Abstract
Background
CXCL10, a member of the CXC chemokine family, is known to mediate chemotaxis, apoptosis, angiogenesis, and cell growth. It is also reportedly involved in tumor development and can affect prognosis in several cancers. However, the precise relationship between CXCL10 and the prognosis of patients with esophageal squamous cell carcinoma (ESCC) is not fully understood.
Methods
We used ESCC tissue microarrays containing samples from 177 patients to test whether the CXCL10 expression status, determined using immunohistochemical analysis, is predictive of prognosis. We also tested whether CXCL10 expression status could serve as a clinically useful marker for evaluating the need for adjuvant chemotherapy after surgery.
Results
We found that high CXCL10 expression in clinical samples was an independent prognostic factor and was predictive of a favorable 5-year overall survival and disease-specific survival (p = 0.0102 and 0.0332, respectively). Additionally, no significant difference was detected between patients in the CXCL10-high group treated with surgery alone and those treated with surgery followed by adjuvant chemotherapy. In the CXCL10-low group, on the other hand, patients treated with surgery followed by adjuvant chemotherapy had better 5-year overall survival than those treated with surgery alone.
Conclusions
High CXCL10 expression is an independent prognostic factor and has the potential to serve as a clinically useful marker of the need for adjuvant chemotherapy after surgery in patients with advanced thoracic ESCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Esophageal cancers rank as the sixth leading cause of cancer death worldwide.1 Indeed, thoracic esophageal squamous cell carcinoma (ESCC) is the most common pathologic condition in many regions of the world; it is characterized by an extremely poor prognosis and rapid clinical progression.2,3 Thanks to recent advances in surgical technique, perioperative management, chemotherapy, and radiotherapy, however, the outcomes of these patients have improved somewhat, though the 5-year survival rate remains unsatisfactory, and the treatment strategy for these patients—surgery, chemotherapy, and/or radiotherapy—is still controversial.4–7
CXCL10 (or interferon gamma–induced protein 10, IP-10) is a member of the CXC chemokine family and is known to mediate chemotaxis, apoptosis, angiogenesis, and cell growth.8 CXCL10 is also reportedly involved in tumor development in several cancers, including brain cancer, breast cancer, colorectal cancer, head and neck cancer, lung cancer, and lymphoma.9–14 According to those reports, CXCL10 has dual effects on tumorigenesis, exhibiting both antitumor and tumor-promoting properties. In ESCC, CXCL10 expression inhibits cancer cell invasion and promotes migration of immune cells to the cancer microenvironment, suggesting that CXCL10 exerts antitumor effects on ESCC progression.15,16 Whether CXCL10 expression correlates with prognosis in patients with ESCC remains unclear, however.
In the present study, therefore, we examined whether CXCL10 expression status correlates with prognosis in patients with advanced ESCC. We also evaluated whether CXCL10 expression status can serve as a clinically useful marker of the need for adjuvant chemotherapy after surgery in these patients.
Materials and Methods
Thoracic ESCC Patients
This study was approved by the ethics committee of the Akita University School of Medicine. All of the participants provided informed consent and signed human subject institutional review board consent forms. Between January 2000 and December 2011, 507 esophageal cancer patients received esophagectomy at Akita University Hospital. Of those, 177 pT2–4 thoracic ESCC patients who had received curative surgery with no preoperative treatment were enrolled onto this study. For all patients, the esophageal cancer stage and the treatment strategy were decided by the esophageal cancer board, which includes radiologists, oncologists, physicians, and surgeons. In addition, the disease was classified according to the World Health Organization 7th tumor, node, metastasis classification system.17 The clinicopathologic features of these patients are summarized in Table 1.
Surgery and Adjuvant Chemotherapy
Our standard operative procedure is right transthoracic esophagectomy with resection of the cardiac portion of the stomach and dissection. Three-field lymph node dissections of the mediastinal (involving the periesophageal region and areas around the trachea and bilateral main bronchus), abdominal (involving the perigastric region and areas around the celiac axis), and cervical (involving the bilateral periesophageal region and supraclavicular region) lymph nodes were performed. Reconstruction commonly involved inserting a gastric tube through the posterior mediastinal route.18
On the basis of the pathologic results, administration of adjuvant chemotherapy was initiated within 2 months after esophagectomy. Some patients declined adjuvant chemotherapy or did not receive it because of their clinical condition. The chemotherapy consisted of protracted infusion of 5-fluorouracil (800 mg/m2 per day ) on days 1–5 and 8–12, combined with cisplatin (80 mg/m2 per day) on days 1 and 8. This protocol was repeated twice with 3-week intervals in between.19
ESCC Tissue Microarray (TMA)
An ESCC TMA was constructed at the Pathology Institute, Toyama, Japan, using 177 paraffin-embedded blocks of primary tumor samples from the study participants, as described elsewhere.19,20 Triplicate cores measuring 0.6 mm in diameter were collected randomly from cancer areas to account for cancer tissue heterogeneity and transferred to the TMA. As a result, the TMA block contained 531 cores (three cores each from 177 paraffin blocks).
Immunohistochemical (IHC) Staining
Four-micron-thick sections from the TMA were deparaffinized in xylene and rehydrated through a graded ethanol series. For antigen retrieval, the slides were first incubated for 10 min in pH 6.0 citrate buffer at 121 °C in an autoclave. Endogenous peroxidase activity was inactivated by incubation for 30 min in 3.0 % H2O2, and protein blocking was accomplished by incubation for 30 min in 10 % normal rabbit serum/Tris (Vector Laboratories, Burlingame, CA). The tissue sections were then incubated over night at 4 °C with goat anti-human CXCL10/IP-10 polyclonal antibody (R&D Systems, Minneapolis, MN), after which the antigen was visualized using a Histofine simple stain kit (Nichirei, Tokyo, Japan) with DAB peroxidase substrate according to the manufacturer’s instructions.
Photomicrographs of the IHC staining were taken for analysis with a NanoZoomer Digital Pathology C9600 (Hamamatsu Photonics, Hamamatsu, Japan) and were viewed with a NanoZoomer Digital Pathology Virtual Slide Viewer (version 1.2.33; Hamamatsu Photonics). For each sample, a staining score was assigned on the basis of the HER2 IHC scoring system (American Society of Clinical Oncology and CAP guidelines) by an investigator masked to the clinical data.21 Positivity for protein expression was given an IHC score of 3+ if more than 30 % of the cell cytoplasm or nuclei stained intensely, 2+ if there was moderate cytoplasmic or nuclear staining in >10 % of cells, and 1+ if there was weak staining. Samples with IHC scores of 3+ or 2+ were judged as high expression and 1+ or − as low expression.
Biostatistical Analysis
Mean, standard deviation, and frequency were used to summarize characteristics of subjects in the CXCL10 high and CXCL10 low groups. The Wilcoxon test (for continuous variables) or χ 2 and Fisher’s exact tests (for categorical variables) were used to evaluate the differences between these groups. Survival length was determined from the date of surgery to the patient’s death or date of last clinical follow-up. Survival curves were constructed by the Kaplan–Meier method, and the differences between curves were analyzed by the log rank test. The Cox proportional hazard regression model was used for univariate and multivariate analyses. Age, gender, lymph node metastasis, pathologic stage, tumor differentiation, and CXCL10 expression status were included in the multivariate model. Statistical analyses were performed using JMP11 (SAS Institute, Cary, NC). Values of p ≤ 0.05 (two-sided) were considered significant.
Results
CXCL10 Expression and 5-Year Overall and Disease-Specific Survival Among Patients with ESCC
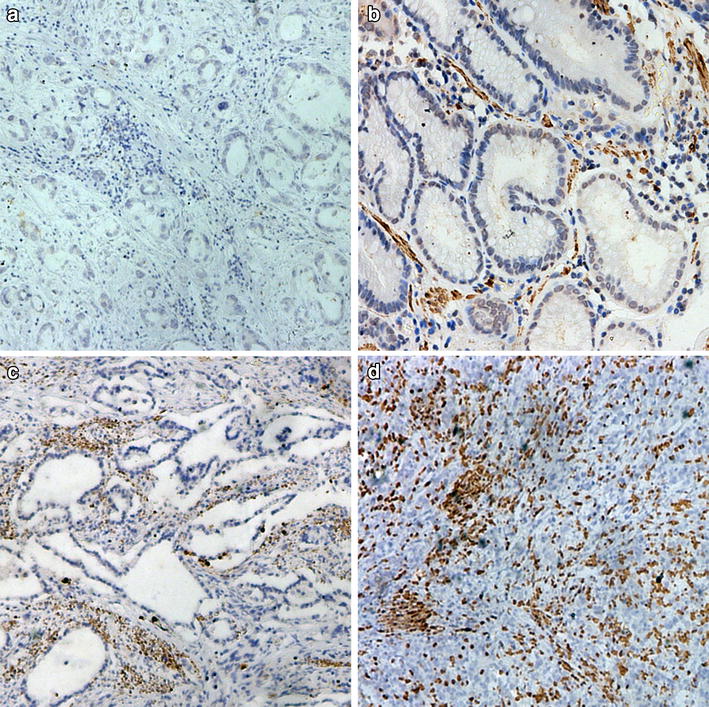
We used IHC analysis of a TMA to evaluate the relationship between CXCL10 expression status and 5-year overall and disease-specific survival (OS and DSS, respectively) among patients with ESCC. Representative photomicrographs of three cores from individual specimens with 3+, 2+, 1+, and − IHC scores are shown in Fig. 1. CXCL10 expression was mainly observed in the cytoplasm of ESCC cells. The Kaplan–Meier curves revealed that patients expressing high levels of CXCL10 had significantly better 5-year OS and DSS than those expressing lower levels of CXCL10 (p = 0.0102 and 0.0332, respectively) (Fig. 2). Consistent with this finding, univariate analysis of age (70 years and older vs. younger), gender, depth of invasion (T2–3 vs. T4), lymph node metastasis (N0–1 vs. N2–3), pathologic disease stage (stage IIIB–IIIC vs. stage IB–IIIA), tumor differentiation (poorly differentiated vs. not poorly differentiated), CXCL10 expression (low vs. high), and treatment (surgery alone vs. surgery followed by adjuvant chemotherapy) showed that lymph node metastatic status, pathologic stage, tumor differentiation, and CXCL10 expression status were all significant prognostic factors affecting 5-year OS (Table 2). In addition, multivariate analysis revealed CXCL10 expression status to be a significant prognostic factor in every combination with age, gender, lymph node metastasis, pathologic stage, and tumor differentiation (Table 3).
IHC staining of CXCL10 in ESCC TMA. Representative photomicrographs showing 3 cores from individual specimens with 3+, 2+, 1+, and − IHC scores for expression of CXCL10. For each condition, 3 whole cores from ESCC TMA are shown at ×100 magnification (scale bar 300 μm), along with corresponding ×400 magnification images (scale bar 100 μm)
Five-year OS, DSS, and CXCL10 expression status. Kaplan–Meier analysis of association between CXCL10 expression status (high or low), determined on basis of IHC staining, and 5-year OS (a) and DSS (b) among ESCC patients. Patients expressing high levels of CXCL10 showed significantly better 5-year OS and DSS than those expressing lower levels (p = 0.0102 and 0.0332, respectively)
Low CXCL10 Expression and Adjuvant Chemotherapy
We also evaluated whether CXCL10 expression status could serve as a clinically useful marker of the need for adjuvant chemotherapy after surgery. In the CXCL10 high expression group, 41 patients were treated with surgery alone and 55 were treated with surgery followed by adjuvant chemotherapy. The Kaplan–Meier curves showed that there was no significant difference in 5-year OS between patients treated with surgery alone and those treated with surgery followed by adjuvant chemotherapy (Fig. 3). On the other hand, in the CXCL10 low expression group, the 50 patients treated with surgery followed by adjuvant chemotherapy had better 5-year OS than the 31 patients treated with surgery alone (p = 0.07). However, the difference did not reach statistical significance.
Adjuvant chemotherapy and 5-year OS among CXCL10 high and low groups. OS among patients in CXCL10 high and low groups treated with surgery alone or with surgery followed by adjuvant chemotherapy. No significant difference between patients treated with surgery alone or surgery followed by adjuvant chemotherapy was found in CXCL10 high group (a). In CXCL10 low group, patients treated with surgery followed by adjuvant chemotherapy had better 5-year OS than those treated with surgery alone (b)
Discussion
In this study, we found that high CXCL10 expression is an independent prognostic factor affecting 5-year OS in patients with advanced ESCC. We also found that CXCL10 expression status has the potential to serve as a clinically useful marker for evaluating the need for adjuvant chemotherapy after surgery in these patients. To our knowledge, this is the first report showing an association between CXCL10 expression status and prognosis in patients with ESCC.
Consistent with the results of this study, low CXCL10 expression was previously found to be an independent adverse prognosticator in stage II and III colorectal cancer patients.22 This may reflect the inhibitory effects of CXCL10 on angiogenesis and its promotion of immune cell migration to the cancer microenvironment. For example, CXCL10 production reportedly correlates inversely with tumor growth in lymphoma, adenocarcinoma of lung, and breast cancer as a result of a marked reduction in tumor-associated angiogenesis.23,24 Several investigators have also shown that CXCL10 exerts significant synergistic antitumor effects through its ability to recruit immature antigen-presenting dendritic cells and activated T cells into the cancer microenvironment in glioma and melanoma.25,26 In ESCC, Lu et al. reported that interleukin 17 could induce ESCC tumor cells to produce inflammatory chemokines, including CXCL10, which were associated with the recruitment of T cells, NK cells, and dendritic cells.15 On the other hand, high CXCL10 expression was predictive of a significantly poorer prognosis in patients with ovarian cancer.27,28 Further, CXCL10 overexpression reportedly promotes tumor growth in breast cancer, colon cancer, basal cell carcinoma, and glioma.29–32 These seemingly contradictory effects of CXCL10 in cancer may reflect the expression status of the two splice variants of its receptor, CXCR3.8 Whereas overexpression of CXCR3-A appears to be involved in promoting breast cancer, CXCR3-B appears to limit tumor growth through angiostatic and/or immunomodulatory actions in several types of cancer.29 However, further study will be needed to clarify the relationship between the CXCR3 splice variants and CXCL10 in ESCC.
In the present study, there was no significant difference in 5-year OS between patients in the CXCL10 high expression group treated with surgery alone and those treated with surgery followed by adjuvant chemotherapy. Among patients in the CXCL10 low expression group, however, those treated with surgery followed by adjuvant chemotherapy had better 5-year OS than those treated with surgery alone. This was despite the fact that the patients treated with surgery followed by adjuvant chemotherapy had more advanced disease than those treated with surgery alone.19 These results suggest that adjuvant chemotherapy after surgery has the potential to improve the 5-year OS of CXCL10 low patients and might serve as one option for personalized treatment of patients with advanced thoracic ESCC. That said, the beneficial effect of postsurgical adjuvant chemotherapy did not reach statistical significance. Further study with a larger patient population and/or with other populations is needed before clinical application.
In conclusion, our findings indicate that high CXCL10 expression is an independent prognostic factor affecting 5-year OS among patients with advanced ESCC. Moreover, CXCL10 expression status has the potential to serve as a clinically useful marker for evaluating the need for adjuvant chemotherapy after surgery.
References
Jemal A, Bray F, Center M, et al. Global cancer statics. CA Cancer J Clin. 2011;61:69–90.
Ando N, Ozawa S, Kitagawa Y, et al. Improvement in the results of surgical treatment of advanced squamous esophageal carcinoma during 15 consecutive years. Ann Surg. 2002;232:225–32.
Law S, Kwong D, Wong KF, et al. Improvement in treatment results and long-term survival of patients with esophageal cancer: impact of chemoradiation and change in treatment strategy. Ann Surg. 2003;238:339–47.
Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study—JCOG9204. J Clin Oncol. 2003;21:4592–6.
Kato K, Muro K, Minashi K, et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for stage II–III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol Phys. 2011;81:684–90.
Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68–74.
Sanuki N, Ishikura S, Shinoda M, et al. Radiotherapy quality assurance review for a multi-center randomized trial of locally advanced esophageal cancer: the Japan Clinical Oncology Group (JCOG) trial 0303. Int J Clin Oncol. 2012;17:105–11.
Liu M, Guo S, Stiles JK. The emerging role of CXCL10 in cancer. Oncol Lett. 2011;2:583–9.
Sun L, Hui AM, Su Q, et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9:287–300.
Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63.
Ki DH, Jeung HC, Park CH, et al. Whole genome analysis for liver metastasis gene signatures in colorectal cancer. Int J Cancer. 2007;121:2005–12.
Ginos MA, Page GP, Michalowicz BS, et al. Identification of a gene expression signature associated with recurrent disease in squamous cell carcinoma of the head and neck. Cancer Res. 2004;64:55–63.
Talbot SG, Estilo C, Maghami E, et al. Gene expression profiling allows distinction between primary and metastatic squamous cell carcinomas in the lung. Cancer Res. 2005;65:3063–71.
Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–47.
Lu L, Pan K, Zheng HX, et al. IL-17A promotes immune cell recruitment in human esophageal cancers and the infiltrating dendritic cells represent a positive prognostic marker for patient survival. J Immunother. 2013;36:451–8.
Yoo JY, Choi HK, Choi KC, et al. Nuclear hormone receptor corepressor promotes esophageal cancer cell invasion by transcriptional repression of interferon-γ-inducible protein 10 in a casein kinase 2–dependent manner. Mol Biol Cell. 2012;23:2943–54.
Sobin LH, Gospodarowicz MK, Wittekind C, International Union Against Cancer. TNM classification of malignant tumours. 7th ed. Chichester: Wiley; 2010.
Sato Y, Motoyama S, Maruyama K, et al. A second malignancy is the major cause of death among thoracic squamous cell esophageal cancer patients negative for lymph node involvement. J Am Coll Surg. 2005;201:188–93.
Sato Y, Motoyama S, Nanjo H, et al. REG1A expression status suggests chemosensitivity among advanced thoracic esophageal squamous cell carcinoma patients treated with esophagectomy followed by adjuvant chemotherapy. Ann Surg Oncol. 2013;20:3044–51.
Sato Y, Marzese DM, Ohta K, et al. Epigenetic regulation of REG1A and chemosensitivity of cutaneous melanoma. Epigenetics. 2013;8:1043–52.
Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45.
Jiang Z, Xu Y, Cai S. CXCL10 expression and prognostic significance in stage II and III colorectal cancer. Mol Biol Rep. 2010;37:3029–36.
Persano L, Crescenzi M, Indraccolo S. Anti-angiogenic gene therapy of cancer: current status and future prospects. Mol Aspects Med. 2007;28:87–114.
Aronica SM, Raiber L, Hanzly M, et al. Antitumor/antiestrogenic effect of the chemokine interferon inducible protein 10 (IP-10) involves suppression of VEGF expression in mammary tissue. J Interferon Cytokine Res. 2009;29:83–92.
Fujita M, Zhu X, Ueda R, et al. Effective immunotherapy against murine gliomas using type 1 polarizing dendritic cells—significant roles of CXCL10. Cancer Res. 2009;69:1587–95.
Jiang XB, Lu XL, Hu P, et al. Improved therapeutic efficacy using vaccination with glioma lysate-pulsed dendritic cells combined with IP-10 in murine glioma. Vaccine. 2009;27:6210–6.
Rainczuk A, Rao JR, Gathercole JL, Fairweather NJ, et al. Evidence for the antagonistic form of CXC-motif chemokine CXCL10 in serous epithelial ovarian tumours. Int J Cancer. 2014;1;134:530–41.
Furuya M, Suyama T, Usui H, et al. Up-regulation of CXC chemokines and their receptors: implications for proinflammatory microenvironments of ovarian carcinomas and endometriosis. Hum Pathol. 2007;38:1676–87.
Datta D, Flaxenburg JA, Laxmanan S, et al. Ras-induced modulation of CXCL10 and its receptor splice variant CXCR3-B in MDA-MB-435 and MCF-7 cells: relevance for the development of human breast cancer. Cancer Res. 2006;66:9509–18.
Kawada K, Hosogi H, Sonoshita M, et al. Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes. Oncogene. 2007;26:4679–88.
Lo BK, Yu M, Zloty D, et al. CXCR3/ligands are significantly involved in the tumorigenesis of basal cell carcinomas. Am J Pathol. 2010;176:2435–46.
Maru SV, Holloway KA, Flynn G, et al. Chemokine production and chemokine receptor expression by human glioma cells: role of CXCL10 in tumour cell proliferation. J Neuroimmunol. 2008;199:35–45.
Acknowledgment
Supported in part by JSPS KAKENHI (Grant 25870072).
Disclosure
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sato, Y., Motoyama, S., Nanjo, H. et al. CXCL10 Expression Status is Prognostic in Patients with Advanced Thoracic Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 23, 936–942 (2016). https://doi.org/10.1245/s10434-015-4909-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4909-1