 adverse reactions related to TACE. The most common adverse reaction was nausea and vomiting (n = 130, 94.9 %). An increase in ALT/aspartate aminotransferase (AST) was present in 56.2 % of the patients (n = 77) and an increase in bilirubin in 51.1 % of the patients (n = 70). Other adverse reactions included pain (23.4 %), leucopenia (39.4 %), increase in GGT (35.8 %), and decrease in albumin (30.7 %).
Survival Analysis
The study was censored on 31 August 2014. The median follow-up period was 28.8 months (95 % confidence interval [CI] 23.0–38.7 months) for the RH group and 43.4 months (95 % CI 37.9–58.6 months) for the PA-TACE group. A total of 174 patients died of HCC (112 RH patients and 62 PA-TACE patients). Tumor recurrence developed for 235 patients (135 RH patients and 100 PA-TACE patients). The 1-, 2-, 3-, and 5-year RFS rates were respectively 69.3, 55.5, 46.7, and 35.0 % for the PA-TACE group and 47.0, 36.2, 34.1, and 30.3 % for the RH group (log-rank, χ2 = 6.309; p = 0.012, Fig. 2a). The 1-, 2-, 3-, and 5-year OS rates were respectively 94.2, 78.8, 71.5, and 54.0 % for the PA-TACE group and 78.9, 62.2, 54.1, and 43.2 % for the RH group (log-rank, χ2 = 7.537; p = 0.006, Fig. 2b). In the univariate analysis and Cox’s multivariate analysis of the RH and PA-TACE groups, PA-TACE was an independent risk factor for RFS and OS (p = 0.003; hazard ratio [HR], 0.641; 95 % CI, 0.478–0.861 for RFS vs p = 0.001; HR, 0.602; 95 % CI 0.440–0.822 for OS). The maximum tumor diameter also was also an independent risk factor for both RFS and OS (Table 3).
Discussion
Studies have demonstrated that MiVI is a major independent prognostic factor for HCC patients.2,19 The presence of MiVI is common in clinical practice. It is present in 20 % of tumors 2 cm in size, 30–60 % of nodules 2–5 cm in size, and up to 60–90 % of nodules larger than 5 cm.20 The presence of MiVI is a main obstacle to achievement of good long-term survival rates after RH for patients with HCC.2,21,22 To date, there has been no generally accepted postoperative adjuvant treatment for HCC patients with MiVI. Therefore, we enrolled a large sample of HCC patients with MiVI who received curative RH and evaluated the impact of PA-TACE on this group of patients.
This study found that PA-TACE significantly increased the RFS and OS of HCC patients with MiVI compared with those who received RH alone, and PA-TACE was found to be an independent risk factor of postoperative RFS and OS. Whereas previous RCT and non-RCT studies showed that PA-TACE prolongs survival of patients with macrovascular invasion.10,11 this study provided data showing PA-TACE to be effective in preventing tumor recurrence and prolonging survival in HCC patients with MiVI.
Our results further indicate that PA-TACE significantly reduces the early recurrence rate. The 2-year RFS rate was 55.5 % for the PA-TACE group and 36.2 % for the RH group. After 2 years, this difference decreased, and at the time this study was censored, the RFS was similar between the two groups. This result is similar to the results of a previous study.23 Because early recurrence is mainly related to residual tumor or intrahepatic metastasis, TACE improved the RFS rate because of its therapeutic actions on these tumors.24
The drastic decline in RFS (Fig. 2A) within the first 3 to 4 months in both the RH group and the PA-TACE group may be related to the following issues. First, HCC patients with MiVI experience a very early recurrence pattern.2 Second, some patients presented with HCC recurrence beyond the power of CT/MRI to detect at the first evaluation. The RFS rate in our study was higher than those reported in other studies.9,10,24 The differences in patient selection are a likely explanation because the other studies included high proportions of patients with macrovascular invasion. Because early disease recurrence becomes fatal for the vast majority of patients with HCC, RFS is proposed to be used as a surrogate for OS. In our study, TACE prevented early recurrence of disease and improved both DFS and OS synchronously.
Microvascular invasion shows an aggressive tumor behavior and is closely linked to large tumor burden.25 Patients with MiVI experience a high frequency of fatal recurrence, and MiVI is associated with multiple intrahepatic tumors, macrovascular invasion, and extrahepatic metastasis.16 The beneficial effects of PA-TACE are likely related to the following factors. First, the unsatisfactory therapeutic outcomes of RH for HCC patients with MiVI are the result of residual cancer cells that micrometastase via the bloodstream before or during liver resection.26 – 28 The use of PA-TACE kills or decreases the number of these cells to improve the prognosis. Second, patients with MiVI have a tendency to form micro-metastases before RH.16,29 Thus, 3–4 weeks after RH, when the patients have recovered from the operation and the residual tumors are still very small, TACE is an appropriate and effective treatment.9 Third, most of the blood supply to the HCC is derived from the hepatic artery. Recurrent tumors commonly occur in the liver remnant near the surgical resection margin.30,31 The use of PA-TACE increases the concentration of local anticancer agents to these margins because of increased blood supply to a healing wound, which may be helpful for these patients.
Because MiVI can be assessed accurately only after careful histologic assessment of the whole surgically resected specimen,32 adjuvant treatment after RH becomes important for these patients due to the difficulty in preoperative diagnosis of MiVI. Two additional points are important in clinical practice. First, an accurate finding of MiVI by histopathologic examination of the whole surgical specimen is the key to diagnosis of MiVI. Second, markers such as alpha-1-fucosidase and histone H4 can be used to help in the detection of MiVI.3,18
This study had the following limitations. First, our data came from a single study center and were acquired retrospectively. A prospective, multicenter, randomized clinical trial should be conducted for further validation of the impact PA-TACE has on these patients. Second, the results of this study may not be used for patients with hepatitis C- or alcohol-related HCC.33 Third, the ideal drug and dosage of PA-TACE are not known. Kwok et al.34 also failed to provide a recommendation on the best adjuvant protocol for preventing tumor recurrence of HCC after curative resection in a small cohort study. In conclusion, PA-TACE may be beneficial for HCC patients with MiVI.
References
EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43.
Lim KC, Chow PK, Allen JC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254:108–13.
Pote N, Alexandrov T, Le Faouder J, et al. Imaging mass spectrometry reveals modified forms of histone H4 as new biomarkers of microvascular invasion in hepatocellular carcinomas. Hepatology. 2013;58:983–94.
Jonas S, Bechstein WO, Steinmuller T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–6.
Lee YH, Hsu CY, Huang YH, et al. Vascular invasion in hepatocellular carcinoma: prevalence, determinants, and prognostic impact. J Clin Gastroenterol. 2014;48:734–41.
Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–42.
Lau WY, Lai EC, Leung TW, et al. Adjuvant intra-arterial iodine-131-labeled lipiodol for resectable hepatocellular carcinoma: a prospective randomized trial-update on 5-year and 10-year survival. Ann Surg. 2008;247:43–8.
Tournoux C, Paoletti X, Barbare JC. Treatment outcomes for hepatocellular carcinoma using chemoembolization in combination with other therapies: Cancer Treat Rev. 2007;33:762–3.
Li JQ, Zhang YQ, Zhang WZ, et al. Randomized study of chemoembolization as an adjuvant therapy for primary liver carcinoma after hepatectomy. J Cancer Res Clin Oncol. 1995;121:364–6.
Zhong C, Guo RP, Li JQ, et al. A randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for stage IIIA hepatocellular carcinoma. J Cancer Res Clin Oncol. 2009;135:1437–45.
Peng BG, He Q, Li JP, et al. Adjuvant transcatheter arterial chemoembolization improves efficacy of hepatectomy for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Surg. 2009;198:313–8.
Li Q, Wang J, Sun Y, et al. Efficacy of postoperative transarterial chemoembolization and portal vein chemotherapy for patients with hepatocellular carcinoma complicated by portal vein tumor thrombosis: a randomized study. World J Surg. 2006;30:2004–11; discussion 2012–3.
Ke-Wei L, Tian-Fu W, Xi L, et al. The effect of postoperative TACE on prognosis of HCC with microscopic venous invasion. Hepatogastroenterology. 2012;59:1944–6.
Poon RT, Ng IO, Lau C, et al. Tumor microvessel density as a predictor of recurrence after resection of hepatocellular carcinoma: a prospective study. J Clin Oncol. 2002;20:1775–85.
Yin L, Li H, Li AJ, et al. Partial hepatectomy vs transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan criteria: a RCT. J Hepatol. 2014;61:82–8.
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2.
Shi J, Lai EC, Li N, et al. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17:2073–80.
Wang K, Guo W, Li N, et al. Alpha-1-fucosidase as a prognostic indicator for hepatocellular carcinoma following hepatectomy: a large-scale, long-term study. Br J Cancer. 2014;110:1811–9.
Du M, Chen L, Zhao J, et al. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer. 2014;14:1471–2407.
Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200.
Shimada K, Sano T, Sakamoto Y, et al. A long-term follow-up and management study of hepatocellular carcinoma patients surviving for 10 years or longer after curative hepatectomy. Cancer. 2005;104:1939–47.
Kamiyama T, Nakanishi K, Yokoo H, et al. Recurrence patterns after hepatectomy of hepatocellular carcinoma: implication of Milan criteria utilization. Ann Surg Oncol. 2009;16:1560–71.
Izumi R, Shimizu K, Iyobe T, et al. Postoperative adjuvant hepatic arterial infusion of Lipiodol containing anticancer drugs in patients with hepatocellular carcinoma. Hepatology. 1994;20:295–301.
Fan J, Zhou J, Wu ZQ, et al. Efficacy of different treatment strategies for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2005;11:1215–9.
Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early- and late-phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–7.
Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141:330–9.
Yamanaka N, Okamoto E, Toyosaka A, et al. Prognostic factors after hepatectomy for hepatocellular carcinomas: a univariate and multivariate analysis. Cancer. 1990;65:1104–10.
Sun YF, Xu Y, Yang XR, et al. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013;57:1458–68.
Tsai TJ, Chau GY, Lui WY, et al. Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery. 2000;127:603–8.
Yoshida Y, Kanematsu T, Matsumata T, et al. Surgical margin and recurrence after resection of hepatocellular carcinoma in patients with cirrhosis: further evaluation of limited hepatic resection. Ann Surg. 1989;209:297–301.
Matsumata T, Kanematsu T, Takenaka K, et al. Patterns of intrahepatic recurrence after curative resection of hepatocellular carcinoma. Hepatology. 1989;9:457–60.
Schlitt HJ, Neipp M, Weimann A, et al. Recurrence patterns of hepatocellular and fibrolamellar carcinoma after liver transplantation. J Clin Oncol. 1999;17:324–31.
Chan HL, Tse CH, Mo F, et al. High viral load and hepatitis B virus subgenotype ce are associated with increased risk of hepatocellular carcinoma. J Clin Oncol. 2008;26:177–82.
Kwok PC, Lam TW, Lam PW, et al. Randomized controlled trial to compare the dose of adjuvant chemotherapy after curative resection of hepatocellular carcinoma. J Gastroenterol Hepatol. 2003;18:450–5.
Acknowledgments
This work was supported by grants of the Science Fund for Creative Research Groups (No. 81221061), The State Key Project on Diseases of China (2012zx10002016016003), The China National Funds for Distinguished Young Scientists (no. 81125018), Chang Jiang Scholars Program (2013) of China Ministry of Education, The National Key Basic Research Program “973 Project” (No. 2015CB554000), The New Excellent Talents Program of Shanghai Municipal Health Bureau (No. XBR2011025), Shanghai Science and Technology Committee (No. 134119a0200), and SMMU Innovation Alliance for Liver Cancer Diagnosis and Treatment (2012).
Disclosures
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jing Jian Sun, Kang Wang, and Cun Zhen Zhang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, J.J., Wang, K., Zhang, C.Z. et al. Postoperative Adjuvant Transcatheter Arterial Chemoembolization After R0 Hepatectomy Improves Outcomes of Patients Who have Hepatocellular Carcinoma with Microvascular Invasion. Ann Surg Oncol 23, 1344–1351 (2016). https://doi.org/10.1245/s10434-015-5008-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-5008-z