Abstract
Background
Advanced gastric cancer in the upper or middle third of the stomach is routinely treated with a total gastrectomy, albeit in some cases with higher morbidity and mortality. The aim of this study was to describe the morbimortality and survival results in total gastrectomy in a single center.
Methods
This retrospective study included patients with gastric adenocarcinoma treated with a total gastrectomy at a single Brazilian cancer center between January 1988 and December 2011. Clinical, surgical, and pathology information were analyzed through time, with three 8-year intervals being established. Prognostic factors for survival were evaluated only among the patients treated with curative intent.
Results
The study comprised 413 individuals. Most were male and their median age was 59 years. The majority of patients had weight loss and were classified as American Society of Anesthesiologists 2. A curative resection was performed in 336 subjects and a palliative resection was performed in 77 subjects. Overall morbidity was 37.3 % and 60-day mortality was 6.5 %. Temporal analysis identified more advanced tumors in the first 8-year period along with differences in the surgical procedure, with more limited lymph node dissections. In addition, a significant decrease in mortality was observed, from 13 to 4 %. With a median follow-up of 74 months among living patients, median survival was 56 months, and 5-year overall survival was 49.2 %. Weight loss, lymphadenectomy, tumor size, and T and N stages were prognostic factors in multivariate analysis.
Conclusions
Total gastrectomy is a safe and feasible treatment in experienced hands. Advances in surgical technique and perioperative care have improved outcomes through time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Surgical treatment of gastric cancer demands an R0 resection, which can be obtained with a distal or total gastrectomy. The latter is indicated in lesions of the proximal third, middle third, or distal third where the proximal margin cannot be achieved with a distal resection.1
However, the morbidity and mortality associated with total gastrectomy has represented a limiting factor for its execution by some surgical groups. Many studies in the literature show mortality rates ranging between 0 and 15 %.2,3 In addition to complications typically associated with more complex surgical procedures, such as respiratory and urinary tract infections, in total gastrectomies dehiscence of the esophagojejunal anastomosis may occur in 1.3–15.9 % of cases and be associated with higher morbidity and mortality.4
The aim of this study was to describe the results of morbimortality and survival in total gastrectomy for adenocarcinoma at a single Western cancer center for over 20 years.
Methods
Patients
This was a retrospective study that included gastric cancer patients treated with a total gastrectomy in a single Brazilian cancer center between January 1988 and December 2011.
Exclusion criteria were proximal or distal gastrectomy, gastric stump tumors, concomitant esophagectomy and incomplete data in medical records.
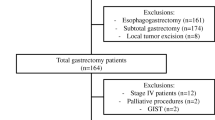
In the study period, 1,143 patients had a gastric resection. Not included in the study were 561 patients who underwent a distal gastrectomy, 56 in which gastrectomy was performed for tumors of the gastric stump, 76 in which the resection was a total esophagectomy with partial gastrectomy or total esophagogastrectomy, and 37 patients whose charts had incomplete data. Thus, the sample comprised 413 individuals who had a total gastrectomy, 336 with curative intent.
Variables
Clinical characteristics studied included age, sex, body mass index (BMI), weight loss, and American Society of Anesthesiologists (ASA) preoperative risk score.5
The extent of gastric resection and lymphadenectomy, reconstruction technique, resection of adjacent organs, operative time, blood transfusion, and intensive care unit (ICU) and hospital length of stay were the surgical variables evaluated. D1 or D2 lymphadenectomy was assigned according to medical record information, following the guidelines of the 2010 Japanese Gastric Cancer Association.1 After resection, anastomosis was performed manually, with interrupted non-absorbable sutures, or mechanically, by use of a circular stapler number 21 or 25, followed by a second non-absorbable interrupted suture. The type of postoperative nutritional support was also reported, and postoperative morbidity was described. For data clarification, we chose to stratify complications using the modified Clavien–Dindo classification.6 Postoperative mortality comprised patients who died within 60 days after surgical resection.
During the study period, patients received different regimens of multimodality treatment with chemotherapy and radiotherapy. While it is recognized that different results may have been obtained with the respective modalities, we opted to not break them down as they were not one of the research objectives of this study.
Studied histopathological variables included lesion site, Lauren’s histology type, number of dissected positive lymph nodes and TNM staging, according to the 7th edition of the American Joint Committee on Cancer (AJCC) manual.7
A historical comparison of previously mentioned characteristics and outcomes was made at an established 8-year time interval for each.
Survival
Overall survival was defined as the interval in months between the date of surgery and the date of death, for any cause, or the date of last appointment. For studying prognostic factors, only patients treated with curative intent data were analyzed.
Lost to follow-up was defined as absence in two consecutive follow-up visits, without any information regarding patient outcome after this period.
Of the 413 individuals in the study, 282 were included in the survival analysis. The other 131 were excluded due to postoperative death (27), loss to follow-up (38) or non-curative resection (66).
Statistical Analysis
The measure of the quantitative variables was expressed by the adequate measure of central tendency (mean or median) and its respective standard deviation or interquartile range. Comparison of clinicopathological characteristics was performed according to the type of variable. For qualitative data, the Student’s t test was used. To analyze the relationship between categorical variables, we used the Pearson’s Chi square test or the Fisher’s exact test in cases of frequency lower than five within a table larger than 2 × 2.
Survival analysis was undertaken using the Kaplan–Meier product-limit estimator, and the comparison of curves was obtained through the log-rank test. Variables with a p value <0.20 were selected for multiple analysis using the Cox proportional hazards model.
Results
Clinical Characteristics
In the analyzed period, 413 patients with gastric adenocarcinoma were treated with a total gastrectomy. Median age was 59 years, ranging between 20 and 89 years. Male predominance accounted for 260 cases. The median BMI of patients at the time of surgery was 23.4 kg/m2 (12.9–44.4 kg/m2). Weight loss was reported by 242 individuals, and 127 quantified it as greater than 10 %. ASA preoperative risk criteria identified a majority of subjects as ASA 2 (274) and ASA 3 (74).
Surgical Treatment
Gastrectomy was followed by a Roux-en-Y reconstruction in 408 patients. Anastomosis was mechanical in 358 individuals. The resection was characterized as curative in 336 patients and palliative in 77 patients. The median surgical time was 360 min (120–900 min), median ICU stay was 2 days (0–65 days), and median hospitalization was 11 days (2–144). Blood transfusion was required in 140 patients (33.9 %), with a median of 1 UI of red blood cells (1–5 IU).
D2 lymphadenectomy was performed on 299 patients (72.4 % of the sample) and D1 on 37 treated with curative intent. In all individuals undergoing a palliative gastrectomy, lymph node dissection was limited. Resection of adjacent organs occurred in 158 patients, with the majority undergoing splenectomy (146) or distal pancreatectomy (46).
In 322 subjects, some mode of postoperative nutritional support was adopted. The most common was the administration of enteral nutrition by nasoenteral tube (199 individuals) or jejunostomy (104). The investigation of the integrity of the anastomosis with imaging or oral administration of methylene blue was performed on 320 patients.
Some postoperative morbidity was observed in 154 individuals (37.3 %). The most common clinical complication was pneumonia in 36 cases (8.7 %), and the most common surgical complication was intracavitary abscess in 34 patients (8.2 %). Esophagojejunal anastomosis fistula was diagnosed in 18 subjects (4.4 %), the same number of cases in which a pancreatic fistula occurred. Through Clavien classification, there was a predominance of minor complications (I and II), which occurred in 85 patients. Another 42 patients had grade III and IV complications.
Postoperative mortality was 6.5 % (27 cases). The main causes of death were esphagojejunal fistula (six patients), pneumonia (five patients), enteric fistula (four patients), and intracavitary abscess from other causes (four patients).
Some form of multimodal treatment was adopted in 124 patients, with perioperative chemotherapy and adjuvant chemoradiotherapy being the most frequent.
Pathology
Intestinal-type tumors were the most frequent (203 patients), while 174 cases were diffuse-type. The most common tumor site was the stomach body (282 individuals), and in 96 subjects the lesion was in the gastroesophageal junction. The reported median size was 5.5 cm (0.5–19.0 cm).
Regarding staging, most of the patients had T4a lesions (252). Lymph node metastasis was detected in 283 individuals with a median of three positive lymph nodes, and most cases staged as N2 and N3a (81 and 84, respectively).
Temporal Comparison
Over the 24 years of the study, there was a progressive increase in the indication of total gastrectomy (Fig. 1).
The comparison between the populations and postoperative results in the three periods studied is found in Table 1.
Progressive increase in BMI and a decrease in the occurrence of weight loss were observed. It was also noted that resections during the first time period were less frequently associated with D2 lymphadenectomy, and had a lower number of dissected lymph nodes and a higher frequency of blood transfusions. The lesions were more advanced in T and N categories. Conversely, multimodal treatment methods were most often instituted in the last period.
Overall postoperative morbidity stratified by the Dindo–Clavien classification was similar between groups. Among the most common complications, pneumonia and esophagojejunal fistulas decreased through time but this result was not statistically significant; however, mortality was significantly reduced over the years (13.1 % vs. 4.1 % vs. 4.5 %; p = 0.019).
Patients in the later years were more exposed to a multidisciplinary treatment modality. Perioperative chemotherapy was administered in 43 individuals, and the other 51 had adjuvant chemoradiotherapy. In the previous 16 years, selected patients received adjuvant chemotherapy.
Survival
Median follow-up among living patients was 74 months (9–292 months), and 18 months (1–168 months) among those who died.
Median survival of individuals treated with curative resection was 56 months, and 5-year overall survival was 49.2 %. Patients receiving non-curative resection had median survival of 9 months, with 3-year survival of 6.1 %; no patients were alive at 5 years. The 5-year overall survival for different stages was 97 % for stage IA, 74.4 % for stage IB, 63 % for stage IIA, 53.2 % for stage IIB, 56.5 % for stage IIIA, 32.5 % for stage IIIB, and 15.6 % for stage IIIC.
Prognostic factors associated with worse overall survival were age ≥70 years, weight loss, ASA 3 and 4, lymphadenectomy D0/D1, resection of adjacent organs, size ≥5.0 cm, linitis plastica, T3/T4 and N2/N3 stages, and the surgical procedure having occurred in the first 8-year period (Table 2).
Multivariate analysis identified weight loss >10 %, D0/D1 lymphadenectomy, lesion size ≥5.0 cm, and T3/T4 and N2/N3 stages as independent predictors of poor prognosis (Table 3).
Discussion
This series is one of the largest cohorts of gastric cancer patients who were submitted to a total gastrectomy in a single Western center. Trends regarding subjects’ profile and treatment over time were observed. In the early years, patients had lower BMI and significant weight loss (>10 %) in about 40 % of cases. Tumors were larger and more frequently had serosa invasion. This temporal change may be related to the population’s better access to diagnostic tools, and therefore to an earlier diagnosis.
The average surgical treatment has also changed. The annual volume of procedures, despite some variations, has consistently increased in recent years. Between 2005 and 2011, in five of the seven years, more than 21 total gastrectomies were performed per year, whereas in previous years this had only occurred in 1993 and 1994. Although 21 procedures is the number used to characterize high-volume centers in gastrectomies,8 the fact that it had already been exceeded only with total resections can justify better technical standardization and, consequently, better results.
The best exemplification of this evolution is the extent of lymph node dissection. In the early years of this series a limited lymphadenectomy was performed in half of the cases, while in the latest scenario, 83 % of patients were treated with D2 lymphadenectomy. This reality follows the evolution of the controversy on the extent of lymphadenectomy in gastric cancer surgery. In the late 1990s, the results of two European randomized trials showed no survival gain with a D2 dissection.9,10 A better understanding of the factors that led to these results, particularly with the update in the Dutch trial, which showed a decrease in disease-related deaths and also in locoregional recurrence,11 coupled with a higher adherence to the recommendations of Japanese Gastric Cancer Association (JGCA),1,12 led to a D2 dissection becoming routinely performed in recent years.
The rate of complications described (37.3 %) is quite similar to a recent large population study with 1,165 individuals treated with total gastrectomies, with an overall morbidity of 36 %.13 It is higher than that observed in an Asian study that established a risk model for morbimortality in total gastrectomy attending 20,011 patients, in which overall morbidity was 26.2 %.14 However, the incidence of some important specific complications was similar. In this Japanese study, there were 6.4 % of intracavitary abscesses, 4.4 % of esophagojejunal fistulas, and 3.7 % instances of pneumonia. In our series, pneumonia occurrence was higher (8.7 %) but the incidence of abscesses and esophagojejunal fistulas (8.2 and 4.4 %, respectively) was similar. Pulmonary infections reduced through the years, which can be explained by improvements in areas such as preoperative physiotherapy treatment in high-risk patients, and better ICU care. Esophagojejunal fistulas tended to decrease with the routine use of staplers, especially in the last period.
These changes help explain the gradual decrease in postoperative mortality over the years. In the first 8-year period, it was similar to that observed in the randomized trials of D1 versus D2 lymphadenectomy (over 10 %). In the last 8 years, the mortality rate approached that of Eastern studies, i.e. around 1 %.14,15 Another factor that likely contributed to this difference was the higher number of extended resections, such as pancreatectomy and splenectomy, which was much more frequent in the early years and has been associated with increased postoperative morbidity 16 and poor survival in the literature. 17–19 In a recent study assessing morbidity and mortality in total and subtotal gastrectomy, mortality after total gastrectomy was 5.4 % but this number was significantly higher among patients undergoing pancreatectomy, splenectomy, and associated colectomy (11.9, 9.9, and 12.3 %, respectively).20 Lastly, postoperative mortality was also improved because patients in the later years more frequently had tumors that were not so advanced, had less weight loss, and were in better nutritional and general conditions.
The survival results observed in our study were slightly lower than in another study with a similar case selection, which was conducted in Italy and showed 5-year survival of patients treated with curative intent of 61.8 %.21 Some factors may explain this difference, such as the greater amount of extended resections (38 vs. 18 %) and D1 lymphadenectomy in our series, in which more limited lymph node dissection was a prognostic factor for poor survival. Regarding survival in patients undergoing non-curative resection, the results were similar to most studies that contained data specific to total gastrectomies 22 and lower than those observed in some series with exclusive chemotherapy as treatment for metastatic patients.23
In the analysis of prognostic factors for survival, besides TNM staging and lymphadenectomy, weight loss greater than 10 % and lesion size were significant in multivariate analysis. The first was related to morbidity and postoperative mortality in a recent study 20 and poor survival in another publication,24 whereas tumor size seems to be the most important prognostic factor in patients whose lesions have serous infiltration.25,26
Several limitations can be characterized in our study. Its retrospective design and data collection from medical records prevents a more accurate analysis of postoperative morbidity, although the numbers reported are consistent with other studies in the literature. The loss to follow-up of almost 10 % of patients also represents a high number and that may have influenced the survival results. However, we believe that the evaluation of treatment over time has allowed the identification of factors that have contributed to the improvement of postoperative, intra-hospital, and late evolution.
Conclusions
Total gastrectomy is a safe and feasible treatment in experienced centers. Advances in surgical technique and perioperative care have allowed the progressive decline of morbidity and mortality, with increasingly higher survival rates, which match those observed in centers where high-quality standard surgical treatment is performed.
References
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113–23.
McCullouch P, Ward J, Tekkis PP, ASCOT Group of Surgeons, British Oesophago-Gastric Cancer Group. Mortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort study. BMJ. 2003;327:1192–7.
Sano T, Sasako M, Yamamoto S, et al. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy. Japan Clinical Oncology Group study 9501. J Clin Oncol. 2005;22:2767–73.
Meyer L, Meyer F, Dralle H, et al. Insufficiency risk or esophagojejunal anastomosis after total abdominal gastrectomy for gastric carcinoma. Langenbecks Arch Surg. 2005;390:510–6.
American Society of Anesthesiologists. ASA physical status classification system. Available from: http://www.asahq.org/. Accessed 30 Aug 2014.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications. Ann Surg. 2004;240:205–13.
Sobin LH, Gospodarowicz MK, Wittekind C. International Union Against Cancer (UICC) TNM classification of malignant tumours. 7th edition. New York: Wiley-Liss; 2010, pp. 73–76.
Altini M, Carretta E, Morgagni P, et al. Is a clear benefit in survival enough to modify patient access to the surgery service? A retrospective analysis in a cohort of gastric cancer patients. Gastric Cancer. 2014. doi: 10.1007/s10120-014-0346-2.
Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Br J Cancer. 1999;79:1522–30.
Bonnenkamp JJ, Hermans J, Sasako M, et al; Dutch Gastric Cancer Group. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908–14.
Songun I, Putter H, Kranenbarg EMK, Sasako M, van de Velde CJH. Surgical treatment of gastric cancer: 15-year follow-up results of the randomized nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–49.
Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1–5.
Bartlett EK, Roses RE, Ketz RR, Drebin JA, Fraker DL, Karakousis GC. Morbidity and mortality after total gastrectomy for gastric malignancy using the American College of Surgeons National Surgical Quality Improvement Program database. Surgery. 2014;156:298–304.
Watanabe M, Miyata H, Gotoh M, et al. Total gastrectomy risk model: data from 20,011 Japanese patients in a nationwide internet-based database. Ann Surg. 2014;00:1–6.
Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–62.
Weitz J, Jaques DP, Brennan M, Karpeh M. Association of splenectomy with postoperative complications in patients with proximal gastric and gastroesophageal junction cancer. Ann Surg Oncol. 2004;11:682–9.
Martin RC 2nd, Jaques DP, Brennan MF, Karpeh M. Extended local resection for advanced gastric cancer: increased survival versus increased morbidity. Ann Surg. 2002;236:159–65.
Hartgrink H, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477–90.
Coimbra FJF, Costa WL Jr, Montagnini AL. The interaction between N-category and N-ratio as a new tool to improve lymph node metastasis staging in gastric cancer: results of a single cancer center in Brazil. Eur J Surg Oncol. 2011;37:47–54.
Papenfuss W, Kukar M, Oxenberg DO, et al. Morbidity and mortality associated with gastrectomy for gastric cancer. Ann Surg Oncol. 2014;21:3008–14.
Pacelli F, Papa V, Rosa F, et al. Four hundred consecutive total gastrectomies for gastric cancer: a single-institution experience. Arch Surg. 2008;143:769–75.
Mahar AL, Coburn NG, Singh S, Law C, Helyer LK. A systematic review of surgery for non-curative gastric cancer. Gastric Cancer. 2012;15 Suppl 1:25–37.
Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46.
Maconi G, Manes G, Porro GB. Role of symptons in diagnosis and outcome of gastric cancer. World J Gastroenterol. 2008;14:1149–55.
Quan J, Zhang R, Liang H, et al. The impact of tumor size on survival of patients with pT4aN0M0 gastric cancer. Am Surg. 2013;79:328–31.
Bilci A, Uygun K, Seker M, et al. The effect of tumor size on overall survival in patients with T3 gastric cancer: experience from 3 centers. Onkologie. 2010;33:676–82.
Disclosures
Wilson L. Costa Jr, Felipe J.F. Coimbra, Héber S.C. Ribeiro, Alessandro L. Diniz, André Luís de Godoy, Igor Correia de Farias, Maria Dirlei F.S. Begnami, and Fernando Augusto Soares report no disclosures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Costa, W.L., Coimbra, F.J.F., Ribeiro, H.S.C. et al. Total Gastrectomy for Gastric Cancer: An Analysis of Postoperative and Long-Term Outcomes Through Time. Ann Surg Oncol 22, 750–757 (2015). https://doi.org/10.1245/s10434-014-4212-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-4212-6