Abstract
Background and Aims
Compliance with S-1 adjuvant chemotherapy is not satisfactory, and the aim of the present study was to clarify risk factors for the continuation of S-1 after gastrectomy.
Methods
This retrospective study selected patients who underwent curative D2 surgery for gastric cancer, were diagnosed with stage II/III disease, had a creatinine clearance >60 ml/min, and received adjuvant S-1 at our institution between June 2010 and March 2014. The time to S-1 treatment failure (TTF) was calculated.
Results
Fifty-eight patients were selected for the present study. When the TTF curves stratified by each clinical factor were compared using the log-rank test, lean body-mass loss (LBL) of 5 % was regarded as a critical cutoff point. Univariate Cox’s proportional hazard analyses demonstrated that LBL was a significant independent risk factor for continuation. The 6-month continuation rate was 91.7 % in patients with an LBL < 5 %, and 66.3 % for patients with an LBL > 5 % (p = 0.031).
Conclusions
The present study demonstrated that LBL might be an important risk factor for a decrease in compliance to adjuvant chemotherapy with S-1 in patients with stage II/III gastric cancer who underwent D2 gastrectomy. A multicenter, double-blinded, prospective cohort study is necessary to confirm whether LBL would affect adjuvant S-1 continuation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastric cancer is the fourth most common human malignant disease, and the second most frequent cause of cancer-related death worldwide.1 Complete tumor removal is essential for curing gastric cancer; however, more than half of the patients develop recurrent disease even after curative surgery.
Two large phase III trials in Eastern Asia showed the effectiveness of adjuvant chemotherapy for gastric cancer. One was a Japanese phase III trial [Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer (ACTS-GC)],2 and the other was a Korean phase III trial [Capecitabine and Oxaliplatin Adjuvant Study in Stomach Cancer (CLASSIC)].3 Although the ACTS-GC trial clearly demonstrated that S-1 is effective as adjuvant chemotherapy for Japanese patients who have undergone curative D2 gastrectomy for gastric cancer and were diagnosed with pathological stage II or III disease, the proportion of patients with treatment failure was 65.8 % at 12 months after surgery. Moreover, among patients who received treatment for 12 months, a reduction of dose was necessary in 46.5 % of patients.2 The most common cause of withdrawal was adverse events. The efficacy of adjuvant chemotherapy will be decreased when treatment is insufficient, as has been confirmed in breast cancer.4
We recently demonstrated that body-weight loss >15 % at 1 month after surgery was an independent risk factor for the continuation of S-1 adjuvant chemotherapy.5 In that study, almost 10 % of patients had been evaluated by this measure as being at high risk of continuation.5 However, the high-risk population has decreased as a result of recent progress in perioperative care using the Enhanced Recovery After Surgery (ERAS) program.6–8 A more sensitive measure is therefore needed to identify patients at high risk for S-1 continuation.
Body composition, specifically the body proportions of lean and adipose tissues and organs, is one of the phenotypic factors that may affect the metabolism and toxicity of chemotherapy drugs.9,10 Changes in body composition may be a more sensitive marker than body-weight loss for chemotherapy-induced toxicities or continuation. Generally, computed tomography (CT) has been used to measure body composition;11 however, CT examinations 1 month after surgery are not routinely performed. Instead, bioelectrical impedance appears to provide a non-invasive, safe, and rapid method for evaluating body composition.12
Based on these points, we re-analyzed the risk factors for continuation of S-1 adjuvant chemotherapy by including the measures obtained by a bioelectrical impedance method in gastric cancer patients who had undergone gastrectomy.
Patients and Methods
Patients
Patients were selected from the prospective database of the Department of Gastrointestinal Surgery, Kanagawa Cancer Center, Yokohama, Japan, according to the following criteria; (1) histologically-proven gastric adenocarcinoma; (2) patients underwent a curative gastrectomy for gastric cancer as a primary treatment between June 2010 and March 2014; (3) stage II or III disease was diagnosed pathologically according to the 14th edition of the General Rules for Gastric Cancer published by the Japanese Gastric Cancer Association;13 (4) the patient had a creatinine clearance >60 ml/min;14 (5) the patient did not experience weight loss before surgery; and (6) the patient had undergone a body composition analysis within 1 week before surgery and at 1 month after surgery.
Surgical Procedures and Perioperative Care
All patients received distal or total gastrectomy with nodal dissection for gastric cancer. In principle, a D1 or D1+ lymphadenectomy is indicated for cT1N0 tumors, and D2 is applied for cN+ or cT2–T4 tumors, regardless of the approach.15 Spleen-preserving D2 total gastrectomy was permitted in this study.
Patients were treated using the ERAS protocol after gastrectomy. Details of this protocol have been reported in a previous study.16 In brief, patients were allowed to eat until midnight on the day before the surgery and were required to drink the contents of two 500-ml plastic bottles containing oral rehydration solution until 3 h before surgery. The nasogastric tube was removed immediately after surgery. Oral intake was initiated on postoperative day (POD) 2, beginning with water and an oral nutritional supplement. Patients began to eat solid food on POD 3, starting with rice gruel and soft food on POD 3 and advancing in three steps to regular food intake on POD 7. Patients were discharged when they had achieved adequate pain relief and soft food intake, had returned to their preoperative mobility level, and exhibited normal laboratory data on POD 7.
S-1 Adjuvant Chemotherapy
Patients received S-1 chemotherapy at 80–120 mg/body/day according to body surface area (BSA): BSA < 1.25 m2, 80 mg/day; 1.25 < BSA < 1.5 m2, 100 mg/day; 1.5 m2 < BSA, 120 mg/day2. The planned period of S-1 treatment was 1 year, with the exception of patients who were registered in the Optimal Period of Adjuvant S-1 chemotherapy trial (OPAS-1, a multicenter, phase III trial for pathological stage II gastric cancer patients who underwent D2 gastrectomy to confirm non-inferiority of recurrence-free survival in the test arm of four courses of S-1 adjuvant chemotherapy against the control arm of eight courses of S-1) after February 2012.17 Doses were modified in accordance with the following guidelines: when adverse reactions appeared, the dose was reduced from 120 to 100 mg/day or from 100 to 80 mg/day, or administration was temporarily discontinued. Treatment was discontinued when the patient showed a recurrence of disease or adverse reactions that were uncontrollable even by dose modification or temporary withdrawal of drug administration.
Body Composition Analysis
The segmental body composition was analyzed using the Tanita MC-190EM bioelectrical impedance analyzer (Tanita, Tokyo, Japan), which provides relative information regarding the amount of lean and fat tissue in the trunk area and each limb, as well as the overall body composition and hydration status. Body weight and composition were evaluated by a bioelectrical impedance analyzer within 1 week before surgery and at 1 month after surgery.
Evaluation and Statistical Analysis
Lean body-mass loss (LBL) was defined as: % loss of lean body mass = (preoperative lean body mass − lean body mass at 1 month after surgery) × 100/preoperative lean body mass. The preoperative lean body mass was measured within 1 week before surgery. Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria, version 3.0. The time to S-1 treatment failure (TTF) and the proportion of treatment failures at 3 and 6 months after surgery were calculated using the Kaplan–Meier method, and were compared using the log-rank test. In this study, when patients were administered S-1 for more than 6 months after surgery, they were treated as censored cases at 6 months. When S-1 was discontinued at less than 6 months after surgery, we defined that the event had occurred on the last day of S-1 treatment based on the protocol due to the adverse events, the patient’s refusal due to the development of adverse events, the patient’s refusal due to other reasons than adverse events, disease recurrence, or the patient’s death. The Cox proportional hazard model was used to perform univariate analyses to determine the risk factors for S-1 continuation. To determine the optimal cutoff values for the loss of lean body mass and serum albumin for the risk factor analyses, the TTF was examined by stratifying these values. A p value < 0.05 was defined as being statistically significant. The SPSS software package (v11.0 J Win; SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
This study was approved by the Institutional Review Board of the Kanagawa Cancer Center.
Results
Background of Patients
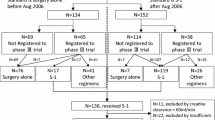
A total of 465 patients underwent surgical resection between June 2010 and March 2014. A flow diagram of the study selection criteria is shown in Fig. 1; 58 patients were eligible for the present study. Seven patients had been registered in the OPAS-1 trial in the same period, while 51 received S-1 treatment as part of general clinical practice.
Patients’ age ranged between 36 and 80 years (median 67 years). Twenty-nine patients were male, and 29 were female. Sixteen patients received neoadjuvant chemotherapy as part of clinical trials, while only one patient experienced body-weight loss over 15 % at 1 month after surgery. LBL was significantly related with body-weight loss in this cohort (r = 0.500, p = 0.001 by Pearson’s correlation coefficient; Fig. 2). Five patients had pathological stage I (all stage I patients had received neoadjuvant chemotherapy), 20 patients had stage II, and 33 patients had stage III.
Optimal Cutoff Value
The TTF stratified by each clinical factor was compared using the log-rank test. A loss of lean body mass of 5 % was defined as the optimal critical point for classification after considering the 3- and 6-month proportion of patients with treatment failure (Table 1).
Risk Factors
Each of the clinicopathological factors were categorized as shown in Table 2, and were analyzed for their association with the risk of treatment failure. Univariate analyses demonstrated that a loss of lean body mass was the only significant risk factor for the continuation of treatment (Table 2). Figure 3 shows that the proportion of patients with treatment failure at 6 months was 91.7 % in patients with a loss of lean body mass < 5, and 66.3 % in those with a loss of lean body mass ≥5 %. Table 3 shows details of the patients who stopped S-1 due to any events. The reasons for discontinuation included the prespecified rules of the protocol due to adverse events in five patients, the patient’s refusal due to adverse events in two patients, disease recurrence in two patients, and death in none of the patients. When comparing the loss of lean body mass between the patients with any reason for discontinuation and those without discontinuation, the loss of lean body mass was higher in the patients with any reason for discontinuation. The median loss of lean body mass was −6.1 % (range −17.9 to 4.6 %) in the patients with any reason for discontinuation, while in the patients without discontinuation, the median loss of lean body mass was −4.1 % (range −13.6 to 1.0 %).
Toxicity
All 58 patients were evaluated for toxicities of grade 2, 3, or 4 (Table 4). There were no grade 4 toxicities. Although there were no significant differences in both incidences of grade 2 and 3 toxicities between the LBL > 5 % group and the LBL < 5 % group (76.9 vs. 83.0 % in grade 2, and 42.9 vs. 18.9 % in grade 3), the p value for grade 3 was marginal (p = 0.050).
Discussion
This report first demonstrated that a loss of lean body mass ≥5 % at 1 month after surgery was a significant risk factor for the continuation of S-1 adjuvant chemotherapy, even though these patients did not develop body-weight loss ≥15 % at 1 month after surgery. To confirm the present results, a multicenter, double-blinded, prospective cohort study is necessary to confirm whether the LBL would affect adjuvant S-1 continuation.
A previous study showed that lean body mass decreased after gastrectomy. Kiyama et al. evaluated 108 patients who received gastrectomy, and found that a loss of muscle mass occurred immediately after surgery.18 The decrease in lean body mass may therefore be due to surgical stress.
There are several possible reasons why the loss of lean body mass affected the continuation of S-1 adjuvant chemotherapy. First, the lean tissue compartment is composed of metabolic tissues, such as the liver and kidneys, intracellular and extracellular water, and the skeletal muscles, which contribute a high proportion of the overall lean body mass. Low relative muscularity or a low overall lean body mass is apparently related to chemotherapy-induced toxicity and cancer survival.19,20 Current evidence suggests that the changes in lean body mass are a better measure for normalizing the doses of drugs that are distributed to and metabolized in lean tissues. Prado et al. reported that the severe depletion of skeletal muscle (sarcopenia) in breast cancer patients serves as a predictor of fluorouracil toxicity.21 Similar findings were also reported by Aslani et al.22 Actually, in the present study, the incidence of grade 3 toxicities tended to be higher in the LBL > 5 % group than the LBL < 5 % group (p = 0.050). These results might suggest that LBL relates to severe toxicities of S-1.
In our previous study, body-weight loss after surgery was an independent risk factor for the continuation of S-1 adjuvant chemotherapy; body-weight loss of 15 % was regarded as a critical cutoff point in that study. In this cohort, only one patient met this criterion, likely due to the improved postoperative management protocol. On the other hand, 21 patients could be regarded as an at-risk population, using a loss of lean body mass >5 % in the present study. Moreover, LBL was significantly related with body-weight loss in this cohort. Thus, the loss of lean body mass would be a more sensitive risk factor than the overall body-weight loss for the continuation of S-1 adjuvant chemotherapy.
There are some possible limitations associated with this study that should be kept in mind when interpreting the results. First, we investigated only the 6-month continuation of S-1 adjuvant chemotherapy. The ACTS-GC demonstrated efficacy for S-1 adjuvant chemotherapy when it was administered for 1 year after surgery; however, patients who were registered in the OPAS-1 trial after February 2012 received S-1 treatment for 6 months after surgery, according to the protocol. Therefore, we could not calculate the 1-year continuation rate of S-1 adjuvant chemotherapy in this study population. Second, this was a retrospective, non-blinded, single-center study. The present results may be observed by chance because of the small sample size in a single hospital. Multivariate analysis could not be applied in the present study because there were less than ten events among the small sample size. Moreover, we cannot rule out the possibility that our study included observer bias which physicians may overestimate S-1 toxicity and hesitate to continue S-1 chemotherapy in patients with severe loss of lean body mass. To confirm the present results, it would be necessary to conduct a multicenter, double-blinded, prospective cohort study where patients who receive S-1 and physicians who administer S-1 were blinded from LBL after surgery. In that study, physicians must strictly follow the protocol which defined S-1 administration of dose modification, delay and stop, initiation, and termination, which are the same regardless of LBL. Third, the event of recurrence may overestimate the difference of S-1 continuation by LBL. When we analyzed the subgroup after exclusion of patients who failed to continue S-1 administration due to recurrence, the hazard ratio of the LBL > 5 % group was 3.355 (95 % confidence interval 0.801–14.048), with p value of 0.078. Although the difference did not reach statistical significance, possibly due to the small number of the patients, the same trend was confirmed. Fourth, in this study, the segmental body composition was analyzed using a bioelectrical impedance analyzer, which considered not only the muscle mass but also the liver and kidney mass. The mass of visceral organs would not be changed by surgery, and the major contributor to the change in lean body mass would be muscle; the bioelectrical impedance analyzer cannot directly measure the muscle mass. On the other hand, CT or magnetic resonance imaging (MRI) have been proven to be accurate for measuring human body composition. For example, Mitsiopoulos et al. validated MRI and CT measurements of adipose tissue embedded within muscle (interstitial adipose tissue) and surrounding muscle (subcutaneous adipose tissue). They found that MRI- or CT-measured interstitial and subcutaneous adipose tissue correlated well with cadaver-measured interstitial and subcutaneous adipose tissue.11 Therefore, the results of the present study need to be validated by MRI and CT measurements.
Conclusions
The present study demonstrated that LBL might be an important risk factor for a decrease in compliance to adjuvant chemotherapy with S-1 in patients with stage II/III gastric cancer who underwent D2 gastrectomy. A multicenter, double-blinded, prospective cohort study is necessary to confirm whether the LBL would affect adjuvant S-1 continuation.
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20.
Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomized controlled trial. Lancet. 2012;379:315–21.
Bonadonna G, Valagussa P. Dose-response effect of adjuvant chemotherapy in breast cancer. N Engl J Med. 1981;304:101–5.
Aoyama T, Yoshikawa T, Shirai J, Hayashi T, Yamada T, Tsuchida K, et al. Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2013;20:2000-6.
Fein M, Fuchs KH, Thalheimer A, Freys SM, Heimbucher J, Thiede A. Long-term benefits of Roux-en-Y pouch reconstruction after total gastrectomy: a randomized trial. Ann Surg. 2008;247:759–65.
Lassen K, Soop M, Nygren J, Cox PB, Hendry PO, Spies C, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144:961–969.
Yamada T, Hayashi T, Aoyama T, Shirai J, Fujikawa H, Cho H, et al. Feasibility of enhanced recovery after surgery in gastric surgery: a retrospective study. BMC Surg. 2014;14:41.
Morgan DJ, Bray KM. Lean body mass as a predictor of drug dosage. Implications for drug therapy. Clin Pharmacokinet. 1994;26:292-307.
Roubenoff R, Kehayias JJ. The meaning and measurement of lean body mass. Nutr Rev. 1991;49:163-75.
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115-22.
Lorenzo AD, Andreoli A. Segmental bioelectrical impedance analysis. Curr Opin Clin Nutr Metab Care. 2003;6:551-5.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12.
Aoyama T, Yoshikawa T, Hayashi T, Kuwabara H, Mikayama Y, Ogata T, et al. Risk factors for 6-month continuation of S-1 adjuvant chemotherapy for gastric cancer. Gastric Cancer. 2012;16:133-9.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14: 113-23.
Yamada T, Hayashi T, Cho H, Yoshikawa T, Taniguchi H, Fukushima R, et al. Usefulness of enhanced recovery after surgery protocol as compared with conventional perioperative care in gastric surgery. Gastric Cancer. 2012;15:34–41.
Yoshikawa T, Rino Y, Yukawa N, Oshima T, Tsuburaya A, Masuda M. Neoadjuvant chemotherapy for gastric cancer in Japan: a standing position by comparing with adjuvant chemotherapy. Surg Today. 2014;44:11-21.
Kiyama T, Mizutani T, Okuda T, Fujita I, Tokunaga A, Tajiri T, et al. Postoperative changes in body composition after gastrectomy. J Gastrointest Surg. 2005;9:313-9.
Prado CM, Baracos VE, McCargar LJ, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res. 2007;13:3264-8.
Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patientswith solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629-35.
Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15:2920-6.
Aslani A, Smith RC, Allen BJ, Pavlakis N, Levi JA. The predictive value of body protein for chemotherapy induced toxicity. Cancer. 2000;88:796-803.
Acknowledgments
This work was supported in part by a non-governmental organization, the Kanagawa Standard Anti-Cancer Therapy Support System. The authors express their sincere gratitude to Mrs. Rika Takahashi for her excellent data management.
Disclosure
Toru Aoyama, Taiichi Kawabe, Hirohito Fujikawa, Tsutomu Hayashi, Takanobu Yamada, Kazuhito Tsuchida, Norio Yukawa, Takashi Oshima, Yasushi Rino, Munetaka Masuda, Takashi Ogata, Haruhiko Cho, and Takaki Yoshikawa declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Toru Aoyama and Takaki Yoshikawa have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Aoyama, T., Kawabe, T., Fujikawa, H. et al. Loss of Lean Body Mass as an Independent Risk Factor for Continuation of S-1 Adjuvant Chemotherapy for Gastric Cancer. Ann Surg Oncol 22, 2560–2566 (2015). https://doi.org/10.1245/s10434-014-4296-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-4296-z