Abstract
Background
Perioperative chemotherapy improves survival in patients with advanced esophagogastric cancer, but the optimal treatment regimen remains unclear. More intensive chemotherapy may improve outcome, but also increase toxicity and complications.
Methods
A total of 843 patients were included in this retrospective study and stratified in 4 groups: doublet therapy with cisplatin or oxaliplatin and 5-fluorouracil (groups A/B) or triplet therapy with additional epirubicin or taxane (groups C/D). The influence of the different neoadjuvant chemotherapy regimens on response, prognosis, and complications was assessed.
Results
Clinical and pathological response were associated with longer overall survival (OS; p < 0.001). No significant differences regarding response or OS were found, but there was a trend toward better outcome in group D (taxane-containing triplet). In the subgroup of 669 patients with adenocarcinomas of the esophagogastric junction (AEG), patients who had received taxane-containing regimens had a significantly longer OS (p = 0.037), but taxane use was not an independent factor in multivariate analysis. Triple therapy with taxanes did not result in a higher complication rate or postoperative mortality.
Conclusions
Although no superior neoadjuvant chemotherapy regimen was identified for patients with esophagogastric adenocarcinoma, taxane-containing regimens should be further investigated in randomized trials, especially in patients with AEG tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastric and esophageal cancer are serious worldwide health problems,1,2 with 39,590 new cases estimated for the year 2013 in the United States alone.3 Surgery is the only curative option for most of these patients, but many patients relapse, and 5-year survival remains low.4 Although there is currently no global consensus,5 perioperative chemotherapy is an accepted standard for patients with locally advanced gastroesophageal adenocarcinoma in Europe6,7 and the United States8 and improves 5-year overall survival (OS) by about 13 %.9,10 The optimal perioperative therapy regimen is still a matter of debate, and controversy exists whether the inclusion of anthracyclines, taxanes, or radiation is beneficial for the patients.7,11,12 Patients in the MAGIC study received triple chemotherapy with epirubicin, cisplatin, and 5-FU,9 while patients in the FNCLCC/FFCD study were treated with cisplatin/5-FU only.10 There have been no randomized studies comparing different chemotherapy regimens. In the palliative setting, a meta-analysis suggested that the addition of the anthracyclin epirubicin to cisplatin and 5-FU might result in longer OS and that the traditionally used cisplatin can be replaced by oxaliplatin without a loss in efficacy and with less toxicity.13 The addition of docetaxel to platin/5-FU increased response rates and survival, but also toxicity.14–16 In other types of cancer, e.g., breast cancer, more intensive neoadjuvant therapy that increases clinical and pathological response rates can also result in improved survival,17 and we hypothesize that this might also be the case in esophagogastric adenocarcinoma.
In this study, we analyzed a large cohort of patients resected for esophagogastric adenocarcinoma (AEGI/II/III by Siewert classification and gastric cancer) who had received a variety of different neoadjuvant chemotherapy regimens. The aim of our retrospective study was to assess the influence of the different preoperative chemotherapy regimens on patients’ response, complication rate, and prognosis.
Patients and Methods
Patients
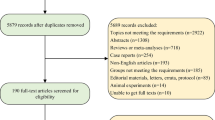
A total of 1051 patients with gastroesophageal adenocarcinomas were treated with neoadjuvant chemotherapy between 1987 and 2011 in two academic centers (Department of Surgery, Technische Universität München, Klinikum rechts der Isar, Munich and Heidelberg University Hospital) (Fig. 1). Of these, 843 patients who had received neoadjuvant doublet chemotherapy with either cisplatin/5-fluorouracil (5-FU) or oxaliplatin/5-FU (groups A and B) or triplet therapy with additional epirubicin (group C) or a taxane (group D) were included in this study. Neoadjuvant treatment was initiated in T3/T4/Nany cM0/x patients. Clinicopathological and follow-up data were collected in a prospective database and analyzed retrospectively. The study was approved by the institutional review board (S-635/2013).
Staging and Clinical Response
Initial staging included upper endoscopy with biopsies and computed tomography (CT) of the chest and abdomen. Clinical response was evaluated after completion of neoadjuvant chemotherapy before resection as described in Ref. 18 and defined as at least partial response in endoscopy (<75 % residual tumor) and CT scan (decrease >50 % in wall diameter).
Chemotherapy
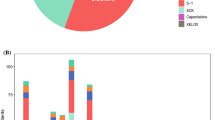
The choice of neoadjuvant chemotherapy was at the discretion of the attending oncologist and depended on the patient’s general condition, but also changed with the available evidence and study results. We stratified the patients into 4 groups (Fig. 1). The 417 patients in group A received cisplatin and 5-FU. The PLF protocol19 was used in 412 patients, the other patients received modified versions of the protocol. The 54 patients in group B were treated with oxaliplatin and 5-FU. The majority of patients (n = 25) were treated according to the OLF protocol,20 17 patients received the FLO-protocol,21 7 patients EOX without epirubicin,22 and 5 patients other oxaliplatin/5-FU-combinations. The 190 patients in the epirubicin, platin, and 5-FU group C received EOX (n = 141), ECF (n = 18), ECX (n = 8), EOF (n = 5), and EPLF (n = 14; PLF protocol19 with 30 mg Epirubicin/m day 1 of week 2, 4, and 6), or combinations of these protocols. The 182 patients in group D were treated with a combination of a taxane (docetaxel or paclitaxel) with platin and 5-FU. Most patients (n = 123) were treated according to the paclitaxel-PLF-protocol,23 20 patients received docetaxel-PLF,16 and 39 patients the FLOT protocol.15 A total of 101 patients had received various other chemotherapy protocols and were excluded from this analysis (Fig. 1).
Surgery
Surgery was performed 2–4 weeks after completion of chemotherapy. In patients with AEG I, a right abdominothoracic en bloc esophagectomy with a 2-field lymphadenectomy (LAD) (Ivor-Lewis procedure) with intrathoracic anastomosis or a transhiatal extended esophagectomy with cervical anastomosis was performed including the abdominal lymph nodes along the celiac axis and suprapancreatic region (according to a D2-LAD for gastric carcinoma). In case of AEG II and III, the standard procedure was a transhiatal extended gastrectomy with an extended D2-LAD (lymph node groups 1+2 according to the Japanese Research Society for Gastric Cancer) including a left retroperitoneal LAD. For patients with gastric cancer a total gastrectomy with a D2-LAD was performed. In some patients with distal gastric cancer a subtotal gastrectomy was performed.
Histopathological Response
All resection specimens were histologically examined in the Departments of Pathology, University of Heidelberg and the Technische Universität München, Munich. Histopathologic examination included (y)pTNM-categories, R-category, tumor differentiation, and growth pattern according to Laurén. The results were converted to the UICC classification, seventh edition, 2010, when previous editions had been used. Tumor regression was classified according to the scoring system by Becker et al.24 Tumors with less than 10 % residual tumor (Becker grade 1a/1b) were classified as responders.
Statistical Analysis
Patients were stratified according to the different neoadjuvant chemotherapy regimens, and the influence of the chemotherapies on various clinical parameters was analyzed. Overall survival and disease-free survival (DFS) were calculated from time of diagnosis till death/diagnosis of recurrent disease or last follow-up date using Kaplan–Meier curves. Survival analysis was performed using Kaplan–Meier estimates, log-rank tests, and Cox proportional hazards regression analysis. To compare discrete data, we used the χ2 test. A p value of less than 0.05 was considered statistically significant. Analyses were performed using the SPSS statistical package (version 20.0; SPSS Inc., Chicago, IL) using 2-sided tests.
Results
Patient Characteristics
A total of 843 patients were included in this study (Fig. 1). Median follow-up was 33 months, and median overall survival (mOS) was 39.1 months. The majority of patients (71.8 %) had tumors of the esophagogastric junction (AEG, Siewert types I, II, or III); the remaining patients had gastric cancer of the corpus or antrum (23.5 %) or cancer of the whole stomach (4.6 %) (Table 1). Median survival was 39.3 months for patients with AEG I, II, or III tumors and 39.9 months for patients with gastric cancer. Patients treated in Munich (n = 575) and Heidelberg (n = 268) had comparable mOS (40.4 vs 32.9 months, p = 0.403). A total of 138 patients received adjuvant chemotherapy.
Clinical and Pathological Response
Clinical response18 was a statistically significant predictor of longer OS (p < 0.001). We did not observe patients with complete clinical response. Patients with a partial clinical response to neoadjuvant therapy (n = 265) had a mOS of 101.7 months compared with 26.7 month for those with minor response, no change, or progressive disease. Likewise, pathological response was associated with survival. While mOS in the 54 patients with complete pathological regression (cPR, Becker category 1a) was not yet reached, patients classified as regression 1b (n = 171), regression 2 (n = 217), and regression 3 (n = 405) had a mOS of 77.8, 43.1 and 25.4 months, respectively (p < 0.001). Histopathological responder (regression 1a/b) had a median survival of 92.2 months compared with 27.7 months for histopathological nonresponder (p < 0.001).
Clinical and histopathological response were also of prognostic relevance within the subgroup of patients with AEG tumors (p < 0.001). In gastric cancer patients, clinical response was also predictive for longer survival (p = 0.003), but histopathological response was not (p = 0.330).
Chemotherapy Regimens and Outcome
Groups A Versus B Versus C Versus D
First we compared the 4 groups of patients who received either a platin-based doublet therapy (groups A and B) or a triplet therapy with epirubicin or taxane (groups C and D, respectively).
Planned chemotherapy was completed by 74 % of patients, ranging from 70.4 % in the cisplatin group A to 83.2 % in the epirubicin group C (Table 2). Interestingly, there was no significant difference in mOS between patients who completed the planned neoadjuvant therapy (n = 623, 41.6 months) and those who, for various reasons, did not receive the full number of chemotherapy cycles (n = 219, 36.7 months, p = 0.362).
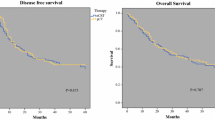
The best clinical response rate (34.8 %) was observed in group D; however, the differences to the other groups were not statistically significant (Table 2). We also found the longest mOS (53.9 months) in group D compared with 36.4, 24.3, and 44.2 months in group A, B, and C, respectively, without reaching statistical significance (p = 0.224, Fig. 2a). Disease-free survival was also not significantly different (p = 0.190). Significantly more patients in group A were found to have metastatic disease during the operation. There were no significant differences in R-status, ypT category, or ypN category (Table 2).
Patient survival according to different chemotherapies. a Group A versus B versus C versus D. b Platin/5-FU doublet therapy (groups A and B) vs triplet chemotherapy (groups C and D). c Therapy without taxane (groups A, B, and C) versus therapy with taxane (group D). Therapy without taxane vs therapy with taxane separately in patients with AEG tumors (d) and patients with gastric cancer (e). Results are shown as Kaplan–Meier survival curves
The rate of histopathological responders (Becker category 1) was similar in the different groups. Unexpectedly, the pCR rate (Becker category 1a) in the taxane group D was only 6.2 %. Within group D, the pCR rates of the patients receiving paclitaxel-PLF (n = 121), docetaxel-PLF (n = 19), and FLOT (n = 38) were 6.6, 10.5, and 2.6 %, respectively. The differences in these subgroups were not significant.
While the rate of surgical complications in the different groups was similar, patients in group B who had received oxaliplatin and 5-FU had a significantly higher rate of nonsurgical complications and the highest 30-day and in-hospital mortality (p = 0.003 and 0.040, Table 2).
Doublet Chemotherapy Versus Triplet Chemotherapy
No significant differences in mOS (36.1 vs 45.6, p = 0.098, Fig. 2b), clinical response (p = 0.927), or pCR (p = 0.287), were found between patients receiving doublet (groups A and B) or triplet (groups C and D) regimens. Overall complications were not significantly different, but the nonsurgical complication rate was higher in the triplet chemotherapy group. Mortality rates were similar in both groups.
Addition of Taxanes
When we compared patients receiving a triple therapy containing a taxane (group D) with the combined groups A, B, and C, we found a trend for better clinical response (34.8 vs 28.0 %, p = 0.078) and longer survival (55.9 vs 37.1 months, p = 0.068, Fig. 2c) in the taxane group (Table 3). The ypT stages were slightly lower in the taxane group (p = 0.035, Table 3). DFS was significantly longer in the taxane group (31.5 vs 28.0 months, p = 0.038). In multivariate analysis, however, taxane therapy was not an independent factor (p = 0.185). The pathological response rates were similar (p = 0.836). The overall complication rate was similar, but the anastomotic leakage rate was higher in the taxane group. However, in the taxane group there were significantly more patients that were treated by esophagectomy (50.5 vs 31.8 %, p < 0.001). When the patients were analyzed according to their type of surgery, there was no difference in anastomotic leakage rate (esophagectomy: leakage rate 26.2 % without taxane versus 30.4 % with taxane, p = 0.485; transhiatal extended gastrectomy: 6.9 vs 9.3 %, p = 0.564). Mortality was not significantly different.
Separate Analysis of AEG and Gastric Cancer
When we analyzed patients with AEG tumors separately, we found a significantly improved mOS (p = 0.037, Fig. 2d) and DFS (p = 0.041) for patients treated with taxanes. In multivariate analysis, T1/2, N0, M0, histopathological and clinical response were independent factors in regard to OS, but taxane therapy was not (p = 0.114; hazard ratio 0.770; 95 % confidence interval 0.556–1.064, Table 4). Since patients in group D tended to be younger and had a higher proportion of male patients, we used a model adjusted for age and sex. In this calculation, the OS difference was no longer statistically significant (p = 0.147). Again, there was no difference in overall or surgical complications.
In the subgroup of patients with gastric cancer the addition of taxanes did not significantly improve OS (47.9 months with taxane vs 37.9 months without taxane, p = 0.898, Fig. 2e), DFS (p = 0.649) or other outcome parameters, but there was a trend toward better pathological response (28.6 vs 16.3 % responders, p = 0.086). However, the subgroup of gastric cancer patients treated with taxanes was rather small (n = 37).
Discussion
Locally advanced gastric cancer and adenocarcinoma of the esophagogastric junction still have high recurrence rates after perioperative therapy and curative resection.25–27 In addition to optimization of surgical techniques and quality, better perioperative strategies are necessary to increase long-term survival of these patients.1,4,28 With 843 included patients, this is to our knowledge the largest series evaluating the influence of different preoperative chemotherapy regimens on the patient’s outcome. The patients had a rather extensive disease burden, with 25 % ypN3 disease, 17.5 % M1 disease, and 24.6 % R1/R2/Rx resections. Since patients treated with older chemotherapy regimens such as EAP29,30 or radiation were excluded, the analyzed patient cohort was large, homogenous, treated with standardized chemotherapy and surgery at two academic expert centers, and had a long follow-up in a prospectively generated database.
We were unable to identify a superior chemotherapy regimen that significantly improved clinical response, pathological response, or OS in the complete cohort. The potentially more potent triple combinations with epirubicin or taxane did not significantly improve outcome. However, there was a trend for better clinical response and survival when the taxane-triplet therapy group D was compared with the combined other groups.
It has been previously reported that AEG tumors are more likely to respond to preoperative chemotherapy compared with distal gastric cancer31,32 and that patients with AEG tumors have a greater benefit from preoperative chemotherapy.33 When we analyzed patients with AEG tumors and those with distal gastric cancer separately, the trend for better clinical response in the taxane group was maintained, and OS was significantly better. In contrast, patients with gastric cancer did not benefit from taxanes; however, the number of gastric cancer patients treated with taxanes was limited. Importantly, we did not find any evidence supporting the concern that more intensive chemotherapy containing taxanes results in higher surgical complication rates or mortality.
Our study confirms that both clinical and pathological response25,26,34–37 to neoadjuvant chemotherapy are strongly associated with patients’ survival, so that both might be a valid tool to compare the effects of different chemotherapy regimens There was a trend for a better clinical response in AEG patients treated with taxane-triplet therapy, but the pCR rate in the taxane-treated patients was only 6.2 %. This is in contrast to some recent studies that reported impressive pCR rates of 15–17 % for these patients.36,38 We have no conclusive explanation for this result, although the histopathological workup probably influences the observed pCR rate. In our series, the whole tumor bed was paraffin embedded and analyzed by experienced pathologists.24,34,37
This is a retrospective analysis and therefore has all the disadvantages of this study type, but since there are only few randomized phase III studies9,10,39 some questions have to be answered retrospectively. The choice of the applied chemotherapy regimens in our cohort was partly influenced by the patients’ age and general condition. Oxaliplatin was chosen instead of cisplatin in older patients and in patients with impaired kidney function, probably explaining the higher nonsurgical complication rate and postoperative mortality in group B. Patients in the taxane group D were younger, and the statistical significance of the OS difference was lost when a model adjusted for age and sex was used. Our database does not systematically include patients who progressed under neoadjuvant chemotherapy and were never referred to the surgeon. Another limitation of our study might be the long study period. However, basic staging including endoscopy and CT in all patients remained unchanged over the long period. Additionally, since the beginning of the study period, high surgical standards including radical surgery with an intra-abdominal D2 lymphadenectomy were applied, and therefore the influence of the time factor is probably limited.
The study’s limitations clearly highlight the demand for randomized clinical trials. The ongoing FLOT4-study (NCT01216644) that compares perioperative epirubicin-containing triple chemotherapy with the docetaxel-containing FLOT protocol for esophagogastric cancer stages >cT1 will clarify whether the observed trend for better results with taxanes in our analysis will be confirmed in a randomized controlled trial. Our analysis showed no hint for a benefit of an addition of epirubicin to platin/5-FU; the results of the British OEO5 trial will provide more solid evidence regarding the value of epirubicin in the neoadjuvant setting.
However, other questions will remain open even after these studies. It is generally accepted that the addition of radiotherapy increases the rates of complete histopathological responses11 compared with chemotherapy alone, but whether this local effect leads also to increased survival remains unclear.27 The value of the adjuvant part of perioperative chemotherapy is also not clear. In our cohort, mainly patients in group C that were treated in analogy to the MAGIC study9 received adjuvant chemotherapy after resection. This confounder could potentially favor group C; however, we did not find a significant benefit for triple therapy with epirubicin. However, both studies, MAGIC and FFCD, had in common that in only about 50 % of patients postoperative adjuvant chemotherapy was delivered,9,10 so that the prognostic relevance of the adjuvant part of chemotherapy remains unanswered so far.
In conclusion, our retrospective analysis did not demonstrate superiority of a specific chemotherapy protocol for neoadjuvant therapy of all patients with gastroesophageal adenocarcinoma. The tendency for better results with taxane-containing regimens, especially in patients with proximal adenocarcinomas, raises the hope that these protocols will show a significant benefit in the currently ongoing randomized trials.
References
Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477–90.
Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–12.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30.
Wilke H, Lordick F, Meyer HJ, Stahl M. (Neo)-adjuvant chemo(-radio) therapy for adenocarcinomas of the gastroesophageal junction and the stomach in the West. Dig Surg. 2013;30:112–8.
Sehdev A, Catenacci DV. Perioperative therapy for locally advanced gastroesophageal cancer: current controversies and consensus of care. J Hematol Oncol. 2013;6:66.
Okines A, Verheij M, Allum W, Cunningham D, Cervantes A. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v50–v54.
Lutz MP, Zalcberg JR, Ducreux M, et al. Highlights of the EORTC St. Gallen International Expert Consensus on the primary therapy of gastric, gastroesophageal and oesophageal cancer—differential treatment strategies for subtypes of early gastroesophageal cancer. Eur J Cancer. 2012;48:2941–53.
Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Cancer Netw. 2013;11:531–46.
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–21.
van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84.
Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–92.
Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010;3:CD004064.
Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–7.
Al-Batran SE, Hartmann JT, Hofheinz R, et al. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol. 2008;19:1882–7.
Lorenzen S, Hentrich M, Haberl C, et al. Split-dose docetaxel, cisplatin and leucovorin/fluorouracil as first-line therapy in advanced gastric cancer and adenocarcinoma of the gastroesophageal junction: results of a phase II trial. Ann Oncol. 2007;18:1673–9.
Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15:640–7.
Heger U, Bader F, Lordick F, et al. Interim endoscopy results during neoadjuvant therapy for gastric cancer correlate with histopathological response and prognosis. Gastric Cancer. 2014;17:478–88.
Ott K, Sendler A, Becker K, et al. Neoadjuvant chemotherapy with cisplatin, 5-FU, and leucovorin (PLF) in locally advanced gastric cancer: a prospective phase II study. Gastric Cancer. 2003;6:159–67.
Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8:797–805.
Al-Batran SE, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435–42.
Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46.
Bader FG, Lordick F, Fink U, et al. Paclitaxel in the neoadjuvant treatment for adeno carcinoma of the distal esophagus (AEG I). A comparison of two phase II trials with long-term follow-up. Onkologie. 2008;3:366–72.
Becker K, Mueller JD, Schuhmacher C, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98:1521–30.
Fields RC, Strong VE, Gonen M, et al. Recurrence and survival after pathologic complete response to preoperative therapy followed by surgery for gastric or gastrooesophageal adenocarcinoma. Br J Cancer. 2011;104:1840–7.
Ott K, Blank S, Becker K, et al. Factors predicting prognosis and recurrence in patients with esophago-gastric adenocarcinoma and histopathological response with less than 10 % residual tumor. Langenbecks Arch Surg. 2013;398:239–49.
Vallböhmer H, Hölscher AH, DeMeester S, et al. A multicenter study of survival after neoadjuvant radiotherapy/chemotherapy and esophagectomy for ypT0N0M0R0 esophageal cancer. Ann Surg. 2010;252:744–9.
Schuhmacher C, Reim D, Novotny A. Neoadjuvant treatment for gastric cancer. J Gastric Cancer. 2013;13:73–8.
Fink U, Schuhmacher C, Stein HJ, et al. Preoperative chemotherapy for stage III–IV gastric carcinoma: feasibility, response and outcome after complete resection. Br J Surg. 1995; 82:1248–52.
Schuhmacher CP, Fink U, Becker K, Busch R, Dittler HJ, Mueller J, Siewert JR. Neoadjuvant therapy for patients with locally advanced gastric carcinoma with etoposide, doxorubicin, and cisplatinum. Closing results after 5 years of follow-up. Cancer. 2001;91:918–27.
Lorenzen S, Blank S, Lordick F, Siewert JR, Ott K. Prediction of response and prognosis by a score including only pretherapeutic parameters in 410 neoadjuvant treated gastric cancer patients. Ann Surg Oncol. 2012;19:2119–27.
Reim D, Gertler R, Novotny A, et al. Adenocarcinomas of the esophagogastric junction are more likely to respond to preoperative chemotherapy than distal gastric cancer. Ann Surg Oncol. 2012;19:2108–18.
Ronellenfitsch U, Schwarzbach M, Hofheinz R, et al. Preoperative chemo(radio)therapy versus primary surgery for gastroesophageal adenocarcinoma: systematic review with meta-analysis combining individual patient and aggregate data. Eur J Cancer. 2013;49:3149–58.
Becker K, Langer R, Reim D, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg. 2011;253:934–9.
Lowy AM, Mansfield PF, Leach SD, Pazdur R, Dumas P, Ajani JA. Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg. 1999;229:303–8.
Lorenzen S, Thuss-Patience P, Al-Batran SE, et al. Impact of pathologic complete response on disease-free survival in patients with esophagogastric adenocarcinoma receiving preoperative docetaxel-based chemotherapy. Ann Oncol. 2013;24:2068–73.
Langer R, Ott K, Feith M, Lordick F, Siewert JR, Becker K. Prognostic significance of histopathological tumor regression after neoadjuvant chemotherapy in esophageal adenocarcinomas. Mod Pathol. 2009;22:1555–63.
Homann N, Pauligk C, Luley K, et al. Pathological complete remission in patients with oesophagogastric cancer receiving preoperative 5-fluorouracil, oxaliplatin and docetaxel. Int J Cancer. 2012;130:1706–13.
Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Springfeld, C., Wiecha, C., Kunzmann, R. et al. Influence of Different Neoadjuvant Chemotherapy Regimens on Response, Prognosis, and Complication Rate in Patients with Esophagogastric Adenocarcinoma. Ann Surg Oncol 22 (Suppl 3), 905–914 (2015). https://doi.org/10.1245/s10434-015-4617-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4617-x