Abstract
Purpose
This study examined the prognosis of the “node-negative with eLNs ≤ 15” designation and the additional value of incorporating it into the pN1 designation in the seventh edition of the N classification.
Methods
From January 2000 to September 2010, a total of 1258 gastric cancer patients (patients with eLNs > 15 or node-negative with eLNs ≤ 15) undergoing radical gastric resection were enrolled in this study. We incorporated node-negative patients with eLNs ≤ 15 into pN1 and compared this designation with the current 7th edition UICC N stage for 3, 5-year overall survival by univariate and multivariate analysis. Homogeneity, discriminatory ability, and monotonicity of gradients in the hypothetical N stage and the UICC N stage were compared using linear trend χ2, likelihood ratio χ2 statistics, and Akaike information criterion (AIC) calculations.
Results
Node-negative patients with eLNs ≤ 15 had worse survival compared with those with eLNs > 15. In univariate and multivariate analyses, the hypothetical N stage showed superiority to the 7th edition pN staging. The hypothetical staging system had higher linear trend and likelihood ratio χ 2 scores and smaller AIC values compared with those for the TNM system, which represented the optimum prognostic stratification.
Conclusions
Node-negative patients with eLNs ≤ 15 can be considered to be incorporated into the pN1 stage in the 7th edition of the TNM classification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastric cancer is the fourth most common malignant tumor worldwide, with about one million new cases of stomach cancer estimated to have occurred. It is also the second leading cause of cancer-related death in both men and women.1 It has been widely demonstrated that the status of lymph node metastasis is one of the most important prognostic factors for gastric cancer. The 7th edition of AJCC/UICC TNM classification designates pN0 when all examined lymph nodes (eLNs) are negative, regardless of the total number of eLNs. Several studies have found that the number of eLNs was associated with the prognosis of node-negative gastric cancer and have recommended a proper number of eLNs for node-negative gastric patients.2–4 However, few studies have investigated how to accurately classify node-negative with insufficient numbers of eLNs in gastric cancer patients. The purpose of the present study was to determine the association between the number of eLNs and the prognosis of node-negative gastric cancer patients and to establish an optimal classification in the TNM staging system.
Methods
Patients
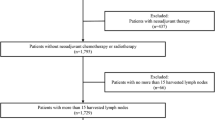
Continuous clinical-pathological data from 2085 cases of gastric cancer patients who underwent surgical resection from January 2000 to September 2010 at Sun Yat-sen University Cancer Center were analyzed retrospectively. The screening eligibility criteria included a postoperative survival time of ≥3 months. Patients who were undergoing neoadjuvant chemotherapy or chemoradiotherapy were excluded from the study. Because patients with palliative surgery had low LN counts as the goal was not a curative surgery but more to relieve symptoms, patients who were undergoing palliative surgery also were excluded from the study. Among the potential participants, 56 were lost to follow-up, 257 were undergoing palliative surgery, and 122 were undergoing neoadjuvant chemotherapy or chemoradiotherapy. Thus, the screening patients eligible for this study were 1650. Because AJCC/UICC TNM staging system recommend eLNs > 15 for accurate staging except for pN0 designation, node-positive with eLNs ≤ 15 also were excluded from the study. A total of 169 patients had 1–2 metastatic lymph nodes (MLNs) with eLNs ≤ 15, 173 patients had 3–6 MLNs with eLNs ≤ 15, and 71 patients had 7–15 MLNs with eLNs ≤ 15. Thus, 1258 patients were eligible for this study. Curative gastrectomy and lymphadenectomy (D2) were performed by experienced surgeons following the JGCA guidelines.5
All nodal material was separately dissected from the specimen at the end of the procedure by the surgeon. Each lymph node was submitted in a separate container labeled according to the site of origin and then analyzed by the pathologist. For all LNs, one to two sections were routinely examined histopathologically. Sometimes serial sections were cut from node area with the purpose of achieving definitive diagnosis and staging.2
Lymph node metastasis was classified according to the 7th edition UICC N stage (N0: no metastasis; N1: 1–2 MLNs; N2: 3–6 MLNs; N3a: 7–15 MLNs; N3b: ≥ 16 MLNs).6 We chose 15 eLNs as the best cutoff point for node-negative patients based on previous literature, clinical experience, and convenience in clinical application.4 We incorporated node-negative patients with eLNs ≤ 15 into pN1. Therefore, the hypothetical N classification was as follows: hypothetical N0: no metastasis with eLNs > 15; hypothetical N1: 1–2 MLNs or no metastasis with eLNs ≤ 15; N2–N3b were the same as 7th edition UICC N stage.
Postoperative follow-up included clinical and laboratory examinations every 3 months for the first 2 years at our outpatient department, every 6 months from the third to the fifth years, and annually thereafter until at least 5 years after the operation or until the patient died, whichever came first. Overall survival (OS) was defined as the time from operation to death or last follow-up. Disease-specific survival (DSS) was defined as the time from operation to death caused by disease progression or treatment-related complications. OS and DSS were used as measures of prognosis. The median follow-up for the entire cohort was 61.00 (range 3–156) months. The follow-up was closed in December 2014.
Statistical Analysis
The 3, 5-year survival rate was calculated using the Kaplan–Meier method. Statistical comparisons of different factors were made with the log-rank test. In multivariate analysis, forward stepwise regression analysis was performed with a Cox proportional hazards model. P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 18.0 (SPSS Inc., Chicago, IL).
To compare the homogeneity of the hypothetical N classification with that of the 7th edition UICC N system, the likelihood ratio χ 2 test related to the Cox regression model was used. The discriminatory ability and monotonicity of gradient assessments were measured with the linear trend χ 2 test. The Akaike information criterion (AIC) value within a Cox proportional hazard regression model was also calculated for each system to measure its discriminatory ability. The AIC statistic was defined by \({\text{AIC}} = - 2 {\text{ log maximum likelihood}} + 2 \times {\text{the number of parameters in the model}}\). A smaller AIC value indicates a more desirable model for predicting outcome.7–12
Results
The mean ± SD number of pathologically confirmed eLNs for the enrolled 1258 patients was 24.1 ± 12.1 (range 3–79). The 3, 5-year survival rate (3-year OS, 5-year OS) of all enrolled patients was 74.0, 70.6 % separately, and 888 patients were alive when the follow-up was completed. The median OS of all patients after surgery was 37.0 months.
As shown in Fig. 1, the survival of patients with >15 eLNs was significantly higher than those with ≤15 eLNs (3-year OS, 94.6 vs. 84.0 %, P < 0.001; 3-year DSS, 94.6 vs. 84.4 %, P < 0.001; 5-year OS, 93.4 vs. 78.6 %, P < 0.001; 5-year DSS, 93.7 vs. 79.4 %, P < 0.001).
OS and DSS curves for UICC N0/N1 gastric cancer patients who underwent radical surgical resection. a Log-rank test for overall survival: UICC N0 with eLNs > 15 versus UICC N0 with eLNs ≤ 15, P < 0.001; UICC N0 with eLNs > 15 versus UICC N1 with eLNs > 15, P < 0.001; UICC N0 with eLNs ≤ 15 versus UICC N1 with eLNs > 15, P = 0.969. b Log-rank test for disease-specific survival: UICC N0 with eLNs > 15 versus UICC N0 with eLNs ≤ 15, P < 0.001; UICC N0 with eLNs > 15 versus UICC N1 with eLNs > 15, P < 0.001; UICC N0 with eLNs ≤ 15 versus UICC N1 with eLNs > 15, P = 0.973; eLNs: Examined lymph nodes
The survival curve according to the 7th edition of AJCC/UICC N stage is shown in Fig. 2. The 5-year OS and 5-year DSS of node-negative patients with eLNs ≤ 15 were 78.6 and 79.4 %, and those of N1 with eLNs > 15 were 79.9 and 80.6 % as previously mentioned. We found that both the 5-year OS and 5-year DSS of node-negative patients with eLNs ≤ 15 were very similar to N1 with eLNs > 15. We also found that 3-year OS and 3-year DSS of node-negative patients with eLNs ≤ 15 were very similar to N1 with eLNs > 15 (node-negative with eLNs ≤ 15, 3-year OS 84.0 %, 3-year DSS 84.4 %; N1 with eLNs > 15, 3-year OS 82.0 %, 3-year DSS 82.7 %). Thus, hypothetical N1 (n = 396) was obtained from the sum of N1 with eLNs > 15 (n = 139) and node-negative patients with eLNs ≤ 15 (n = 257). The hypothetical N0 (n = 317) was only designated for node-negative patients with eLNs > 15.
Comparison of survival curves between hypothetical staging system and 7th edition AJCC/UICC staging system. a Log-rank test for 7th edition AJCC/UICC N stage: N0 versus N1, P = 0.027; N1 versus N2, P = 0.006; N2 versus N3a, P < 0.001; N3a versus N3b, P < 0.001. b Log-rank test for hypothetical N stage: hypothetical N0 versus hypothetical N1, P < 0.001; hypothetical N1 versus N2, P < 0.001; N2 versus N3a, P < 0.001; N3a versus N3b, P < 0.001. c Log-rank test for 7th edition AJCC/UICC TNM stage: IA versus IB, P = 0.021; IB versus IIA, P = 0.004; IIA versus IIB, P = 0.449; IIB versus IIIA, P = 0.068; IIIA versus IIIB, P = 0.002; IIIB versus IIIC, P = 0.001. d Log-rank test for hypothetical TNM stage: IA versus hypothetical IB, P = 0.975; hypothetical IB versus hypothetical IIA, P < 0.001; hypothetical IIA versus hypothetical IIB, P = 0.469; hypothetical IIB versus hypothetical IIIA, P = 0.012; hypothetical IIIA versus hypothetical IIIB, P < 0.001; hypothetical IIIB versus hypothetical IIIC, P < 0.001
The following 11 factors were found to have statistically significant associations with the 3-year OS, 3-year DSS, 5-year OS, and 5-year DSS upon univariate analysis: age, tumor location, tumor size, histological grade, type of gastrectomy, lymphatic vascular invasion, UICC T stage, UICC N stage, hypothetical N stage, TNM staging, and hypothetical TNM staging (Table 1; Supplementary Tables 1–3). All of the aforementioned variables were included in a multivariate Cox proportional hazards model to adjust for the effects of covariates. In our study, the UICC N stage and hypothetical N stage were highly correlated. Therefore, two separate multivariate models, one with UICC N and the other with hypothetical N stage, were run to avoid problems with the presence of multicollinearity. In that model, we demonstrated that age, histological grade, type of gastrectomy, lymphatic vascular invasion, UICC T stage, UICC N stage, and hypothetical N stage remained independent prognostic factors for 5-year OS, 3-year OS (Table 2; Supplementary Tables 4–6).
In multivariate analysis, 95 % confidence interval (CI) of hypothetical N stage did not cover zero in each group, whereas the 95 % CI of “UICC N1 versus UICC NO” covered zero with P > 0.05 for 5-year OS (95 % CI 0.952–2.301; Supplementary Table 6). Thus, classification using the hypothetical N stage provided well-discrimination risk groups, whereas classification using UICC N provided poorly separated risk groups.
A comparison of the performance of the AJCC/UICC N stage and the hypothetical N stage, as assessed by the linear trend χ 2, likelihood ratio χ 2, and the AIC tests is described in Table 3. Compared with the AJCC/UICC N stage, the hypothetical N stage had better homogeneity (higher likelihood ratio χ 2 score, 304.860 vs. 299.295), discriminatory ability, and monotonicity of gradients (higher linear trend χ 2 score, 314.418 vs. 295.911). Furthermore, in our study, the hypothetical N stage had a smaller AIC value (4243.832 vs. 4260.239), representing the optimum prognostic stratification.
Discussion
The 7th edition AJCC/UICC TNM staging system designates pN0 as any gastric cancer with all eLNs negative, regardless of the total number of eLNs. However, several studies have reported that an insufficient number of eLNs is associated with poor prognosis for node-negative gastric cancer patients.2–4 Thus, several researchers have recommended different numbers of eLNs. Jiao XG et al. reported that dissection of >15 eLNs is recommended during lymphadenectomy to improve the long-term survival.4 Xu et al. also suggested that patients with lymph node-negative gastric cancer who underwent D2 dissection should have at least 16 LNs examined, especially in advanced gastric cancer.2 Baiocchi et al. suggested retrieval of more than 25 nodes can stratify node-negative gastric cancer patients accurately.3 However, few studies have shown how to classify node-negative gastric cancer patients with less than the recommended numbers of eLNs. The purpose of the present study was to determine the association between the number of eLNs and the prognosis of node-negative gastric cancer patients, and more importantly, this study incorporated node-negative gastric cancer patients with less than the recommended number of eLNs into an optimal classification in the TNM staging system.
In summary, we can conclude that (1) in N stage, the 3, 5-year survival rate of node-negative gastric patients with eLNs ≤ 15 was worse than those with eLNs > 15 and was similar to N1 with eLNs > 15; (2) we validated our results utilizing the linear trend χ 2, likelihood ratio χ 2, and the AIC tests to confirm the homogeneity, discriminatory ability, and monotonicity of gradients in our novel classification. Compared with the 7th edition N stage, the hypothetical N stage performed better for homogeneity, discriminatory ability, and monotonicity of gradients.
The hypothetical TNM staging mainly performs two advantages: (1) It can predict the prognosis of patients with gastric cancer more accurately; (2) It can select some patients who need postoperative adjuvant chemotherapy according to the eligibility criteria of CLASSIC trial and ACTS GC trial. In this study, a total of 48 patients (3.2 %) with stage IB changed to IIA stage. According to the results of CLASSIC trial and ACTS GC trial, postoperative adjuvant chemotherapy is recommended for these patients.13,14
There are several possible factors affecting the number of exLN: innate number of LNs for each patient, extent of the surgery, retrieval technique, enthusiasm to find more LNs, fat volume of the specimens, nodal status, and so on. The reasons for poor prognosis of node-negative patients with ≤15 eLNs may be as follows: 1) metastatic lymph nodes may be missed during retrieval or pathological examination in node-negative patients with ≤15 eLNs; 2) inadequate dissection of lymph nodes could lead to poor prognosis of node-negative gastric cancer patients because lymph node metastasis, micrometastasis, or lymphovascular invasion may exist.
We acknowledge several limitations in this study. Our sample population is from a single institution experience, is relatively small compared with the worldwide gastric cancer collaboration database, and is based on a retrospective study. On the other hand, the strengths of this study are that the surgical procedures, pathologic examinations, and patient follow-up were uniform throughout the entire study period. Our present study demonstrated that the prognosis of node-negative patients with ≤15 eLNs was worse than those with >15 eLNs, and for the first time, investigated the incorporation of node-negative patients with ≤15 eLNs into the N1 stage in postoperative samples of gastric cancer. We suggested that the designation of pN0 should be used if all lymph nodes are negative when total number examined more than 15 (not include 15). pN1 should be designed if there is metastasis in one to two regional lymph nodes or negative in all lymph nodes when total number examined less than 15.
References
Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917.
Xu D, Huang Y, Geng Q, et al. Effect of lymph node number on survival of patients with lymph node-negative gastric cancer according to the 7th edition UICC TNM system. PloS One. 2012;7(6):e38681.
Baiocchi GL, Tiberio GA, Minicozzi AM, et al. A multicentric Western analysis of prognostic factors in advanced, node-negative gastric cancer patients. Ann Surg. 2010;252(1):70–3.
Jiao XG, Deng JY, Zhang RP, et al. Prognostic value of number of examined lymph nodes in patients with node-negative gastric cancer. World J Gastroenterol. 2014;20(13):3640–8.
Japanese Gastric Cancer A: Japanese classification of gastric carcinoma: 2nd English Edition. Gastric Cancer. 1998;1(1):10–24.
Sobin LH, Gospodarowicz MK, Wittekind C, et al. TNM classification of malignant tumours, 7th edn. Chichester, NJ: Wiley; 2010.
Harrell FE, Jr, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA.1982;247(18):2543–6.
Wunder JS, Healey JH, Davis AM, et al. A comparison of staging systems for localized extremity soft tissue sarcoma. Cancer. 2000;88(12):2721–30.
Kee KM, Wang JH, Lee CM, et al. Validation of clinical AJCC/UICC TNM staging system for hepatocellular carcinoma: analysis of 5,613 cases from a medical center in southern Taiwan. Int J Cancer. 2007;120(12):2650-5.
Cho YK, Chung JW, Kim JK, et al. Comparison of 7 staging systems for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Cancer. 2008;112(2):352–61.
Hsu PK, Wu YC, Chou TY, et al. Comparison of the 6th and 7th editions of the American Joint Committee on Cancer tumor-node-metastasis staging system in patients with resected esophageal carcinoma. Ann Thorac Surg. 2010;89(4):1024-31.
Wang W, Xu DZ, Li YF, et al. Tumor-ratio-metastasis staging system as an alternative to the 7th edition UICC TNM system in gastric cancer after D2 resection: results of a single-institution study of 1343 Chinese patients. Ann Oncol. 2011; 22(9):2049-56.
Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–20.
Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–21.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (81172080 and 81201773), the Research Fund for the Doctoral Program of Higher Education of China by the Ministry of Education (20100171110084 and 20120171120114), and the Science and Technology Project of Guangdong Province (2011B031800181). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors have declared no conflicts of interest related to this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Bofei Li, Yuanfang Li and Wei Wang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2015_4578_MOESM2_ESM.tif
Supplementary material 2 (TIFF 6865 kb) Supplementary figure 1. Flowchart of study design. SYSUCC: Sun Yat-senUniversity Cancer Center; MLNs: Metastatic lymph nodes; eLNs: Examined lymphnodes.
Rights and permissions
About this article
Cite this article
Li, B., Li, Y., Wang, W. et al. Incorporation of N0 Stage with Insufficient Numbers of Lymph Nodes into N1 Stage in the Seventh Edition of the TNM Classification Improves Prediction of Prognosis in Gastric Cancer: Results of a Single-Institution Study of 1258 Chinese Patients. Ann Surg Oncol 23, 142–148 (2016). https://doi.org/10.1245/s10434-015-4578-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4578-0