Abstract
Fluvastatin (FLV) is known to inhibit the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA), which is over-expressed in various cancers. FLV has been reported to decrease cancer development and metastasis. However, because of low bioavailability, extensive first-pass metabolism and short half-life of FLV (1.2 h), it is not appropriate for clinical application. Therefore, FLV-loaded emulsomes were formulated and optimized using Box–Behnken experimental design to achieve higher efficiency of formulation. Antitumor activity of optimized FLV-loaded emulsomes was evaluated in prostate cancer cells using cell cytotoxicity, apoptotic activity, cell cycle analysis, and enzyme-linked immunosorbent assay. The FLV-loaded emulsomes exhibited a monodispersed size distribution with a mean particle size less than 100 nm as measured by zetasizer. The entrapment efficiency was found to be 93.74% with controlled drug release profile. FLV-EMLs showed a significant inhibitory effect on the viability of PC3 cells when compared to the free FLV (P < 0.0025). Furthermore, FLV-EMLs showed significant arrest in G2/M and increase in percentage of apoptotic cells as compared to free FLV. FLV-EMLs were more effective than free FLV in reducing mitochondrial membrane potential and increase in caspase-3 activity. These results suggesting that FLV-EMLs caused cell cycle arrest which clarifies its significant antiproliferative effect compared to the free drug. Therefore, optimized FLV-EMLs may be an effective carrier for FLV in prostate cancer treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is reported to be the second most diagnosed cancer which leads to critical health problems in men [1, 2]. Promising chemopreventive agents for prostate cancer that include vitamin E, selenium, nonsteroidal anti-inflammatory, and statins were investigated [3, 4]. It is vital to use a novel delivery system such as vesicular delivery system and design a formulation which can potentially lower the cost, dose, dosing frequency, and improve patient during drug administration [5,6,7,8]. The vesicular delivery system is used to enhance the bioavailability of drug [5, 9,10,11]. This approach is also used for acid-labile drugs susceptible to the gastric environment as well as for drugs prone to gastric enzyme degradation. Such delivery systems include liposomes, niosomes, ethosomes, pharmacosomes, proniosomes, and emulsomes (EMLs) [12,13,14,15,16]. Modification of liposomes have shown improved performance and efficacy of the loaded drug [5, 17]. Although many vesicular delivery systems have shown application in delivering poorly soluble drugs [11, 18], EMLs gained much attention since they overcome some additional limitations generally related to these systems [19,20,21,22].
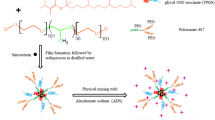
Structurally, EMLs are lipoidal vesicular delivery systems containing a solid fat core internal structure covered by a phospholipid bilayer [19]. This vesicular delivery system enclosed poorly water-soluble drugs inside the solid fat core [23,24,25]. EMLs are suitable vehicles for facilitating prolonged active molecule release, and simultaneously reducing dosing frequency and cost [19]. Further, EMLs are formulated in the nano-size range, and thus making EMLs suitable vehicles for parenteral drug delivery, and since the drug is entrapped, it provides a longer half-life in the systemic circulation [21]. Other advantages attributed to EMLs are higher loading of hydrophobic drugs, as well as relatively higher stability compared to conventional liposomal delivery systems [19, 26]. Since EMLs are solid at room temperature, they can be conveniently formulated in different dosage forms [20]. This versatility and the aforementioned benefits make EMLs especially promising delivery vehicles with the potential of overcoming the hurdles facing drug transport [21, 22, 27].
A major advantage of using EMLs is their potential application in targeting specific tissues and organs, thereby reducing off-target drug effects and lowering effective dose requirements [21, 28, 29]. For example, specific localization of active drug molecules can be achieved by opsonin, a circulating serum factor that uptakes EML vesicles. Localization through this system can be used to treat tumors and other infectious diseases with minimal side effects [24, 28]. For instance, compared to unentrapped conditions, methotrexate in EML vesicles demonstrated a relatively lower rate of tumor proliferation as well as a higher plasma level accompanied by a slower rate of elimination [30]. Thus, altering the pharmacokinetics of the drug using EML vesicles can have an added advantage of lowering the side effects of antineoplastic drugs [28, 30].
Some promising active pharmaceutical ingredients (API) require EML delivery systems to counter challenges as the poor solubility, high rates of first-pass metabolism, and off-target toxicity [19, 25]. Taking advantage of targeted drug delivery using EMLs, active class of molecules such as statins can be directed to treat cancer cells, with studies showing statin-induced antitumor activities, triggering of apoptosis, and cell growth arrest [31,32,33,34,35,36]. Statins, such as fluvastatin (FLV), are known to inhibit HMG-CoA reductase and are commonly used for the treatment of hypercholesterolemia [37]. After oral administration, FLV has poor bioavailability (24–29%) and it undergoes extensive first-pass metabolism with a biological half-life of 1.2 h [38]. These characteristics coupled with its poor solubility in water made delivery by EMLs an ideal choice.
The study aims to develop nano sized FLV EML formulation with a higher entrapment efficiency to provide controlled and sustained release of the poorly soluble active drug molecule applying design of experiment methodology. The effects of FLV, phosphatidylcholine (PC) concentration, and pH of the hydration medium on FLV-EML size and entrapped FLV efficiency were assessed. Furthermore, the anticancer activities including cell viability, cell cycle, apoptotic activity, mitochondrial membrane potential, and caspase-3 of the FLV EMLs and of free FLV were investigated in PC3 prostate cancer cells.
Materials and Methods
Materials
Fluvastatin was received as a gift sample from EIPICO Pharmaceutical Industries Company (EIPICO, Cairo, Egypt). MitoProbeTM TMRM Kit was from (Thermo Fisher Scientific, CA, USA). Caspase-3 and Annexin V-FITC Apoptosis Kit were from (BD Bioscience, San Jose, CA, USA). Phospholipon®90 H was procured from Lipoid GmbH (Frigenstr, Germany).
Formulation and Optimization of FLV-EMLs
Experimental Box-Behnken design was implemented for the formulation of FLV-EMLs using Design-Expert software (Version 12; Stat-Ease Inc., Minneapolis, MN, USA). Concentration of FLV (FLV %w/w), phosphatidylcholine (PC %w/w), and pH of the hydration medium were examined as the three independent variables. FLV-EML size and FLV entrapment efficiency were examined as the dependent responses. The software produced 17 runs and the levels of variables for the experimental runs and their observed responses were presented in Tables I and II. Model fit analysis was accomplished to determine the response best fitting model. Analysis of variance (ANOVA) was then applied for statistical analysis of the responses data at 95% level of significance. To demonstrate the interaction between the explored variables, two-dimensional contour and three dimensional surface plots were plotted. Numerical optimization following desirability approach was employed for predicting the variable levels for the optimal EML formulation. The optimization aim was to minimize FLV-EML size and maximize FLV entrapment. The predicted optimized formulation was prepared and further characterized.
FLV-EMLs were prepared as previously reported [21, 39]. Specified amounts of tripalmitin (2% w/v), PC, FLV, and cholesterol (4% w/v) were dissolved in chloroform:methanol (2:1, v/v). The dried film was developed after evaporation of the organic solution. After that, film hydration with buffer, at the specified pH, was carried out and then ultra-sonicated for further characterization studies.
FLV-EMLs In Vitro Release
FLV-EMLs and free FLV release were performed using cellulose tube diffusion technique. In due course of this experiment, cellulose tube was soaked in the release media overnight before starting the experiment. The cellulose tube was filled with an accurately weighed amount of the FLV-EMLs equivalent to 100 mg FLV. The 900 mL of PBS (pH 7.4) were used as a dissolution media at 37 ± 0.5°C in which cellulose tube containing EMLs was immersed and rotated in the medium at 100 rpm. A 2 mL of released media were collected at 0.5, 1, 2, 4, 6, 8, 12, and 24 h, followed by replacement with an equivalent volume of media. Samples were collected and filtered prior to analysis using HPLC method. These analyses were performed in triplicates, and the mean was considered for further evaluation. The release data were applied into various kinetic models that include zero order, first order, Higuchi release, and Peppas model to interpret kinetics of drug release. The in vitro release model equations are given in Table III.
Cell Viability
To find out the cytotoxicity of FLV-EMLs and free FLV in PC3 cells, MTT assay was performed. PC3 cells were grown in RPMI-1640 medium containing glutamine (1%), penicillin-streptomycin, and 10% FBS at 37 °C in a humidified 5% CO2 incubator. About 2 × 105cells/well were seeded into each 96-well plate. The cells that adhere overnight in wells were applied with free FLV and FLV-loaded EMLs at concentrations of 100, 25, 6.25, 1.56, and 0.39 μg/mL, respectively. The culture medium was removed, and MTT solution was added and incubated for 4h at 37°C. The plates were later removed, and 100μL/well of dimethyl sulfoxide (DMSO) was added, and colorimetric measurements were made using a microplate reader.
Cell Cycle Assessment
Flow cytometry was executed using Annexin V-FITC Reagent to study the effect of FLV-EMLs and FLV on the PC3 cells seeded in 6-well plates. Cell’s density was 3 × 105 cells/well incubated for 24 h. After that, treatments were incubated with cells for further 48 h. Cells were then collected, washed with PBS, and stained for characterization of cell-cycle phase distribution using flow cytometry.
Annexin V–FITC Apoptosis Assay
The apoptotic effects of FLV-EMLs and FLV on PC3 cells were assayed based on manufacturer’s protocol. Briefly, PC3 cells 3 × 105 cells/well (6-well plates) were incubated for 24 h then treated with different formulations and incubated for 48 h. PC3 cells were centrifuged, separated, washed with buffer (PBS), and finally resuspended in 1× binding buffer (500 μL). Annexin V-FITC and propidium iodide stained the cells that were analyzed by flow cytometry.
Mitochondrial Membrane Potential
The mitochondrial membrane potential (MMP) of PC3 cells was calculated according to the manufacturer’s instructions using MitoProbeTM TMRM Assay Kit. Briefly, PC3 cells 5 × 104 cells/well (96-well plates) were treated, after 24-h incubation, with different formulations and kept for 48 h. The medium was eradicated, and cells were incubated in darkness for 30 min at 37 °C with 20 μM tetramethylrhodamine, methyl ester (TMRM). Finally, the solution was substituted and analyzed by flow cytometry with a live cell imaging buffer (FACScalibur, BD Bioscience, USA).
Caspase-3 Assay
The activity of caspase-3 was evaluated using colorimetric kit as per the protocol provided by the manufacturer. Briefly, PC3 cells 5 × 104 cells/well (96-well plates) were treated, after 24-h incubation, with different formulations and incubated for 48 h. Cells were washed, lysed in lysis buffer, and centrifuged at 10,000 g for 1 min. The supernatant was eventually incubated with a caspase-3 substrate and measured using a microplate reader.
Statistical Analysis
Graphpad Prism software, non-parametric one-way analysis of variance (ANOVA) with Tukey’s post hoc was used to analyze the results. Each experiment was independently carried out in triplicates. A substantial difference was found to be P < 0.05. The findings are expressed in terms of means ± SD.
Results and Discussion
FLV-EML Experimental Design
Sequential Model Selection and Diagnostic Analysis
FLV-EML size and efficiency of FLV entrapped were fitted to the two-factor interaction (2FI) model (Table IV). According to the greatest multiple correlation coefficient (R2) and the smallest predicted residual error sum of squares (PRESS), best fitting model was chosen. The logical agreement between the predicted and adjusted R2 for each response is considered another indication for the model validity. In addition, satisfactory signal-to-noise ratio was expressed by accuracy >4 implying that the chosen model is satisfactory for the experimental design space navigation [40].
Diagnostic plots were developed to estimate the consistency of the 2FI model’s fit and guarantee its credibility for both responses (Fig. 1). Randomly and reliably distributed points were shown by externally studentized residuals versus run plots, suggesting that both responses were not affected by any lurking variable. Furthermore, the reasonable linearity of the observed versus predicted values of the plots of both responses shows that the responses observed correspond well with the predicted ones [41].
Statistical Analysis Particle Size (Y1) Data
Particle size for nano-sized drug delivery systems is a key factor that could significantly affects the drug release and consequently its permeation across the biological barriers. FLV-EMLs exhibited average size ranging from 83.64 ± 1.89 to 212 ± 6.91 nm (Table II). ANOVA for FLV-EML size affirmed the quadratic model significance as evidenced by the F-value of 17.59 computed in the ANOVA analysis for the particle size ensures that the selected 2FI model is significant (P < 0.0001). The chance that an F-value could be attributed to noise is 0.01%. Further, the lack of fit F-value of 3.16 (P = 0.1422) indicates non significance, i.e., the data fit to the selected model. The sequential 2FI model (Eq. 1) was generated.
ANOVA disclosed that the linear terms (X1 and X2) referring to both the drug and PC concentrations play a significant role in controlling the particle size (P = 0.0013 and 0.0008, respectively). Also, the interaction term X2X3 referring to the interaction between PC concentration and hydration medium pH was highly significant on the particle size at the same level (P < 0.0001). The contour and response surface plots (Fig. 2) illustrate the effects of the studied variables on size of FLV-EMLs. The size of FLV-EMLs was significantly increased with the increase in PC concentration. This observation is asserted by X2 positive coefficient in the coded equation. The increase in the size of FLV-EMLs with the PC concentration increase is possibly related to increased number of the multiple bilayers formed during EML preparation [22, 42]. On the contrary, the particle size was decreased significantly at higher drug concentrations (Fig. 2). This is related possibly to the effect of increased FLV concentration that possibly favors the formation of uni/oligo-lamellar vesicles (characterized by small particle size) and reduces the number of multiple bilayer vesicles (large particle size) formed during EML preparation.
Statistical Analysis for Entrapment Efficiency (Y2) Data
FLV-EMLs showed adequate entrapment efficiency ranging from 77.98 ± 1.78 to 95.34 ± 3.90. Data fitting to the 2FI model was ensured by the computed F-value of the model (F = 17.74, P < 0.0001). Data fitting to the model was also affirmed by the non-significant lack of fit shown by F-value of 2.07 (P = 0.2510). The sequential 2FI (Eq. 2) was generated.
ANOVA results declared the significance of the linear terms X2 and X3 corresponding to PC concentration and hydration medium pH on the entrapment efficiency (P = 0.0003, 0.0131, respectively). All the binary interaction terms (X1X2, X1X3, and X2X3) were significant at the same level (P = 0.0085, < 0.0001, and 0.0356, respectively). The response surface and contour plots for the assessed variable effects on FLV entrapment is shown in Fig. 3. The results revealed that FLV entrapment decreases with the increase in PC concentration that is related to the possibility, discussed earlier in particle size part, that increased FLV concentration could favor the formation of uni/oligo-lamellar vesicles (characterized by small particle size). On the other hand, FLV entrapment increased with the increase in pH of hydration medium. Our postulation for this result could be related to the increase in pH of the medium leads to increase in the negative charges on PC that leads to possibility of the alignment of polar groups (-ve) for FLV with polar head of PC. In addition, the hydrophobic portion of the FLV molecule aligns with the hydrophobic (fatty acid chain) of PC molecule. Accordingly, the increased entrapment is related to FLV molecule alignment within the bilayer rather than the charge on the molecule. This also could confirm the previous particle size section finding of increased concentration of FLV leads to decrease in particle size.
Optimization of FLV-EMLs
The optimum levels of the variables were predicted by applying responses constraints. The computed desirability was 0.985. The prepared optimized formulation was subjected to characterization. No major residual error was found indicating the validity of numerical optimization for this study. The optimized formulation levels are demonstrated in Table V. The results indicated that the optimized formula shows EE 93.74%. These results indicated that the optimized FLV-EMLs shows FLV drug loading 4.87%.
In Vitro Release Study of FLV-EMLs
Representation of release profile of FLV in PBS from FLV and FLV-EMLs is made in Fig. 4. From the data, it is evident that the release profile and extent of FLV release was enhanced from the delivery system of FLV. The release profile of FLV from the EMLs showed an initial burst release, followed by gradual release until 99.3 ± 5.2% within the time frame of 48 h, whereas the release of FLV (83.3 ± 2.3%) after 48 h from the FLV-raw. The ability of EMLs to efficiently solubilize FLV is attributed to the lipophilic nature of its components. The EML capability to enhance the solubility of other poorly water-soluble drugs has been previously reported [27]. In addition, the lipophilic nature and the structure of EMLs that mimics the cell membrane nature augments the ability of the nanocarrier to deliver FLV through the cellular barriers compared with raw FLV [21, 24]. The model that best fit the release data was selected on the basis of correlation coefficient (R2). As evidenced by the higher R2 value (0.992), the release of FLV from emulsomes followed a first-order kinetic model.
Cell Viability
Statin’s nano-formulations has been reported to inhibit several cancer cell growths such as breast MCF-7, colorectal HCT-116, and prostate PC3 cancer cells [33, 35, 36, 43,44,45]. For example, FLV combined with alpha lipoic acid and ellagic acid in a nanostructured lipid carrier (NLC) formula exhibited significant cytotoxic effect against PC3 cells when compared to the free drug [4]. Herein, the antiproliferative effects of the FLV-EMLs and FLV were examined on PC3 cells using MTT assay. After incubation at 48-h time period, a significant cytotoxic effect was observed at the highest tested concentration of FLV (P < 0.05) (Fig. 5). This anti-proliferative effect of FLV is attributed to HMG-CoA reductase inhibition in the biosynthesis of cholesterol. The depletion in mevalonate augments basic cell pathways including cell survival and proliferation [33,34,35,36]. Finally, at the same concentration, FLV-EMLs caused a high significant inhibitory effect on the viability of PC3 cells when compared to the free drug FLV (P < 0.0025) (Fig. 5). The FVL-EMLs have shown better cytotoxicity due to better uptake and internalization of drug overcoming the cellular barriers. However, Placebo-E caused a non-significant cytotoxic effect suggesting the safe and successful use of this system for the delivery of FLV to PC3 cancer cells. The improved data of the encapsulated drug when compared to the free drug is attributed to the efficiency of the nano-formula (EMLs) for drug internalization and overcoming the cellular barriers when compared with the free FLV [46, 47].
Cell viability of FLV-EMLs, FLV-raw or placebo-E on PC3 cells after incubation at 48-h time period using MTT assay. Results are expressed as a percentage of the studied group compared to the control. The data are expressed as the mean ± SE of three independent experiments. *P < 0.05 significantly different from the control; #P < 0.0001 significantly different from the control; $P < 0.0025 significantly different from FLV
Cell Cycle Analysis
In order to understand how FLV-EMLs are able to inhibit PC3 cell proliferation, cell cycle analysis was examined. As shown in Fig. 6, control PC3 cells exhibited proliferative profile with about 50% at G0/G1 phase and 6.8% at S phase and 2.9% at pre-G1 phase. FLV treatment boosted the G1 phase number of cells to 55.8% and decreased S phase population to 3.9%, providing evidence of G1 arrest. A similar pattern of cellular DNA disruption was reported previously with FLV treatment in PC3 cells [4]. FLV-EMLs caused a significant upsurge in G1 phase when compared with the control (P < 0.05). As compared to control and FLV, this was also correlated with a concomitant substantial reduction in the S phase cells (31.4%) and pre-G1 (G0) phase cell aggregation (5.7%) for FLV-EMLs (Fig. 6). Furthermore, a significant arrest in G2/M was followed suggesting that FLV-EMLs caused cell cycle perturbation which clarifies its significant antiproliferative effect compared to the free drug. Similar findings were observed by others [32, 48].
Distribution of phases of the cell cycle in a control, b placebo-E, c FLV, and d FLV-EMLs after 48-h treatment in PC3 cells. The data are expressed as the mean ± SE of three independent experiments. *P < 0.05 significantly different from the control; #P < 0.05 significantly different from placebo-E; $P < 0.05 significantly different from FLV
Annexin V–FITC Apoptosis Assay
To investigate if cytotoxicity of FLV-EMLs was secondary to apoptotic or necrotic cell death, Annexin V-FITC Apoptosis Assay was performed. As shown in Fig. 7, FLV-EML-treated cells labeled with both FITC and PI showed a significant increase in FITC-positive cells % (early apoptosis), PI positive cells (necrotic cells), and total apoptotic/necrotic cells compared to control, FLV, and placebo-E. This mechanism of cell death might be attributed to the cell cycle profile as apoptosis would be more related in arrested cells in the G1 phase, while necrosis would be more related in cells arrested in the G2/M phase [32, 49]. This particularly coincides with the present data which showed that FLV-EML-treated PC3 cells were arrested in the G1 and the G2/M phases, thus explaining the cell death mechanisms.
Analysis of apoptosis and necrosis in a control, b placebo-E, c FLV, and d FLV-EMLs after 48-h treatment in PC3 cells. The data are expressed as the mean ± SE of three independent experiments. *P < 0.05 significantly different from the control; #P < 0.05 significantly different from placebo-E; $P < 0.05 significantly different from FLV
Mitochondrial Membrane Potential
Mitochondrial damage has been associated with loss of membrane potential, increase membrane permeability transition, and the release of apoptotic factors. In order to determine if FLV-EML-induced apoptotic effect was due mitochondrial damage, MMP was evaluated. As shown in Fig. 8, FLV did not alter the mitochondrial membrane as compared to control and placebo-E. However, PC3-treated cells with FLV-EMLs had a significant decrease in MMP compared to control, FLV, and placebo-E suggesting that FLV-EMLs induced apoptosis via MMP depletion. Similar observation was also found by another researcher [49, 50].
Changes in MMP of PC3 cells after 48-h incubation with FLV-EMLs, FLV, or placebo-E in PC3 cells. The data are expressed as the mean ± SE of three independent experiments. *P < 0.05 significantly different from the control; #P < 0.05 significantly different from placebo-E; $P < 0.05 significantly different from FLV
Caspase-3 Assay
Activation of cysteine proteases, caspases, plays an essential role in apoptosis signaling pathways. FLV as well as over statins has been shown to activate caspase-3 enzyme in PC3 cells. As presented in Fig. 9, FLV increased caspase-3 by about 2-fold compared with the control. However, FLV-EMLs induced caspase-3 activation by about by 9 and 5-fold compared with the control and FLV, respectively (Fig. 9). These result are accord with data presented by other researchers [50,51,52].
Measurement of caspase-3 activation after 48-h incubation with FLV-EMLs, FLV-raw, or placebo-E in PC3 cells. The data are expressed as the mean ± SE of three independent experiments. *P < 0.05 significantly different from the control; #P < 0.0001 significantly different from the control; $P < 0.0001 significantly different from FLV
Conclusions
The optimized EML formulation, using Box-Behnken design, showed satisfactory FLV-EML size, high FLV entrapment with sustain in vitro FLV release. In vitro experiments established that the FLV-EMLs provided an appropriate system for delivery of FLV to PC3 prostate cancer cells. It improved the cellular cytotoxicity due the disturbance in cell cycle phases, activation of caspase-3, and loss of MMP, all confirmed incidence of apoptosis. Eventually, FLV-EMLS may provide a promising design for targeting PC3 cells in prostate cancer therapy.
Change history
02 June 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1208/s12249-023-02591-y
References
Hsing AW, Chokkalingam AP. Epidemiology of prostate cancer. Prostate Cancer Basic Mech Ther Approaches. Elmer Press. 2005:315–63.
Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist Alphamed Press. 2007;12:20–37.
Bosland MC. Is there a future for chemoprevention of prostate cancer? Cancer Prev. Res. American Association for Cancer Research Inc.; 2016. p. 642–7.
Fahmy UA. Augmentation of fluvastatin cytotoxicity against prostate carcinoma PC3 cell line utilizing alpha lipoic–ellagic acid nanostructured lipid carrier formula. AAPS PharmSciTech. 2018;19:3454–61.
Mahmoudi R, Ashraf Mirahmadi-Babaheidri S, Delaviz H, Fouani MH, Alipour M, Jafari Barmak M, et al. RGD peptide-mediated liposomal curcumin targeted delivery to breast cancer cells. J Biomater Appl SAGE Publications Ltd. 2021;35:743–53.
Mahmoudi R, Hassandokht F, Ardakani MT, Karimi B, Roustazadeh A, Tarvirdipour S, et al. Intercalation of curcumin into liposomal chemotherapeutic agent augments apoptosis in breast cancer cells. J Biomater Appl SAGE Publications Ltd. 2020;35.
Torchilin VP, Levchenko TS. TAT-liposomes: a novel intracellular drug carrier. Curr Protein Pept Sci. 2003;4:133–40.
Quagliariello V, Masarone M, Armenia E, Giudice A, Barbarisi M, Caraglia M, et al. Chitosan-coated liposomes loaded with butyric acid demonstrate anticancer and anti-inflammatory activity in human hepatoma HepG2 cells. Oncol Rep. 2018;41:1476–86.
Khan MI, Madni A, Ahmad S, Mahmood MA, Rehman M, Ashfaq M. Formulation design and characterization of a non-ionic surfactant based vesicular system for the sustained delivery of a new chondroprotective agent. Brazilian J Pharm Sci Faculdade de Ciencias Farmaceuticas (Biblioteca). 2015;51:607–16.
Kaur IP, Rana C, Singh M, Bhushan S, Singh H, Kakkar S. Development and evaluation of novel surfactant-based elastic vesicular system for ocular delivery of fluconazole. J Ocul Pharmacol Ther. 2012;28:484–96.
Nasr M, Mansour S, Mortada ND, Elshamy AA. Vesicular aceclofenac systems: a comparative study between liposomes and niosomes. J Microencapsul Informa UK Ltd UK. 2008;25:499–512.
Jain S, Umamaheshwari RB, Bhadra D, Jain NK. Ethosomes: a novel vesicular carrier for enhanced transdermal delivery of an antiHIV agent. Indian J Pharm Sci Medknow Publications. 2004;66:72–81.
Rajan R, Jose S, Mukund V. Transferosomes—a vesicular transdermal delivery system for enhanced drug permeation. J Adv Pharm Technol Res. 2011;2:138–43.
Lakshmi PK, Kalpana B, Prasanthi D. Invasomes-novel vesicular carriers for enhanced skin permeation. Syst Rev Pharm. 2013:26–30.
Song CK, Balakrishnan P, Shim CK, Chung SJ, Chong S, Kim DD. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: characterization and in vitro/in vivo evaluation. Colloids Surf B: Biointerfaces. Elsevier B.V. 2012;92:299–304.
Azeez NA, Deepa VS, Sivapriya V. Phytosomes: emergent promising nano vesicular drug delivery system for targeted tumor therapy. Adv. Nat. Sci. Nanosci. Nanotechnol. IOP Publishing; 2018. 33001.
Bardania H, Shojaosadati SA, Kobarfard F, Morshedi D, Aliakbari F, Tahoori MT, et al. RGD-modified nano-liposomes encapsulated eptifibatide with proper hemocompatibility and cytotoxicity effect. Iran J Biotechnol. National Institute of Genetic Engineering and Biotechnology. 2019;17:8–13.
El-Nabarawi M, Bendas E, El Rehem R, MY A. Transdermal drug delivery of paroxetine through lipid-vesicular formulation to augment its bioavailability. Int J Pharm. 2013;443:307–17.
Amselem S, Yogev A, Zawoznik E, Friedman D. Emulsomes, a novel drug delivery technology. Proc Control Release Soc. 1994. p. 668–9.
Ucisik M, Sleytr U, Schuster B. Emulsomes meet S-layer proteins: an emerging targeted drug delivery system. Curr Pharm Biotechnol. Bentham Science Publishers. 2015;16:392–405.
Vyas SP, Subhedar R, Jain S. Development and characterization of emulsomes for sustained and targeted delivery of an antiviral agent to liver. J Pharm Pharmacol. 2006;58:321–6.
Alhakamy NA, Badr-Eldin SM, Ahmed OAA, Asfour HZ, Aldawsari HM, Algandaby MM, et al. Piceatannol-loaded emulsomes exhibit enhanced cytostatic and apoptotic activities in colon cancer cells. Antioxidants. MDPI AG. 2020;9:419.
Awan ZA, Fahmy UA, Badr-eldin SM, Ibrahim TS, Asfour HZ, Al-rabia MW, et al. The enhanced cytotoxic and pro-apoptotic effects of optimized simvastatin-loaded emulsomes on MCF-7 breast cancer cells. Pharmaceutics. 2020;12:1–22.
Bolat ZB, Islek Z, Demir BN, Yilmaz EN, Sahin F, Ucisik MH. Curcumin- and piperine-loaded emulsomes as combinational treatment approach enhance the anticancer activity of curcumin on HCT116 colorectal cancer model. Front Bioeng Biotechnol. Frontiers Media S.A. 2020;8:50.
Raza K, Katare OP, Setia A, Bhatia A, Singh B. Improved therapeutic performance of dithranol against psoriasis employing systematically optimized nanoemulsomes. J Microencapsul. 2013;30:225–36.
Pal A, Gupta S, Jaiswal A, Dube A, Vyas SP. Development and evaluation of tripalmitin emulsomes for the treatment of experimental visceral leishmaniasis. J Liposome Res. 2012;22:62–71.
Ucisik MH, Küpcü S, Schuster B, Sleytr UB. Characterization of CurcuEmulsomes: Nanoformulation for enhanced solubility and delivery of curcumin. J Nanobiotechnology. 2013;11:37.
Varshosaz J, Raghami F, Rostami M, Jahanian A. PEGylated trimethylchitosan emulsomes conjugated to octreotide for targeted delivery of sorafenib to hepatocellular carcinoma cells of HepG2. J Liposome Res Taylor & Francis. 2019;29:383–98.
Ucisik MH, Küpcü S, Breitwieser A, Gelbmann N, Schuster B, Sleytr UB. S-layer fusion protein as a tool functionalizing emulsomes and CurcuEmulsomes for antibody binding and targeting. Colloids Surf B: Biointerfaces. 2015;128:132–9.
Paliwal R, Paliwal SR, Mishra N, Mehta A, Vyas SP. Engineered chylomicron mimicking carrier emulsome for lymph targeted oral delivery of methotrexate. Int J Pharm. 2009;380:181–8.
Zhong GC, Liu Y, Ye YY, Hao FB, Wang K, Gong JP. Meta-analysis of studies using statins as a reducer for primary liver cancer risk. Sci Rep. 2016;6:26256.
Zhang W, Wu J, Zhou L, Xie H-Y, Zheng S-S. Fluvastatin, a lipophilic statin, induces apoptosis in human hepatocellular carcinoma cells through mitochondria-operated pathway. Indian J Exp Biol. 2010;48:1167–74.
Ishikawa S, Hayashi H, Kinoshita K, Abe M, Kuroki H, Tokunaga R, et al. Statins inhibit tumor progression via an enhancer of zeste homolog 2-mediated epigenetic alteration in colorectal cancer. Int J Cancer. 2014;135:2528–36.
Higashi T, Hayashi H, Kitano Y, Yamamura K, Kaida T, Arima K, et al. Statin attenuates cell proliferative ability via TAZ (WWTR1) in hepatocellular carcinoma. Med Oncol. 2016;33:123.
Graaf MR, Richel DJ, van Noorden CJF, Guchelaar HJ. Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev. 2004:609–41.
Van Wyhe RD, Rahal OM, Woodward WA. Effect of statins on breast cancer recurrence and mortality: a review. Breast Cancer Targets Ther. 2017. 559–65.
Stancu C, Sima A. Statins: Mechanism of action and effects. J Cell Mol Med. 2001;5:378–87.
El-Helw A-RMA-RM, Fahmy UA. Improvement of fluvastatin bioavailability by loading on nanostructured lipid carriers. Int J Nanomedicine. Dove Press. 2015;10:5797–804.
Fahmy UA, Aldawsari HMHM, Badr-Eldin SMSM, Ahmed OAAOAA, Alhakamy NANA, Alsulimani HHH, et al. The encapsulation of febuxostat into emulsomes strongly enhances the cytotoxic potential of the drug on HCT 116 colon cancer cells. Pharmaceutics. 2020;12:1–17.
Ahmed OAA, Badr-Eldin SM. Development of an optimized avanafil-loaded invasomal transdermal film: ex vivo skin permeation and in vivo evaluation. Int J Pharm. Elsevier B.V.; 2019;570.
Fahmy UA, Badr-Eldin SM, Ahmed OAA, Aldawsari HM, Tima S, Asfour HZ, et al. Intranasal niosomal in situ gel as a promising approach for enhancing flibanserin bioavailability and brain delivery: in vitro optimization and ex vivo/in vivo evaluation. Pharmaceutics. Multidisciplinary Digital Publishing Institute. 2020;12:485.
Badr-Eldin SM, Alhakamy NA, Fahmy UA, Ahmed OAA, Asfour HZ, Althagafi AA, et al. Cytotoxic and pro-apoptotic effects of a sub-toxic concentration of fluvastatin on OVCAR3 ovarian cancer cells after its optimized formulation to melittin nano-conjugates. Front Pharmacol. Frontiers. 2021;11:642171.
Beckwitt CH, Brufsky A, Oltvai ZN, Wells A. Statin drugs to reduce breast cancer recurrence and mortality. Breast Cancer Res. BioMed Central; 2018. 144.
Manthravadi S, Shrestha A, Madhusudhana S. Impact of statin use on cancer recurrence and mortality in breast cancer: a systematic review and meta-analysis. Int. J. Cancer. Wiley-Liss Inc.; 2016. p. 1281–8.
Hindler K, Cleeland CS, Rivera E, Collard CD. The role of statins in cancer therapy. Oncologist AlphaMed. Press. 2006;11:306–15.
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. Nature Publishing Group; 2015. p. 941–51.
Murugan K, Choonara YE, Kumar P, Bijukumar D, du Toit LC, Pillay V. Parameters and characteristics governing cellular internalization and trans-barrier trafficking of nanostructures. Int J Nanomedicine. Dove Medical Press Ltd. 2015;10:2191–206.
Pelaia G, Gallelli L, Renda T, Fratto D, Falcone D, Caraglia M, et al. Effects of statins and farnesyl transferase inhibitors on ERK phosphorylation, apoptosis and cell viability in non-small lung cancer cells. Cell Prolif. 2012;45:557–65.
Li Y, Fu J, Yuan X, Hu C. Simvastatin inhibits the proliferation of A549 lung cancer cells through oxidative stress and up-regulation of SOD2. Pharmazie. 2014;69:610–4.
Md S, Alhakamy NA, Aldawsari HM, Husain M, Kotta S, Abdullah ST, et al. Formulation design, statistical optimization, and in vitro evaluation of a naringenin nanoemulsion to enhance apoptotic activity in A549 lung cancer cells. Pharmaceuticals (Basel, Switzerland). 2020;13(7):152.
Sheikholeslami K, Sher AA, Lockman S, Kroft D, Ganjibakhsh M, Nejati-Koshki K, et al. Simvastatin induces apoptosis in medulloblastoma brain tumor cells via mevalonate cascade prenylation substrates. Cancers (Basel). MDPI AG; 2019;11.
Qi XF, Zheng L, Lee KJ, Kim DH, Kim CS, Cai DQ, et al. HMG-CoA reductase inhibitors induce apoptosis of lymphoma cells by promoting ROS generation and regulating Akt, Erk and p38 signals via suppression of mevalonate pathway. Cell Death Dis. 2013;4:e518.
Khodayar S, Bardania H, Shojaosadati SA. Bagheri F. Optimization and characterization of aspirin encapsulated nano-liposomes. Iran J Pharm Res. 2018;17:11–22.
Acknowledgements
The authors acknowledge with thanks DSR for technical support.
Funding
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. (RG-3-166-41).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1208/s12249-023-02591-y
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alhakamy, N.A., Badr-Eldin, S.M., Aldawsari, H.M. et al. RETRACTED ARTICLE: Fluvastatin-Loaded Emulsomes Exhibit Improved Cytotoxic and Apoptosis in Prostate Cancer Cells. AAPS PharmSciTech 22, 177 (2021). https://doi.org/10.1208/s12249-021-02021-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02021-x