Abstract
To investigate the effect of polyethylene glycol (PEG) molecular weights on circulation time of PEGylated emulsions and the second injection of injected PEGylated emulsions, we studied the effect of molecular weights on the pharmacokinetic behavior of PEG-DSPE (modified emulsions with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-n-[methoxy (polyethyleneglycol)]) and PEG-CHMC (modified emulsions with poly(ethyleneglycol)-cholesteryl carbonate) emulsions in beagle dogs. The “accelerated blood clearance” (ABC) phenomenon was induced. Through this study, the contribution of PEG molecular weights on the ABC phenomenon was further clarified, and the results provided guidance for lessening or eliminating the ABC phenomenon. We injected different PEG-modified emulsions with 10% PEG-modified density into beagle dogs at 2 μmol phospholipids kg−1 and the blood samples were drawn after 1 min, 3 min, 5 min, 10 min, 15 min, 30 min, 60 min, 120 min, 240 min, 360 min, 600 min, and 24 h. Then, concentrations of the drug were assayed using high-performance liquid chromatography (HPLC). The results showed that the circulation times of PEG-DSPE–modified emulsions were significantly different because of the difference in molecular weights, whereas those of the PEG-CHMC modified emulsions were not. The spatial conformation of PEG with small molecular weights (PEG400, PEG600, and PEG800) was more likely to induce a strong ABC phenomenon. The results of our work suggest the interaction of circulation time and PEG molecular weights on the ABC phenomenon, implying that the spatial conformation of PEG has advantages that alleviate the ABC phenomenon. Importantly, the results have implications for the choice of molecular weights of PEG for PEGylated formulations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In recent years, the modification of nanocarriers using polyethylene glycol (PEG) greatly overcame the application barriers that the carrier is easily taken up by the mononuclear phagocyte system (MPS) (1). This strongly promoted the application and development of nanocarrier systems. PEG is used to “coat” (PEGylation) the carrier to improve its surface hydrophilicity and steric hindrance, and to reduce the recognition probability of the MPS, thus prolonging the circulation time in vivo. Therefore, PEG was once the most suitable material for prolonging the circulation time of the carrier, and its development has been regarded as a milestone in the development of nanocarrier applications (2). Since the first PEGylated liposome, Doxil®, was launched in 1995 (3), a variety of PEGylated preparations were used clinically, and a large number of the preparations required repeated administration to achieve the desired therapeutic effect. However, a study by Dams et al. (4) pointed out that when PEGylated liposomes were injected into the same animal repeatedly, their pharmacokinetics change abnormally, in what is called the accelerated blood clearance (ABC) phenomenon. The appearance of the ABC phenomenon not only weakened the long-circulating characteristic of PEGylated preparations, but it also induced an immune response that imposes significant limitations on clinical applications. With further research, more PEGylated carriers, such as solid lipid nanoparticles (5), emulsions (6), polylactic acid (PLA) nanoparticles (7), cationic bovine serum albumin nanoparticles (8), and micelles (9), were verified to induce the ABC phenomenon when they were injected repeatedly.
Although PEGylation provides the carrier “invisible” properties, it also causes an adverse reaction—the ABC phenomenon. Research determined that the first dose of a PEGylated preparation, such as TI-2 antigen, stimulated the splenic marginal zone B cells to produce the anti-PEG IgM. This anti-PEG IgM then combined with the second dose of the PEG preparation to activate the complement system and accelerate identification and phagocytosis by MPS (10). The commonly used PEG-lipid derivatives, with a linkage bond, which is an amide or ether bond, are 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-n-[methoxy(polyethyleneglycol] (PEG-DSPE) (11,12,13), PEG-phosphatidylethanolamine (PEG-PE) (14), and PEG-cholesterol (PEG-CHOL) (15,16,17). These derivatives have high stability in vivo and are not easily degraded, resulting in a persistent action of the PEGylated carriers and the B cells, thereby inducing an intense ABC phenomenon in vivo. Studies found that the ABC phenomenon could be avoided by adjusting the surface properties of the pharmaceutical preparations of the PEG-lipid derivatives. Researchers used a smaller C14-lipid anchor to increase the degree of dissociation of PEG from the carrier surface (10). It was found that the modification of the formulation of the PEG-lipid derivative could significantly attenuate the ABC phenomenon. In response to the reduced phenomenon, we hoped to change the chemical bond between PEG and the lipids, and use human physiological or pathological conditions to cause the PEG chain to fall off from the surface of the liposome upon or after reaching the target site. The cleavable PEG-lipid derivatives were produced under these conditions. The surface of the particle was gradually deviated through the utilization of the characteristics of the physiological environment of the body, thereby increasing the amount of drug reaching the immune response (such as ABC phenomenon) induced by PEG-lipid derivatives. Xu et al. (18) linked the PEG molecules to lipids by ester bonds and these bonds were gradually cleaved under the catalysis of carboxylesterase in vivo, which could weaken the ABC phenomenon. Carboxylesterase is widely distributed in mammalian tissues and circulatory systems, and can effectively catalyze ester bonds and thioester bonds in endogenous and exogenous substances, and hydrolyze the substrate into corresponding free acids (19).
Further experiments determined that the different molecular weights of PEG also have an effect on the behavior of the carrier in vivo. Allen et al. (20) studied the protective effect of PEG-lipids in liposomes of phosphatidylserine (PS). The results indicated that both PEG2000-DSPE and PEG750-DSPE could increase the circulation time of PS liposomes, although that of the former was greater. Another research pointed out that compared with the PEG2000–modified carrier, PEG5000 had a lower protein adsorption capacity, which could extend the circulation time of the carrier to some extent (21). During the ABC phenomenon, the PEG molecular can influence B cells, such as TI-2 antigens because of its three features—large molecular weights, neat surface alignment, and long circulation time for the carrier. Therefore, the PEG molecule is considered an important factor in the ABC phenomenon. Studies have shown that the first injection of 5% PEG2000-DSPE and PEG5000-DSPE modified liposomes, and 5 days after the injection of PEG2000-DSPE modified labeled liposomes, both produced significant ABC phenomena. Therefore, the authors believed that the PEG molecular weights are not essential for the ABC phenomenon. However, Ishida et al. (22) studied the effect factors of the ABC phenomenon and found that it was induced by the first dose of PEG5000-DSPE modified liposome much less than that of the PEG2000-DSPE modified liposome, with liver and spleen accumulations 35.3 ± 3.8% and 78.7 ± 8.8%, respectively. This result indicated that the increase in PEG molecular weights of the first injection weakened the ABC phenomenon induced. The above two contradictory conclusions may be caused by the different experimental animals used: the former study used rats and the latter mice. For this reason, beagle dogs were selected as experimental animals to avoid inconsistencies caused by species-specific differences (23). Furthermore, the immune system of beagle dogs is more sensitive than that of mice; thus, the differences between preparations should be enhanced and therefore improve analyses of results. Wang et al. reported that repeated fragmentation -(CH2CH2O)n- of the PEG molecule may be the key for splenic marginal zone B cells to recognize and produce corresponding antibodies (24). Therefore, we hypothesized that reducing the number of repeated fragments in the PEG-lipid derivatives might weaken the ABC phenomenon. In this study, we prepared emulsions of PEG-CHMC (18) with PEG molecular weights of 400, 600, 800, 1000, 2000, and 5000, and studied the in vivo pharmacokinetic behavior of PEGylated emulsions (referred to as CHMCE) and the ABC phenomenon they induced. The goal of our experiment was to determine whether the circulation time of PEG-CHMC modified emulsions with different molecular weights was improved, and the effects of the ABC phenomenon weakened. By comparison with traditional PEG-DSPE modified emulsions, the effects of different PEG molecular weights on the ABC phenomenon in beagle dogs were clarified for the first time to provide a solution to the ABC phenomenon problem.
MATERIALS AND METHODS
Materials
Tocopheryl nicotinate (TN) was obtained from the Northeast Pharmaceutical Group Co., Ltd. (Shenyang, China), egg phosphatidylcholine (E80, Lipoid GmbH, Germany), and PEGn-CHMC (n = 400, 600, 800, 1000, 2000, and 5000) were synthesized in our laboratory, and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-n-[methoxy(polyethylene glycol)-n] (PEGn-DSPE) was purchased from the Genzyme Corporation (Cambridge, MA, USA). Bovine serum albumin (BSA) was purchased from Biosharp (Seoul, South Korea). Medium-chain triglycerides (MCT) were purchased from the Beiya Medicated Oil Co., Ltd. (Tieling, China), and glucose injections were obtained from Kunming Nanjiang Pharmaceutical Co., Ltd. (Yunnan, China). Horseradish peroxidase (HRP) was purchased from Beijing Biosynthesis Biotechnology Co., Ltd. (Beijing, China). HRP-conjugated goat anti-dog IgM was obtained from Immunology Consultants Laboratory, Inc. (Portland, OR, USA). Sterilized water for injection was obtained from Shijiazhuang Four Medicine Co., Ltd. (Hebei, China). 3-[(3-Cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) was purchased from J&K Chemical Ltd. (Beijing, China). The 96-well plate was obtained from Corning Inc. (New York, USA). All other chemicals were of analytical reagent grade and were used without further purification.
Animals
Male beagle dogs (weight 10–12 kg) were provided by the Experimental Animal Center of Shenyang Pharmaceutical University (Shenyang, China). The animals could obtain water and dog chow freely. Experiments applied on all animals were all carried out consistent with the procedures approved by local Animal Welfare Committee.
The Preparation of PEGylated Emulsions
Two PEGylated emulsions were used: PEG-DSPE and PEG-CHMC. They contained TN, MCT, and E80 in a weight ratio of 1:5:1.165, and E80 to PEG-lipids in a molar ratio of 9:1. The emulsions were modified with PEG400-DSPE, PEG600-DSPE, PEG800-DSPE, PEG1000-DSPE, PEG2000-DSPE, and PEG5000-DSPE, and were named PE400, PE600, PE800, PE1000, PE2000, and PE5000, respectively. Similarly, the emulsions modified with PEGn-CHMC were CHMCE400, CHMCE600, CHMCE800, CHMCE1000, CHMCE2000, and CHMCE5000, respectively. When preparing the emulsions, the mixtures of TN, MCT, E80, and PEG-DSPE or PEG-CHMC were stirred at 55°C constantly. Sterile water as the aqueous phase was heated to the same temperature and then rapidly poured into the above oil phases, making the final total phospholipid concentration 5 μmol mL−1. Through agitating and incubating at 55°C for 10 min, we obtained the crude emulsions. The crude emulsions were sonicated through an experimental ultrasonic cell pulverizer. The operating mode was 200 W × 2 min and 400 W × 6 min (work 1 s, interval 1 s). Then, we obtained the drug-loaded PEGylated emulsions by extruding through 0.22-μm polycarbonate membrane filters and the conventional emulsions (named CE) through a 0.45-μm polycarbonate membrane. Ultimately, we adjusted the osmotic pressure to the isotonic level by injecting 50% glucose. The Nicomp 380 HPL submicron particle analyzer (Particle Sizing Systems, Santa Barbara, CA, USA) was used to determine the particle size distribution and zeta potential.
Pharmacokinetic and the ABC Phenomenon Studies of Various PEGylated Emulsions
Thirty-six male beagle dogs were randomly divided into 12 groups (n = 3). These beagle dogs fasted overnight before drug administration, and 2 mL of blank (blood) was drawn from the left forelimb vein into a heparinized tube. A dose of 2 μmol phospholipid kg−1 of PE and CHMCE was injected via the right forelimb into dogs to study the pharmacokinetics of emulsions. At selected post-injection time points (1 min, 3 min, 5 min, 10 min, 15 min, 30 min, 60 min,120 min, 240 min, 360 min, 600 min, and 24 h), blood was drawn from the left forelimb vein and placed in a heparinized tube. The above process was recorded as the first injection. After a 7-day interval, the beagle dogs were injected with the second dose which was same as the first injection. Table I show the injection protocols of the different PEGylated emulsions. To separate plasma, all blood samples we obtained were centrifuged at 4500 rpm for 10 min and stored at − 20°C before testing.
The TN concentration in the plasma was determined by HPLC. We placed 100 μL plasma sample into a 1.5-mL centrifuge tube, added 100 μL methanol, the 100 μL internal standard (100 μg mL−1 tocopheryl acetate), and 600 μL n-hexane. The mixture was then vortexed for 5 min to completely dissolve the sample. After centrifugation at 10,000 rpm for 10 min, 500 μL of the supernatant was dried using a laboratory CentriVap centrifugal vacuum concentrator. When determined, the evaporated sample was reconstituted with 100 μL of the mobile phase which contains methanol and isopropanol (80/20, v/v). Then, the mixture was blended by vortex for 1 min and centrifuged for 10 min at 10,000 rpm. Then, take supernatant 20 μL for HPLC analysis. The other experimental conditions were as follows: column, Hypersil BDS C18 (200 mm × 4.6 mm, 5 μm, Elite); column temperature, 35°C; flow rate, 1.0 mL min−1; detection wavelength, 264 nm; injection volume, 20 μL; internal standard, tocopherol acetate.
Detection of Anti-PEG IgM Antibodies Levels in Beagle Dog Serum
There was a major factor, anti-PEG IgM, which affects the rapid elimination of PEGylated preparations in vivo. A 2 mL sample of blank blood was taken in heparinized tubes before the first and second injections. After 2 h at room temperature, the blank blood sample was centrifuged at 4500 rpm for 10 min to isolate the serum. To determine the anti-PEG IgM levels in the serum, we used enzyme-linked immunosorbent assay (ELISA) as the determined method (25). Briefly, a 96-well polystyrene plate was added with 50 μL of coating solution (10 nmol PEG2000-DSPE in absolute ethanol) and allowed to air dry overnight at room temperature. Then, 50 mM Tris-buffered saline (0.14 mM NaCl and 1% BSA) as a blocking solution was added to the plate for blocking 1 h. Plasma samples are diluted at a ratio of 1:1000 for used. The plate was washed with Tris-buffered saline (containing 0.05% CHAPS), and then added 100 μL the serum samples to incubate for 1 h. After five washes, the wells were added with the HRP-conjugated goat anti-dog IgM antibody (1 μg mL−1) which was diluted and the sample wells were incubated for 1 h. Afterwards, the 96-well plate was washed five times. The o-phenylene diamine with concentration 1 mg mL−1 (100 μL) was added to initiate the stain. After incubating the samples for 15 min, H2SO4 (100 μL; 2 mol L−1) was added to terminate the reaction. A microplate reader (Bio-Rad Laboratories Ltd., Hertfordshire, UK) was used to measure the absorbance at 490 nm. All of the above experiments were conducted at room temperature.
Determination of the Cleavage Behavior of 10% PEG-CHMC from Surface in Beagle Dog Plasma
CHMCE (50 μL) was added to beagle dog plasma (3.0 mL) in a 7-mL EP tube, which was vortexed for 5 min to mix evenly. The mixture was incubated for 1, 5, 15, 30, 60, and 120 min in a 38°C water bath. Then, ultrafiltration (100 kDa) was conducted after adding distilled water (2.0 mL) and the primary filtrate was separated. The continuous filtrate (1.0 mL) was diluted to 5 mL with distilled water. Measurement of the absorbance at 567.8 nm was conducted using the BaCl2-I2/KI method (26,27), and was recorded as Ai (i = 1, 5, 15, 30, 60, and 120 min).
Another CHMCE (50 μL) sample was added to distilled water (5.0 mL) in a 7-mL EP tube, which was vortexed for 5 min to mix evenly. The primary filtrate (1.0 mL) was diluted to 5 mL with distilled water. The continuous filtrate (1.0 mL) was diluted to 5 mL with distilled water. Measurement of the absorbance at 567.8 nm was conducted using the BaCl2-I2/KI method, which was recorded as At.
The concentrations of PEG (μg mL−1), which was Ci or Ct, were calculated by the standard curve formula. C0 was the amount of PEG cleavage at 0 min in incubation. The percentage of cleavage was calculated using the following equation:
\( \mathrm{PEG}\ \mathrm{cleavage}\ \left(\%\right)=\frac{C_1-{C}_0}{C_t-{C}_0}\times 100\% \)
Statistical Analysis
The experimental data are expressed as mean ± standard deviation (SD). Statistical comparisons were performed using two-tailed unpaired Student’s t test or analysis of variance (ANOVA) with SPSS 16 software (SPSS Inc., Chicago, IL, USA). A two-sided P value of P < 0.05 was considered significant.
RESULTS AND DISCUSSION
The Characteristics of Various PEGylated Emulsions
The two emulsions we prepared, PE and CHMCE, had different size distributions because of the PEG-lipids used as the emulsifier. The average sizes of PE400, PE600, PE800, PE1000, PE2000, PE5000, CE, CHMCE400, CHMCE600, CHMCE800, CHMCE1000, CHMCE2000, and CHMCE5000 were approximately 147.2 nm, 133.4 nm, 149.0 nm, 123.9 nm, 123.8 nm, 141.0 nm, 278.3 nm, 194.2 nm, 203.6 nm, 195.4 nm, 192.6 nm, 158.5 nm, and 139.9 nm, respectively. However, the mean diameter distribution of PE was smaller than that of CHMCE. In addition, the size distribution of the preparations had a low coefficient of variation (CV) of 0.318 to 0.478. All emulsions were negatively charged for E80, PEG-DSPE, and PEG-CHMC. Zeta potential results of PEGylated emulsions indicated that the modifier was successfully applied to the surface of the emulsions (Table II).
The Pharmacokinetic Behavior of PE in Beagle Dogs and the Effects of Different PEG Molecular Weights on the ABC Phenomenon
The Pharmacokinetics of PE
As reported, particles coated with PEG could effectively avoid the uptake of MPS in vivo and prolong the circulation time (2). In this study, we treated dogs with PE400, PE600, PE800, PE1000, PE2000, and PE5000 at a dose of 2 μmol phospholipids kg−1 and evaluated the pharmacokinetic parameters. As shown in Fig. 1, increasing the PEG molecular weights to 2000 and 5000 caused significant changes in the pharmacokinetic profiles. The plasma concentrations (related to the injection dose) of PE2000 and PE5000 at 4 h were 36.95 ± 4.02% and 49.05 ± 3.22%, respectively. However, the percentages of injected TN remaining in other groups were less than 12.2% at 4 h. The pharmacokinetic parameters of all emulsions were statistically analyzed by DAS 2.1.1 software. The results are shown in Table III. The areas under the curve (AUC) of PE from low relative molecular mass to high molecular weight are 839.02, 1098.0, 702.50, 1703.1, 2025.0, and 2172.0 mg min L−1, respectively. The mean residence times (tMRT) were 0.35, 0.35, 0.33, 0.39, 0.43, and 0.45 h.
The ABC Phenomenon Induced by PE with Different PEG Molecular Weights
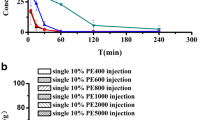
To investigate the effects of PEG molecular weights on PE and the induction phase of ABC phenomenon, PE was administered to the beagle dogs that had different molecular weights PE400, PE600, PE800, PE1000, PE2000, and PE5000 at the first injection. After 7 days, PE2000 as the second dose was administered. The pharmacokinetic parameters of the second dose PE2000 of all groups are shown in Fig. 2. The results of Fig. 2 show that the PEG molecular weights of the emulsions effect the extent of the ABC phenomenon. PE1000, PE2000, and PE5000 induced the most intense ABC phenomenon and the second dose of PE2000 was cleared fastest. The values of ABC index(0–60 min) were 0.17, 0.22, and 0.26, respectively (Table IV). The first injection of PE600 and PE800 induces a moderate effect on the clearance of PE2000, with an ABC index(0–60 min) of 0.34 and 0.33, respectively (Table IV). In contrast, the first injection of PE400 was only slightly affected by the PE2000 (the value of ABC index(0–60 min) is 0.91).
Influence of pre-dose emulsions modified with PEG-DSPE of different molecular weights on the pharmacokinetics of subsequent dose of PE2000. The beagle dogs were given emulsions at a dose of 2 μmol phospholipids kg−1 as the first injection. Then, the beagle dogs were given the second dose after 7 days. b is an enlarged view of a in 0–60 min. Data are shown as mean ± s.d. (n = 3)
Pharmacokinetic Behavior of CHMCE in Beagle Dogs and the Effects of Different PEG Molecular Weights on the ABC Phenomenon
The Pharmacokinetics of CHMCE
CHMCE was intravenously injected into beagle dogs at the same doses (phospholipid dose of 2 μmol kg−1) with PE. The pharmacokinetics of PE is shown in Fig. 3. The results in Fig. 3 show that CHMCE with different PEG molecular weights exhibited a short circulation time and low plasma concentrations compared with corresponding PEs. There were no statistical differences in the pharmacokinetic characteristics of the first doses between the PEG-CHMC modified emulsions (CHMCE400, CHMCE600, CHMCE800, CHMCE1000, CHMCE2000, and CHMCE5000). The TN concentration (related to the injection dose) of all CHMCEs at 15 min after the intravenous injection was below to 18.12 ± 2.42%, and it was consistent at 30 min. Compared with CHMCE, CE has a lower plasma concentration within 20 min. Meanwhile, the result shown in Fig. 3 shows the AUC of CHMCE from 300 to 450 mg min L−1, but the AUC of CE is 271.00. It was speculated that the PEG molecule had been isolated from the surface of the emulsions.
The ABC Phenomenon Induced by CHMCE with Different PEG Molecular Weights
Dogs were treated with CHMCE400, CHMCE600, CHMCE800, CHMCE1000, CHMCE2000, and CHMCE5000, containing the same phospholipid concentration as before as the first dose. And then, the dogs were injected with PE2000 at the same concentration after 7 days. The results in Fig. 4 show that, compared with the control group (pretreated with 10% PE2000), the drug concentrations of the second dose of PE2000 decreased, but not excessively. Among these groups, dogs pretreated with CHMCE400, CHMCE600, and CHMCE800 showed mild clearance of PE2000. The ABC index(0–60 min) in Table IV was 0.59, 0.61, and 0.67, respectively. However, CHMCE5000 induced extensive clearance of PE2000 (ABC index(0–60 min) = 0.40, Table IV).
Influence of pre-dose emulsions modified with PEG-CHMC of different molecular weights on the pharmacokinetics of subsequent dose of PE2000. The beagle dogs were given emulsions at a dose of 2 μmol phospholipids kg−1 as the first injection. Then, the beagle dogs were given the second dose after 7 days. Data are shown as mean ± s.d. (n = 3)
Comparison of the Extent of the ABC Phenomenon Induced by PE and CHMCE with Different PEG Molecular Weights
To clearly determine the ABC phenomenon induced by different molecular weights of PE and CHMCE, we used the parameter AUC(0–60 min) as the ABC evaluation index(0–60 min). The ABC index was calculated by the ratio of the area under the curve (AUC)(0–24 h) for the second injection relative to that of the first injection (7). If the value of ABC index was 1, which indicated no the ABC phenomenon. Furthermore, the results of this experiment (Figs. 2 and 4) showed that AUC(0–60 min) had obvious differences in different groups. Thus, we evaluate the ABC phenomenon with the ratio of AUC(0–60 min). As shown in Table IV, we found that the ABC phenomenon between PE and CHMCE was different. Moreover, PE600, PE800, PE1000, PE2000, and PE5000 had the most marked ABC phenomenon, with the corresponding ABC index(0–60 min) 0.34, 0.33, 0.17, 0.22, and 0.26, respectively, all being below 0.40. In contrast, the ABC index(0–60 min) of PE400 was higher than 0.9, which is very close to 1. This indicated that the first injection of PE400 barely induced the ABC of the second injection of PE2000. Compared with PE, CHMCE induced a slightly higher ABC phenomenon, especially for CHMCE1000 and CHMCE2000, as evidenced by the ABC index(0–60 min) of 0.80 and 0.79, respectively.
Levels of Anti-PEG IgM Between Various PEGylated Emulsions
In recent years, researchers have reputed that the PEGylated nanocarriers of the first injection that entered into the body produced a lower affinity anti-PEG IgM, which is a major factor in the accelerated clearance of the second injection of PEGylated nanocarriers. Therefore, anti-PEG IgM production by PE and CHMCE was determined. In this experiment, the anti-PEG IgM levels were assessed using anti-PEG IgM absorbance ratio on day 7 to day 0 to determine the background values for different beagle dogs (28). Figure 5 results show whether PEG-DSPE modified emulsion or a PEG-CHMC modified emulsion; it produced a certain amount of anti-PEG IgM. It was observed that the amount of anti-PEG IgM was enhanced with the degree of the ABC phenomenon, but there was no statistical difference between the groups. For the first injection of different PEGylated emulsions, the absorbance ratio of anti-IgM was arranged from the highest to the lowest order: CHMCE5000, PE2000, PE5000. Comparison of the parameters between the groups pretreated with CHMCE5000 (**P < 0.01), PE2000 (**P < 0.01), and PE5000 (*P < 0.05) and the control group revealed significant differences. This result was slightly contrary to the aforementioned ABC index(0–60 min). Therefore, we considered that the amount of anti-PEG IgM was not enough to accurately judge the strength of the ABC phenomenon but could be used as a reference.
Anti-PEG IgM production by the first dose of PEGylated emulsions modified with different molecular weights. The method of ELISA was used to determine the level of anti-PEG IgM in the serum, as described in the “Materials and Methods” section. Data are shown as mean ± s.d. (n = 3). P values apply to differences between the control and treated rats. *P < 0.05, **P < 0.01, ***P < 0.001
Fracture Behavior of Different Molecular Weight PEG-CHMC in Beagle Dog Plasma
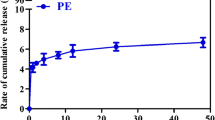
Considering that the PEG molecule is the core factor inducing the ABC phenomenon, and PEG-CHMCE is a cleavable PEG-lipid derivative, we investigated the fracture behavior of PEG-CHMC in beagle dog plasma. The ultraviolet spectrophotometer combined with the BaCl2-I2/KI method was used to determine the cleavage of different molecular weights of PEG-CHMC modified emulsions with dog plasma at different time points. As shown in Fig. 6, PEG400-CHMC was more susceptible to cleavage under the same conditions, and its percentage of cleavage at 1 min was 26.49 ± 1.20%, which was 5 times as much as that of PEG2000-CHMC (5.87 ± 2.00%) and 6 times as much as that of PEG5000-CHMC (4.14 ± 2.10%). Moreover, PEG400-CHMC could achieve 100% fracture at 120 min, whereas PEG2000-CHMC reached 89.97 ± 3.20% fracture at 120 min. In contrast, for PEG5000-CHMC, the percentage of cleavage at 120 min was 12.17 ± 2.01%. Therefore, we concluded that the smaller PEG molecular weights in dog plasma, with the shortening of the PEG chain length, made PEG-CHMC more easily cleaved by plasma esterase. The in vitro rupture test was related to the dose given in vivo, and there was a correlation between the in vivo and in vitro results. Thus, the above results could be applied to the results of the in vivo tests to some extent.
PEG is a neutral, crystalline, thermoplastic polymer that is soluble in water and organic solvents. Water molecules present a tetrahedral lattice by hydrogen-bonded molecules in the liquid state. As a result, PEG’s and water’s tetrahedral lattices are matched exactly, and water molecules form hydrogen bonds with oxygen in the ether bond of the PEG molecular chain. Water molecules are oriented to bind to the PEG molecule, forming a hydration layer wrapped on the surface of the PEG chain. Therefore, the PEG-modified carriers effectively avoided the recognition and uptake of plasma proteins and subsequent clearance in vivo. The results in Figs. 1 and 3 both show the advantages of PEGylation on emulsions over conventional emulsions in terms of circulation time. PEG-CHMC is an emulsion-modified material synthesized by our laboratory. Compared with traditional PEG-DSPE, lipophilic DSPE was replaced by cholesterol and the binding bond with PEG was replaced by the non-cleaving amide bond to the cleavable carbonate bond (Fig. 7 for the structure). Mammals contain a high amount of esterases, and PEG-CHMC is easily degraded because of its carbonate linkages (19). When PEG-CHMC modified emulsions were injected, the surface-modified PEG molecules gradually fall off, causing the carriers to lose the protective layer of PEG and be cleared quickly from the body (Fig. 3). As shown in Fig. 3, PEG-CHMC modified emulsions had a short circulation time in beagle dogs and was reduced to a low concentration within 30 min. For PEG-DSPE modified emulsions, the degree of protection of PEG on the emulsions varies with molecular weights, and the hydration layer thickness on the carriers’ surface increased with the increase in PEG molecular weights (data not shown). This is consistent with the conclusion of Mori et al. (29). Thus, we believed that the stability of these carriers in plasma would be enhanced by increases in the molecular weights of PEG (Fig. 1). The results presented in Fig. 1 show that the circulation time of PE was divided into two levels. The circulation time of PE with molecular weights of 400, 600, 800, and 1000 was significantly shorter than that of 2000 and 5000.
Generally, repeated injection of PEGylated carriers could induce the phenomenon of rapid elimination of ABC in the second injection preparation. We referred to the first injection formulation as the TI-2 antigen with three basic characteristics: large molecular weights, a neat surface alignment, and a long circulation time, which ensured that the TI-2 antigen was associated with B cell surface receptors during a relatively long period of time. These interactions could produce a sustained B cell signal response, stimulating the body to produce antibodies without the need of helper T cells (30,31). To compare the ABC phenomenon induced by the first injection of different preparations intuitively, we made the second injection into PE2000. Analysis of the ABC phenomenon of the PE group and CHMCE group following the second injection of PE2000 showed that CHMCE as the TI-2 type antigen was generally weaker than the PE group because of its short circulation time and lack of a neat surface alignment (gradual shedding of PEG). Therefore, we believed that the effect of circulation time was the first among the three basic characteristics of the TI-2 antigen. Furthermore, the results shown in Fig. 2 indicated that the ABC phenomenon induced by PE was gradually enhanced with the increase of PEG molecular weights, whereas the ABC phenomenon was not induced by PE400 with the shortest circulation time. The calculation of the ABC index in Table IV also proved this point. The ABC index(0–60 min) of PE400 was above 0.90, followed by PE600 and PE800, with PE1000, PE2000, and PE5000 exhibiting the minimum. We analyzed the reason for the influence of circulation time. The longer the circulation time of PE in beagle dogs, the longer the stimulation of spleen B cells and the more anti-PEG IgM produced (Fig. 5). Our team also studied the effects of PEG chain lengths on ABC phenomenon in rats. Considering that the immune system of beagle dogs is more sensitive than that of rats, beagle dogs were used as experimental animals in this study to more accurately reflect the actual influence (32).
If the circulation time of the first injection had important effects on the generation of the ABC phenomenon, why did the CHMCE group with short circulation time still produce a stronger ABC phenomenon? Furthermore, why was the ABC phenomenon induced to different extents when the CHMCE circulation time of different molecular weights was consistent? Figure 4 shows that the ABC phenomenon induced by different molecular weights of CHMCE by the second injection was different. The extent of the ABC phenomenon from strong to weak was CHMCE5000 > CHMCE400, CHMCE600, CHMCE800 > CHMCE1000, CHMCE2000. In general, the ABC phenomenon induced by small molecular weight CHMCE was more intense than that of high molecular weight CHMCE. Was long-chain PEG more susceptible to being attacked by esterase to cleave the PEG molecules and cause surface irregularities? To explore the effect of different molecular weight PEGs on ABC phenomenon, the fracture degree of different molecular weights of PEG-CHMC was investigated. Figure 6 shows the in vitro investigation of the fragmentation behavior of different molecular weights of PEG-CHMC in beagle dog plasma. Under the same conditions, PEGs with small molecular weights were more easily attacked by plasma esterase but the long-chain PEG molecules were intertwined to form a dense network structure, resulting in molecules that made it difficult for esterase to attack the carbonate bond. To summarize, small molecular weight CHMCE still produced an intensive ABC phenomenon under the condition of easy shedding of PEG molecules and reduced PEG-modified density. It was speculated that the spatial conformation of small molecular weight PEG was more likely to stimulate spleen B cells. The long-chain PEG molecules were more likely to stretch freely on the emulsion surface and were more likely to bend and interlace, whereas the small-molecule-PEG tended to stretch (in a “brush” arrangement (20)). Dintzis et al. (33) pointed out that the spleen was more sensitive to the cross-linking produced by linear molecules, so the conformation of PEG molecules on the surface of the emulsion is also an important factor affecting the ABC phenomenon. As for the strong ABC phenomenon of CHMCE5000, it was caused by its relatively long circulation time within 24 h (data were not shown).
CONCLUSION
In this study, we demonstrated that the first dose of PEG-DSPE and PEG-CHMC modified emulsions with different PEG molecular weights could induce the accelerated blood clearance of the second dose of PE2000. Our results revealed PEG molecular weights as an important factor in the first phase of ABC phenomenon. This study showed that the effect of PEG molecular weights could be presented when the effects of circulation time of the first injection on the ABC phenomenon were excluded. The small molecular weight PEG-CHMC induced a stronger ABC phenomenon upon the second injection, suggesting that the spatial conformation of small molecular weight PEG was more likely to stimulate the splenic marginal zone. These findings provide a new insight into the methods that can be employed to weaken or remove the ABC phenomenon induced by PEGylated nanocarriers.
References
Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nature Reviews Drug Discovery. 2003;2(3):214-21.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2(3):214-21.
Lasic DDJN. Doxorubicin in sterically stabilized liposomes. Nature. 1996;380(6574):561.
Barenholz Y. Chapter 13:Doxil® – the first FDA-approved nano-drug: from basics via CMC, cell culture and animal studies to clinical use. Nanomedicines: Design, Delivery and Detection; 2016.
Dams ET, Laverman P, Oyen WJ, Storm G, Scherphof GL, JW, et al. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J Pharmacol Exp Ther. 2000;292(3):1071–9.
Zhao Y, Wang C, Wang L, Yang Q, Tang W, She Z, et al. A frustrating problem: accelerated blood clearance of PEGylated solid lipid nanoparticles following subcutaneous injection in rats. J Pharmacol Exp Ther. 2012;81(3):506–13.
Wang C, Cheng X, Sui Y, Luo X, Jiang G, Wang Y, et al. A noticeable phenomenon: thiol terminal PEG enhances the immunogenicity of PEGylated emulsions injected intravenously or subcutaneously into rats. Eur J Pharm Biopharm. 2013;85(3):744–51.
Ishihara T, Takeda M, Sakamoto H, Kimoto A, Kobayashi C, Takasaki N, et al. Accelerated blood clearance phenomenon upon repeated injection of PEG-modified PLA-nanoparticles. Pharm Res. 2009;26(10):2270–9.
Lu W, Wan J, She Z, Release XJJJoC. Brain delivery property and accelerated blood clearance of cationic albumin conjugated pegylated nanoparticle. J Controlled Release. 2007;118(1):38–53.
Kaminskas LM, Mcleod VM, Porter CJH, Sciences BJBJJoP. Differences in colloidal structure of PEGylated nanomaterials dictate the likelihood of accelerated blood clearance. J Pharm Sci. 2011;100(11):5069–77.
Judge A, Mcclintock K, Phelps J, Maclachlan IJMT. Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol Ther. 2006;13(2):328–37.
Ishida T, Kashima S, Society HKJJoCROJotCR. The contribution of phagocytic activity of liver macrophages to the accelerated blood clearance (ABC) phenomenon of PEGylated liposomes in rats. J Control Release. 2008;126(2):162–5.
Ishida T, Harada M, Wang XY, Ichihara M, Irimura K, Society HKJJoCROJotCR. Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: effects of lipid dose and PEG surface-density and chain length of the first-dose liposomes. J Control Release. 2005;105(3):305–17.
Ishida T, Ichihara M, Wang X, Yamamoto K, Kimura J, Majima E, et al. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J Controlled Release. 2006;112(1):15–25.
Awasthi VD, Garcia D, Goins BA, Pharmaceutics WTPJIJo. Circulation and biodistribution profiles of long-circulating PEG-liposomes of various sizes in rabbits. Int J Pharm. 2003;253(1):121–32.
Iden DL, Acta TMAJBEB. In vitro and in vivo comparison of immunoliposomes made by conventional coupling techniques with those made by a new post-insertion approach. Biochim Biophys Acta. 2001;1513(2):207–16.
Steenpa T, Lung A, Acta RSJBEB. Tresylated PEG-sterols for coupling of proteins to preformed plain or PEGylated liposomes. Biochim Biophys Acta. 2006;1758(1):20–8.
Visser CC, Stevanovic S, Voorwinden LH, Bloois Lv, Gaillard PJ, Danhof M, et al. Targeting liposomes with protein drugs to the blood–brain barrier in vitro. Eur J Pharm Sci. 2005;25(2):299–305.
Xu H, Wang KQ, Deng YH, Biomaterials DWCJ. Effects of cleavable PEG-cholesterol derivatives on the accelerated blood clearance of PEGylated liposomes. Biomaterials. 2010;31(17):4757–63.
Simoes, Chemistry SJJoB. Sterically stabilized pH-sensitive liposomes. Intracellular delivery of aqueous contents and prolonged circulation in vivo. J Biol Chem.1997;272(4):2382–8.
Allen C, Santos ND, Gallagher R, Chiu GNC, Shu Y, Li WM, et al. Controlling the physical behavior and biological performance of liposome formulations through use of surface grafted poly(ethylene glycol). Biosci Rep. 2002;22(2):225–50.
Fang C, Shi B, Pei Y-Y, Hong M-H, Wu J, Sciences H-ZCJEJoP. In vivo tumor targeting of tumor necrosis factor-α-loaded stealth nanoparticles: effect of MePEG molecular weight and particle size. Eur J Pharm Sci. 2006;27(1):27–36.
Ishida T, Ichikawa T, Ichihara M, Sadzuka Y, Release HKJJoC. Effect of the physicochemical properties of initially injected liposomes on the clearance of subsequently injected PEGylated liposomes in mice. J Controlled Release. 2004;95(3):403–12.
Desai NJAJ. Challenges in development of nanoparticle-based therapeutics. Aaps Journal. 2012;14(2):282–95.
Wang XY, Ishida T, Release HKJJoC. Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. J Controlled Release. 2007;119(2):236–44.
Ichihara M, Shimizu T, Imoto A, Hashiguchi Y, Uehara Y, Ishida T, et al. Anti-PEG IgM response against PEGylated liposomes in mice and rats. Pharmaceutics. 2010;3(1):1–11.
Sims GEC, Biochemistry TJSJA. A method for the estimation of polyethylene glycol in plasma protein fractions. Anal Biochem.1980;107(1):60–3.
Xing WG, Wei DZ, He ML, Talanta YCXJ. Discarded free PEG-based assay for obtaining the modification extent of pegylated proteins. Talanta. 2007;71(1):381–4.
Wang C, Cheng X, Su Y, Pei Y, Song Y, Jiao J, et al. Accelerated blood clearance phenomenon upon cross-administration of PEGylated nanocarriers in beagle dogs. Int J Nanomedicine. 2015;10:3533–45.
Mori A, Klibanov AL, Torchilin VP, Letters LHJF. Influence of the steric barrier activity of amphipathic poly(ethyleneglycol) and ganglioside GM1 on the circulation time of liposomes and on the target binding of immunoliposomes in vivo. FEBS Lett. 1991;284(2):263.
Mond JJ, Lees A, Snapper CMJARoI. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13(1):655–92.
Mond JJ, Vos Q, Lees A, Snapper CMJCOiI. T cell independent antigens. Curr Opin Immunol. 1995;7(3):349–54.
Suzuki T, Ichihara M, Hyodo K, Yamamoto E, Ishida T, Kiwada H, et al. Accelerated blood clearance of PEGylated liposomes containing doxorubicin upon repeated administration to dogs. Int J Pharm. 2012;436(1–2):636–43.
Dintzis RZ, Okajima M, Middleton MH, Greene G, Dintzis HMJJoI. The immunogenicity of soluble haptenated polymers is determined by molecular mass and hapten valence. J Immunol. 1989;143(4):1239–44.
Acknowledgments
This study was supported by the Scientific Research Project of Yuncheng University (Grant CY-2017013) and the National Natural Science Foundation of China (Grant Nos. 81573375).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Experiments applied on all animals were all carried out consistent with the procedures approved by local Animal Welfare Committee.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiao, J., Luo, X., Wang, C. et al. Effects of Uncleavable and Cleavable PEG-Lipids with Different Molecular Weights on Accelerated Blood Clearance of PEGylated Emulsions in Beagle Dogs. AAPS PharmSciTech 21, 106 (2020). https://doi.org/10.1208/s12249-020-1640-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-1640-4