Abstract
Background
It is a consensus that radiation therapy (RT) should be applied for all large, deep, high-grade soft tissue sarcomas (STS). Therefore, we investigated the National Cancer Database (NCDB) to study how these guidelines are being followed, to determine what factors may be associated with the decision not to use RT, and to see whether there was an association of RT use and survival.
Methods
We retrospectively analyzed localized high-grade STS patients in the NCDB from 1998 through 2006. They were further stratified into two groups: no radiation (NRT) group and radiation (RT) group. Then, long-term survival between the two groups was evaluated using the Kaplan–Meier (KM) method with comparisons based on the log-rank test. Multiple variables were analyzed between the two groups. Propensity matching was performed secondarily to minimize the influence of confounding variables.
Results
A total of 3982 of 10,290 patients (37.8 %) did not receive RT and 6,308 patients (62.2 %) did receive RT. Patients in the NRT group were more likely to have a below-median education level (median 58.2 % vs. 60.7 %; p = 0.015) and a below-median income level (65.1 % vs. 68.6 %; p < 0.001). In addition, these patients lived farther from their treatment centers (20.2 vs. 14.8 miles, p = 0.002) and were more likely to be uninsured (5.3 % vs. 3.5 %, p < 0.001). They were less likely to receive a radical excision (55.2 % vs. 70.1 %; p < 0.001) and more likely to receive amputation (20.9 % vs. 3.3 %; p < 0.001). The 30-day mortality (1.2 % vs. 0.2 %; p < 0.001) and readmission rate (3.8 % vs. 2.8 %; p = 0.031) were higher for the NRT group. KM analysis showed that long-term survival for patients who did not receive RT was significantly lower, even after propensity score matching (p < 0.001).
Conclusions
This large database review reveals a striking lack of utilization of RT to treat high-grade STS, which correlates with poorer survival even after propensity matching. Lower education and income levels and diminished access to medical care (insurance and distance to the facility) are associated with failing to receive RT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
For the treatment of high-grade soft tissue sarcoma (STS), radiation therapy (RT) is utilized to lower the rate of local recurrence (LR). The effectiveness of RT in decreasing rates of LR has been well-demonstrated.1–4 However, whether a survival benefit results from adjuvant RT utilization remains controversial. In 1982, Rosenberg et al. conducted a prospective, randomized trial in 43 patients with high-grade sarcoma comparing limb-sparing surgery plus radiation therapy versus amputation.5 They found that disease-free survival (DFS) and overall survival (OS) were not significantly different. Therefore, limb-sparing surgery with adjuvant radiation therapy became a standard treatment for high-grade soft tissue sarcoma. In 1998, Yang et al. reported a prospective study of 141 patients randomized to limb-sparing surgery and chemotherapy with or without adjuvant radiation therapy. They found that despite a decrease in LR, there was no significant increase in OS for patients who received adjuvant radiation therapy.3 Interestingly, a recent 20-year follow-up of this trial showed a trend towards improved survival for patients receiving radiation therapy of 5 % at 10 years and 7 % at 20 years, but the sample size of 141 patients was not powered to assess whether survival differences of this size were statistically significant.6 To look at a much larger sample size, in 2010 Koshy et al. retrospectively reviewed 6,960 patients in the surveillance, epidemiology, and end results (SEER) database.7 They found that radiation was associated with improved survival in patients with high-grade STS (3-year OS 73 % vs. 63 %). In a subsequent retrospective study of 983 patients based on the SEER database, Schreiber et al. concluded that the OS and disease-specific survival (DSS) of patients with high grade tumors >5 cm improved after postoperative radiation therapy (3-year OS 73.4 % vs. 55.6 %).8 These conflicting data have raised questions of whether the benefit of treating patients with high-grade STS with radiation therapy is simply to reduce the risk of LR or whether it also may increase the likelihood of long-term survival.9 Moreover, given the potential complications associated with radiation therapy, if radiation therapy does not improve survival, then it perhaps it should be omitted in select sarcoma patients. Indeed, several studies have shown that, in selected patients, appropriate surgery alone can achieve adequate local control and survival, while avoiding the morbidity of radiation therapy.10–16
The clinical practice guidelines for soft tissue sarcoma from both the European Society of Medical Oncology (ESMO) and the National Comprehensive Cancer Network (NCCN), recommend adjuvant RT for all large (>5 cm), deep, high-grade STS.17,18 However, a recent analysis by Horton et al. of 1,354 STS patients in the SEER database showed that only 79 % of patients with high-grade STS were treated with radiation therapy.19 They also found that older patients are more likely not to be treated with radiation therapy and have more cancer-related morbidity and mortality. Therefore, we investigated the National Cancer Database (NCDB), which is larger in size and contains more socioeconomic and medical variables. Our goal was to determine what factors were associated with the decision not to use RT and whether use of RT correlated with patient survival.
Methods
The Institutional Review Board (IRB) of our institution approved this retrospective analysis of STS patients in the NCDB from 1998 through 2011. We accessed the NCDB Participant User File for patients treated at NCDB participating institutions and had tumors in the extremities with International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) topography codes C47.1, C47.2, C49.1, and C49.2. Outdated terminology in use at the time of database entry (for instance, Malignant Fibrous Histiocytoma instead of Undifferentiated Pleomorphic Sarcoma) remains listed by its original name in the database and in this paper.
In order to ensure the quality of survival data, the NCDB requires at least 5 years follow-up period after operation. Thus, the survival data are only available in NCDB for patients who had surgery before 2007; hence, we excluded patients diagnosed after 2006. Patients with known metastatic disease at the time of diagnosis also were excluded. Only patients with a histologic grade higher than 2 and treated with surgery were included. They were further stratified into two groups: no radiation (NRT) group and radiation therapy (RT) group. In the NCDB, instructions for assigning grade for sarcomas are described in the Cancer Statistics (CS) Instructions for Coding Grade memo (seer.cancer.gov/tools/grade/grade-2014-coding-instructions.pdf). Specifically, sarcomas are assigned a grade based on CSv2 SSF 1 using a three-tiered system. Although the grading system of the French Federation of Cancer Centers Sarcoma Group (FNCLCC) is the preferred system, any three-tiered system can be utilized.
The following variables were compared between the two groups: (1) Patient characteristic variables: age, sex, race, Charlson Comorbidity Score (CCS), education level (based on the average education level in the zip code of the patients’ home), income (based on the average income level in the zip code of patients’ home), greater circle distance (the distance from the zip code of patients’ home to the facility), facility type (community cancer program, comprehensive community cancer program, or academic/research program), insurance status (whether the patient is insured or not); (2) Tumor characteristic variables: TNM stage, location of tumor (either upper or lower extremity), tumor size, histologic type of tumor, and tumor grade; (3) Treatment variables: surgery type (local excision/radical excision/limb amputation/major amputation), days from diagnosis to definitive surgery, location of radiation therapy (where the patient received his RT and whether other treatments were given in the same facility), radiation dose (cGy), whether patient received neoadjuvant/adjuvant chemotherapy, and surgical margins (negative/microscopic positive/macroscopic positive); and 4) Endpoint/outcome variables: 30-day mortality rate (whether the patient died within 30 days of surgical discharge), 30-day readmission rate (whether the patient was readmitted within 30 days of surgical discharge), surgical inpatient length of stay (days). It should be clarified that certain limitations exist: (1) depth of tumor and exact anatomic location cannot be determined through the NCDB; (2) facility type refers to the location where surgery was performed; this does not necessarily mean that RT was received at the same facility; and (3) whether RT was given preoperatively or postoperatively could not be determined.
National trends in the incidence of RT were collected from 1998 through 2011. The trends were examined with the Cochran-Armitage test for trend.20 Baseline characteristics and outcomes between groups were compared using Pearson’s Chi square test for categorical variables and analysis of variance (ANOVA) for continuous variables. Multivariable logistic regression was used to predict factors that were associated with the administration of RT. Propensity scores (PS), defined as the conditional probability score of being treated with RT and surgery, were created to control for confounding factors.21,22 Patients were then matched on these propensity scores, using a 1:1 nearest neighbor algorithm, which included the following variables: age, sex, race, comorbidity score, education, income, insurance status, facility type (academic vs. community hospital), tumor size, tumor grade, histology, margin status, procedure type (i.e., extent of resection), and limb location (upper vs. lower extremity). Adjusted medians and proportions between the propensity-matched groups were then compared.
The long-term survival between the two groups was evaluated using the Kaplan–Meier (KM) method with comparisons based on the log-rank test, before and after PS matching. Results are reported as median (interquartile range, IQR), proportions (%), and odds ratios (OR, 95 % CI) as applicable. p values < 0.05 indicate statistical significance, and we controlled for type I error at the level of the comparison. All statistical analyses were performed using R (The R Foundation for Statistical Computing, version 3.0.2, Vienna, Austria).
Results
The relative percentage of RT use in high-grade STS increased from 56 % in 1998 to 69 % in 2011 (p < 0.001; Cochran-Armitage trend test). The rate of RT has steadily increased every year except for the years of 2002 and 2009 (Fig. 1).
As previously described, the NCDB only provides survival data from 1998 to 2006. During this period, 10,290 patients with localized high-grade STS of the extremities were included in this study. Among them, 3982 patients (37.8 %) did not receive radiation therapy and 6308 patients (62.2 %) did receive radiation therapy. The baseline characteristics are shown in Table 1. There were no significant differences in age, sex, race, CCS score, or facility type in which they received their treatment. However, patients who did not receive RT were less likely to have an above-median education level (median 58.2 % vs. 60.7 %; p = 0.015) and an above-median income level (65.1 % vs. 68.6 %; p < 0.001). In addition, these patients had greater circle distance (20.2 vs. 14.8 miles, p = 0.002), which means they tended to live farther from their treatment facilities. They also were more likely to be uninsured (5.3 % vs. 3.5 %, p < 0.001).
In the category of tumor characteristics, no significant differences in TNM stage, tumor location (upper/lower limbs), histologic type, or tumor grade were observed. The median tumor size in NRT group was slightly smaller than the RT group (70 vs. 80 mm; p = 0.008).
In the category of treatment specific variables, patients who did not receive RT were less likely to receive a radical excision (55.2 % vs. 70.1 %; p < 0.001) and more likely to receive an amputation (20.9 % vs. 3.3 %; p < 0.001). The median days to definitive surgery in the NRT and RT group were 24 and 40 days, respectively. The patients who did not receive RT were also less likely to receive neoadjuvant chemotherapy (9.2 % vs. 15.4 %; p < 0.001) and adjuvant chemotherapy (12.2 vs. 14.3 %, p = 0.002).
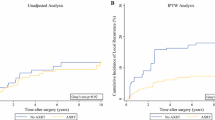
In the category of endpoint/outcome variables, the 30-day mortality (1.2 % vs. 0.2 %; p < 0.001) and readmission rate (3.8 % vs. 2.8 %; p = 0.031) were higher in patients who did not receive RT. Long-term survival was significantly lower for patients who did not receive RT (p < 0.001; Fig. 2). Even after PS matching, patients who did not receive RT still had poorer survival (p < 0.001; Fig. 3).
Independent predictors of RT use in patients with high-grade soft tissue sarcoma (STS) were analyzed using a Chi square test (Table 2). Among them, younger age, patients with lower CCS, and insured patients were more likely to receive RT. Patients with larger tumor size were slightly more likely to receive RT. Academic facilities, compared with community hospitals, were less likely to utilize RT for patients with high-grade STS.
Discussion
The role of radiation therapy has been historically regarded as important in improving rates of local control for STS of the extremities.3,5,23 There are conflicting data regarding whether radiation therapy has a benefit in survival for patients with high-grade STS. The 2010 ESMO guidelines for STS state that RT can improve local control but not overall survival.17 However, after 2010, two studies based on the SEER database observed improved survival for patients receiving RT with sample sizes of 6960 and 983 patients, respectively.7,19 In this study, we used a different database to complement the prior work in the SEER database; in our analysis, we observed improved survival for patients treated with RT in a sample size of 10,290 patients. Unfortunately, the NCDC database does not include information regarding local recurrence. Therefore, we are not able to assess whether the group with increased RT use had higher rates of local control, which may lead to a small, but statistically significant improved survival in such a large sample size. Alternatively, it is conceivable that RT may lead to improved survival independent of its defined role in improving local control. One could hypothesize fundamental biological reasons for a survival benefit associated with RT utilization. For example, there are emerging data that radiation therapy can stimulate the immune system, which could theoretically attack distant disease.24 Indeed, tumor bed wound infection is associated with higher survival rates, possibly due to the induction of antitumor immunity. Therefore, it is possible that RT promotes antitumor immunity that impacts distant disease. Alternatively, it is possible that the association of improved survival in patients with RT is due to confounding variables despite our attempts to address this limitation through propensity matching. There is potential bias from the association of 30-day mortality and readmission rates. These patients were likely sicker and may have had reasons that made them unable to receive RT (e.g., readmission for wound infection). However, this scenario represents only 62 of more than 10,000 patients; therefore, it is not possible for this small subset of patients to introduce significant bias.
According to Horton’s review of the SEER database, 79 % of patients with high-grade STS received radiation therapy.19 In our review, only 62.2 % of patients received radiation therapy. The difference might be due to the fact that their study was limited to limb-sparing surgery and our study included all resection types including amputation. Given the historical role of radiation in improving local control and the high rate of local control often achieved with amputation, patients undergoing amputation (20.9 % in the NRT group) may have been regarded as having no potential benefit from adjuvant radiation therapy. However, even after excluding patients who received amputation (Table 1), only 65.9 % (n = 6,097) patients in our study underwent RT. Therefore, the lower rate of RT utilization in our study might be due to the inclusion of more facilities in the NCDB database compared with the SEER database. In turn, this may reflect a more accurate reflection of RT use nationwide. In addition, in our study, older age is a negative predictor of RT use (Table 2), which is compatible with Horton’s finding. Tumor size, not surprisingly, was a positive predictor of RT use.
The NCDB has more socioeconomic data with which to determine factors correlating with the utilization of radiation therapy. According to Table 1, patients who did not receive RT were more likely to live in zip codes corresponding to lower educational and income levels. After adjustment, neither of these variables were an independent predictor of RT use (Table 2). This may be explained by the fact that these two variables actually represent the average education/income level of the zip code where the patient lived instead of the education/income level of the individual patient. On the other hand, “uninsured” status was an independent negative predictor of RT use (p < 0.001; Table 2). To our surprise, treatment at an academic facility (vs. community facility) was a negative predictor of RT use. We think that the reason might be due to the fact that unplanned incisions are more likely to be performed in the community hospitals and result in a positive margin. This may, in turn, lead to more reexcisions and RT utilization afterwards.
Some authors advocate that no RT is needed for T1 (<5 cm) high-grade STS; the consensus opinion only suggests RT use in large (>5 cm) tumors.25 However, only 65.3 % (n = 4,423) of patients with grade 2 or 3 tumors >5 cm in the NCDB received radiation therapy; whereas 44 % (n = 1,096) of patients with a tumor <5 cm received radiation therapy (Table 1). This suggests that the guidelines are not always followed in treating high-grade tumors, whether large or small.
Limitations of the Study
This study is a retrospective database review, and analysis was limited to the variables included in the database. The survival data were only available from 1998 to 2006. However, we observed a trend towards increasing use of RT after 2006 (Fig. 1). While the rate of RT use increased from 63 % in 2006 to 69 % in 2011, we have no survival data for these patients during that period. Additionally, the database did not include information on local recurrence data. Thus, we were unable to determine whether the incidence of local recurrence in the NRT group was higher than the RT group, and we could not PS match and exclude the confounding effect of local recurrence. The database also did not have information regarding disease-specific survival. Additionally, there was no specific mention of depth or detailed anatomical location of the STS, which could impact the decision to deliver RT. Finally, unlike the SEER database, marriage status was not included. This is notable as Alamanda et al. have shown that married patients tend to have better outcomes than their single peers.26 The limitations mentioned above point towards the need for more prospective data with RT and high-grade STS that are powered to detect survival differences of 5–10 %.
Conclusions
This large database review reveals a striking lack of utilization of RT to treat high-grade STS. In addition, this review finds poorer survival in the patients who did not receive RT. Lower education and income levels, as well as diminished access to medical care (insurance and distance to the facility) among these patients might contribute to this finding. To our knowledge, this study is the largest review of RT use in high-grade STS to date, and the only one that discusses the impact of insurance, income, education, and location status of patients on the likelihood of receiving RT. Our data suggest that the guidelines for treatment of high-grade STS are frequently not followed.
References
Brennan MF, Hilaris B, Shiu MH, et al. Local recurrence in adult soft-tissue sarcoma. A randomized trial of brachytherapy. Arch Surg. 1987;122(11):1289–93.
Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14(5):1679–89.
Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16(1):197–203.
Ballo MT, Zagars GK. Radiation therapy for soft tissue sarcoma. Surg Oncol Clin North Am. 2003;12(2):449–67.
Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196(3):305–15.
Beane JD, Yang JC, White D, Steinberg SM, Rosenberg SA, Rudloff U. Efficacy of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity: 20-year follow-up of a randomized prospective trial. Ann Surg Oncol. 2014;21(8):2484–9.
Koshy M, Rich SE, Mohiuddin MM. Improved survival with radiation therapy in high-grade soft tissue sarcomas of the extremities: a SEER analysis. Int J Radiat Oncol Biol Phys. 2010;77(1):203–9.
Schreiber D, Rineer J, Katsoulakis E, et al. Impact of postoperative radiation on survival for high-grade soft tissue sarcoma of the extremities after limb sparing radical resection. Am J Clin Oncol. 2012;35(1):13–7.
Baldini EH, Raut C. Radiation therapy for extremity soft tissue sarcoma: in the absence of a clear survival benefit, why do we give it? Ann Surg Oncol. 2014;21(8):2463–5.
Weir LM, Vanbergeyk AB, Masri BA, et al. Local excision without radiation for high-grade soft-tissue sarcoma of the extremity and superficial trunk. Sarcoma. 2000;4(3):113–7.
Geer RJ, Woodruff J, Casper ES, Brennan MF. Management of small soft-tissue sarcoma of the extremity in adults. Arch Surg. 1992;127(11):1285–9.
Karakousis CP, Proimakis C, Walsh DL. Primary soft tissue sarcoma of the extremities in adults. Br J Surg. 1995;82(9):1208–12.
Rydholm A, Gustafson P, Rooser B, et al. Limb-sparing surgery without radiotherapy based on anatomic location of soft tissue sarcoma. J Clin Oncol. 1991;9(10):1757–65.
Baldini EH, Goldberg J, Jenner C, et al. Long-term outcomes after function-sparing surgery without radiotherapy for soft tissue sarcoma of the extremities and trunk. J Clin Oncol. 1999;17(10):3252–9.
Fabrizio PL, Stafford SL, Pritchard DJ. Extremity soft-tissue sarcomas selectively treated with surgery alone. Int J Radiat Oncol Biol Phys. 2000;48(1):227–32.
Al-Refaie WB, Habermann EB, Jensen EH, Tuttle TM, Pisters PW, Virnig BA. Surgery alone is adequate treatment for early stage soft tissue sarcoma of the extremity. Br J Surg. 2010;97(5):707–13.
Casali PG, Blay JY. Soft tissue sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v198–203.
National-Comprehensive-Cancer-Network. Soft Tissue Sarcoma Version 2.2014. 2014. http://www.nccn.org/professionals/physician_gls/PDF/sarcoma.pdf. Accessed 28 Aug 2014.
Horton JK, Gleason JF Jr, Klepin HD, Isom S, Fried DB, Geiger AM. Age-related disparities in the use of radiotherapy for treatment of localized soft tissue sarcoma. Cancer. 2011;117(17):4033–40.
Lachin JM. Power and sample size evaluation for the Cochran-Mantel-Haenszel mean score (Wilcoxon rank sum) test and the Cochran-Armitage test for trend. Stat Med. 2011;30(25):3057–66.
Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55.
Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33–8.
Sadoski C, Suit HD, Rosenberg A, Mankin H, Efird J. Preoperative radiation, surgical margins, and local control of extremity sarcomas of soft tissues. J Surg Oncol. 1993;52(4):223–30.
Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol. 2012;2:153.
Pisters PW, Pollock RE, Lewis VO, et al. Long-term results of prospective trial of surgery alone with selective use of radiation for patients with T1 extremity and trunk soft tissue sarcomas. Ann Surg. 2007;246(4):675–81.
Alamanda VK, Song Y, Holt GE. Effect of marital status on treatment and survival of extremity soft tissue sarcoma. Ann Oncol. 2014;25(3):725–9.
Acknowledgment
The authors thank Sadie Hawkins and Virgil Leonard for assistance with the preparation of this manuscript. The data used in this study are derived from a de-identified National Cancer Data Base file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators. The authors thank Cindy Green, PhD, for her review of the statistical methodology utilized in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Alexander L. Lazarides is the co-first author.
Rights and permissions
About this article
Cite this article
Hou, CH., Lazarides, A.L., Speicher, P.J. et al. The Use of Radiation Therapy in Localized High-Grade Soft Tissue Sarcoma and Potential Impact on Survival. Ann Surg Oncol 22, 2831–2838 (2015). https://doi.org/10.1245/s10434-015-4639-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4639-4