Abstract
The main intent of this treatise was to encapsulate tamoxifen citrate (TMXC) into polymeric micellar delivery system and evaluate the influence of TMXC-loaded micelles as a promising carrier on the in vitro cytotoxicity and cellular uptake of TMXC in treatment of breast cancer. Different formulae of polymeric micelles loaded with TMXC using mixtures of different Pluronic polymers were fabricated by thin-film hydration method and evaluated for morphology, drug entrapment efficiency, particle size, surface charge, in vitro liberation of TMXC, uptake by cancer cell lines, and cytotoxic effect against breast cancer cell lines such as MCF-7. The optimal TMXC-loaded micelles exhibited nano-sized particles and entrapped about 89.09 ± 4.2% of TMXC. In vitro liberation study revealed an extended TMXC escape of about 70.23 ± 5.9% over a period of 36 h. The optimized TMXC-loaded micelles formula showed enhanced cellular uptake of TMXC by 2.28 folds and showed a significant cytotoxic effect with MCF-7 breast cancer cells compared to TMXC solution. The obtained yield proposed that Pluronic micelles could be a promising potential delivery system for anticancer moieties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Breast cancer is a prevalent tumor amongst women (1) and represents the foremost reason of cancer’s mortality in females (2). Treatment of breast cancer includes local treatment such as surgery which depends on the type and stage of tumor. Also, systemic drugs can be used to reach cancer cells at any part of the body. These systemic drugs comprise chemotherapy, hormonal therapy, and immunotherapy. Hormonal therapy is used to prevent attachment of progesterone and estrogen to their receptors in some types of breast cancer that affected by hormonal level. Some of these hormonal drugs are named as estrogen blocker which works by stopping estrogen from helping breast cancer cells to grow such as tamoxifen, toremifene, and fulvestrant (3).
Tamoxifen citrate (TMXC) is the most commonly used chemotherapy for siege of breast cancer (4). Although TMXC exhibits favorable outcomes, not all treated patients get the hopeful result. High doses of TMXC presented numerous hormonal effects even dependent and independent (5). However, most anticancer drugs that can successfully diminish the neoplastic cells exhibit remarkable side effects mainly because of absence of precise targeting ability (6).
Nanocarriers have got extensive concern as a promising carrier for chemotherapy in respect to their capability to carry drugs preferentially into tumor tissue (7,8) through enhanced permeation and retention phenomenon (EPR) effect (9). As a result of nano platforms accessible for chemotherapeutic agent, polymeric micelles based on biocompatible polymers have been attracting great attention, due to small particle size, several degrees of functionalization, and great flexibility of fabrication (10).
Pluronic® (poloxamer) copolymers, widely used as nanocarriers, are surfactant moieties. These moieties consist of one lipophilic poly(propylene oxide) (PPO) and two lipophobic poly(ethylene oxide) (PEO) arranged in a PEO–PPO–PEO triblock (11). Poloxamer triblock can self-arranged into a spherical micelle temple constructed by propylene oxide (PO) as a lipophobic internal core and ethylene oxide (EO) as a lipophobic external coat (12,13). This feature facilitates the encapsulation of the poorly water-soluble drugs in the lipophilic core, which diminish the cytotoxicity of chemotherapeutics and sustained activity in patient due to longer circulation in the blood (14). This new block exhibits several advantages such as solubility increments, metabolic stability, and sustained the drug duration in circulation. Pluronic copolymers are known as inert carrier with low cytotoxicity. Also, they can reduce multi-drug-resistant (MDR) tumors mechanism to anticancer agents in vitro and in vivo through inhibition of P-gp (15). Size of micelles exemplifies another important feature of the polymeric micelles which make them effective and promising carriers for drugs (16).
The nano-size of polymeric micelles, as well as long circulatory character, permits polymeric micelles to accumulate inside tumor due to leaky vasculature of compromised tissues and EPR effect. Nanocarriers like micelles cannot diffuse out from interstitial spaces after internalization via EPR effect. On the other hand, the smaller moieties can move into and leave from interstitial spaces via diffusion mechanism (17). Some types of chemotherapy-loaded polymeric micelles became available in markets, such as paclitaxel that was loaded with polymeric micelles under commercial name of Genexol®-PM (18).
In this study, to avoid the drawbacks of a micellar system that consists of single surfactant such as large particle size, low drug capacity, and low stability, a mixed Pluronic micellar system was composed of two types of Pluronic polymers with different HLB values to reach the ideal kinetic and thermodynamic stabilities for the formed micelles. The obtained micellar system could be a promising delivery system for TMXC as one of the most common and cheap anticancer drugs. The main intent of this treatise was to encapsulate TMXC into nano-polymeric micellar delivery system and evaluate the influence of TMXC-loaded micelles as carrier delivery system on the in vitro cytotoxicity and cellular uptake of TMXC in treatment of breast tumors.
MATERIALS AND METHODS
Materials
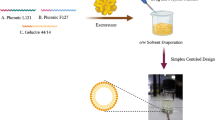
Tamoxifen citrate (purity 98.9%) was kindly gifted by MUP Pharmaceutical company, Egypt. Pluronic P123® (P123) (M. wt. 5700 g/mol) and Pluronic P84® (P84) (M. wt. 4200 g/mol) were supplied as a gift from BASF Corporation (Ludwigshafen, Germany) without further purification. Pluronic F127 (M. wt. 12,600 g/mol) was purchased from Sigma-Aldrich (St. Louis, USA). Methanol (HPLC grade) was purchased from Sigma-Aldrich, Germany. MCF-7 cell line was obtained as a gift from the National Cancer Institute (NCI), Egypt. RPMI1640 cell line culture media was purchased from Lonza Bioscience (Biological products company) (Morristown, NJ 07960, USA). The remaining used chemicals were of high analytical grade and were consumed in experimental work as supplied from vendors without any further purification.
Equilibrium Solubility of TMXC
Equilibrium solubility of TMXC in water and solutions of Pluronic polymers was carried out according to shake flask method (19). Briefly, a known excess of TMXC was added to 10 ml of highly purified water and the aqueous solutions of P84, P123, and P127 that were prepared with different concentrations (2, 4, 6, 8, and 10%). The obtained dispersions were stirred at 37°C for 72 h followed by filtration using a 0.45-μm Millipore syringe filter to remove excess un-dissolved TMXC. The filtrates were diluted with deionized water and examined by a UV spectrophotometer at 277 nm.
Fabrication of TMXC-Loaded Polymeric Micelles
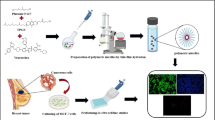
TMXC-loaded polymeric micelles were fabricated by conventional thin-film hydration method that was mentioned earlier (20) with little modifications. In brief, TMXC and Pluronic polymers were dissolved in methanol with weight ratios of 1 for TMXC to 10, 20, 30, 40, and 50 for Pluronic. Under low pressure in a rotary vacuum evaporator set, the solvent was withdrawn (IKA, HBIO basic, RV10B S99, Deutschland, Germany) at 50°C at 200 rpm, until the intended thin film of drug-loaded polymer appeared. This thin film was kept overnight at room temperature to remove solvent residues and totally dried. The film was shaken for 30 min at 50°C with certain volume of deionized water. The obtained TMXC-loaded micellar dispersion was filtered through a 0.22-μm cellulose acetate membrane followed by lyophilization using 5% mannitol as cryoprotectant.
In Vitro Characterization of TMXC-Loaded Micelles
Entrapment Efficiency %
Free un-entrapped TMXC was isolated by a dynamic dialysis method for calculation of entrapment efficiency (EE%) (21). The micellar dispersion was kept in a dialysis tube (MWCO: 12–14 kDa, Livingstone, NSW, Australia) and totally dialyzed for 30 min for numerous times, each time of dialysis was achieved in 100 ml of deionized water. The dialysis process was carried out until no further drug could be detected in the solution, which was confirmed by last run of dialysis cycle for more 0.5 h after zero content of free TMXC. The remaining dialyzed dispersion including TMXC-loaded micelles was collected, diluted with anhydrous methanol, and investigated for TMXC content via UV at λmax 277 nm after suitable dilution with methanol. Each experiment was carried out three times and the average value was deduced.
Determination of Critical Micelle Concentration (CMC)
The CMC of individual polymers (P84 and P123) as well as mixed polymeric micelles formulae that were prepared from (P123 and P84) with different ratios (20:80, 40:60, 50:50, 60:40, 80:20, and 90:10 w/w) in deionized water was examined by UV-vis spectroscopy at 366 nm using a hydrophobic probe of iodine (22). Firstly, a mixture of 0.5 g I2 was dissolved with 1.0 g of KI in 50 ml of deionized water to prepare the standard solution of KI/I2. Different concentrations of individual and mixed polymers in the range of 0.00001–0.1% were mixed with 25 μl of standard solution. The previous mixtures were kept in darkness for 12 h at ambient temperature, and then, the absorbance was investigated at 366 nm. Each experiment was repeated in triplicate and the average of absorbance values was plotted versus the logarithm of polymer concentration. The obtained plot revealed CMC for each polymer.
Particle Size and Zeta Potential
The particle size and zeta potential were examined, and size distribution was measured and expressed as polydispersity index (PDI) using dynamic light scattering integrated in a Zetasizer Nano-ZS (Malvern Instruments/Worcestershire, UK). At room temperature, three separate measurements were taken for each sample from the non-invasive back scatter technique where the detector sets at 173° (23).
Morphology of TMXC-Loaded Micelles
The morphology of formulated TMXC-loaded micelle dispersion (PM6) was investigated with transmission electron microscopy (TEM) and atomic force microscopy (AFM).
Transmission Electron Microscopy
A droplet of TMXC-loaded micellar dispersion (PM6) was placed on a carbon-coated copper grid mesh (300-mesh), and the excess fluid was expelled by an absorbent filter paper. The sample was stained with previously prepared solution of 1% sodium phosphotungstate solution, the excess dye was removed by filter paper, and sample was dried for 1–2 min. Using a Tecani-G20 microscope (FEI, The Netherlands) equipped with super twin lens (a LaB6 electron source/60 kV), the stained sample was probed and visualized.
Atomic Force Microscope
The 3D surface image for TMXC-loaded micellar dispersion was visualized by AFM under normal atmospheric conditions. One drop of the sample dispersion was loaded on a silicon wafer and dried by air. The analysis was carried out using high-resonant-frequency pyramidal cantilevers with silicon probe. The cantilever had a nominal force constant of 0.35–6.06 N/m with a scan speed of 2 Hz. The AFM images were examined using non-contact mode software.
Fourier Transform Infrared Spectroscopy
IR spectra of free TMXC, individual polymers (P84 and P123), blank micelle, and TMXC-loaded micelles (PM6) were determined using Fourier transform infrared spectroscopy by the KBr disc technique. The freeze-dried samples were mixed with a highly dried KBr powder with ratio of 1:9 and pressed to transparent discs. The obtained spectra were located in the spectral region 4000–400 cm−1.
Differential Scanning Calorimetry (DSC)
Thermal properties of weighed samples ranged from 3.5 to 5 mg of free TMXC, individual polymers (P84 and P123), mannitol, blank micelle, and TMXC-loaded micelle (PM6) were characterized using a differential scanning calorimeter. The experiment was achieved under 25 ml/min flow rate of nitrogen gas to avoid sample damage through oxidation and 10°C/min heating rate. All samples were treated with the same scenario, whereas heating profile from room temperature to 200°C. Then, samples were cooled down to 25°C in aluminum pan that was utilized as a reference.
In Vitro Release of TMXC from Polymeric Micelle
In vitro release study of TMXC from the polymeric micelles was performed using a dialysis bag method in phosphate-buffered saline (pH = 7.4) solution as the release medium containing 0.1% Tween 80 (24). The dialysis tube (MWCO: 12–14 kDa, Livingstone, NSW, Australia) was steeped in the release medium overnight. Micellar dispersion equivalent to 3 mg TMXC was introduced into the bag which tied at both ends and sunken into 40 ml release medium at 37°C ± 0.5°C with stirring rate at 100 rpm with a small magnetic stirring bar (25). At predetermined time intervals of 0.5, 1, 1.5, 2, 4, 6, 8, 12, 24, and 36 h, 1 ml of the release medium was withdrawn and replaced immediately with fresh medium. The amount of released drug was analyzed spectrophotometrically at λmax 279.5 nm. For comparison, the release of TMXC from methanolic solution was performed under the same conditions. The released drugs from micelles and free TMXC formulations were both done in triplicate. The release data were suited to the release kinetic models (zero-order, first-order, and Higuchi diffusion equations) to determine the release kinetics. Correlation coefficient values were compared for selection of the most appropriate release model that is best fits the data (26).
Effect of Storage on Stability of TMXC-Loaded Micelles
The lyophilized TMXC-loaded micelle derived from the optimal formula (PM6) was kept at 25°C for 3 and 6 months. Samples of lyophilized powder (0.5 g) were redispersed in deionized water (10 ml) and the obtained dispersions were evaluated for time-dependent changes in entrapment efficiency, particle size, and zeta potential during the storage period (27).
In Vitro Cytotoxicity and Cellular Uptake Studies
Cell Line
Human breast cancer cell line (MCF-7) was incubated in T-75 tissue culture surface treated flasks using suitable culture media of RPMI 1640 that was supplemented with 10% heat inactivated fetal bovine serum (FBS) and antibiotics of penicillin/streptomycin mixture (0.1 mg/ml). Cells were grown in a 5% CO2 incubator at 37°C and 95% air. Cells were observed for appropriate exponential growth phase (sub-confluence) by sub-culturing the cells once reached 70–80% confluence with fresh media (28).
In Vitro Cytotoxicity Assessment
In vitro cytotoxicity of blank micelles and TMXC-loaded polymeric micelle (PM6) was carried out compared to free TMXC solution by sulforhodamine B (SRB) assay (29,30). Briefly, aliquots of 100 μl cell suspension (5 × 103 cells) were seeded in 96-well plates and incubated in growth media for 24 h.
After incubation, 200 μl of different concentrations ranged from 1 to 20 μg/ml of blank micelles, TMXC-loaded polymeric micelles (PM6), and free TMXC solution was added to the attached cells and incubated for 72 h at 37°C. Then, 150 μl of 10% trichloroacetic acid (TCA) was added to the cells for fixation and incubated for 1 h at 4°C. TCA solution was withdrawn, and the cells were washed with distilled water five times. The washed cells were incubated with SRB solution (70 μl, 0.4% w/v) in a dark place for 10 min at 25°C. The plates were washed here times with acetic acid and dried overnight. Thereafter, the protein-bound SRB was dissolved in 150 μl of 10 mM TRIS buffer and the absorbance was analyzed at 540 nm using a BMG LABTECH®- FLUO star Omega microplate reader (Ortenberg, Germany). Finally, the cell viability percentage was estimated from the following equation.
where OD is the optical density of the treated and control cells. From the equation, the concentration of drug that causes 50% of cell death (IC50) was calculated by the GraphPad Prism Software (version 8.02, USA). Each experiment was done on 3 wells.
Cellular Uptake Study
MCF-7 cancers cells were planted in 96-well plates, at cell density of 5 × 103 cells/well in 200 μl of RPMI 1640 culture medium and kept for 24 h for surface attachment prior to the starting of experiments (31). After adding of free TMXC (5 μg/ml) and an equivalent amount of TMXC-loaded micelles, cells were incubated in at 37°C under 5% CO2. Untreated cell with the same density was used as negative control. After 3- and 6-h incubation, the growth media was withdrawn and the cells washed with phosphate-buffered saline (PBS, pH 7.4) three times (32). The collected media and washing PBS were examined for TMXC concentration using LC/MS/MS (Agilent Technologies, Inc., Santa Clara, CA). The calculated decrease in TMXC /TMXC micelle concentration was utilized to investigate the cellular uptake using the following equation:
where C0 is the initial concentration of TMXC added and ECt is the extracellular concentration of TMXC at time = t.
Liquid Chromatography-Mass Spectroscopy (LC-MS) Assay of TMXC
The collected media and washing PBS were examined for TMXC concentration using an LC Agilent 1200 series LC/MS/MS system, and an Agilent 6410 Quadrupole mass spectrometer (Agilent Technologies, Inc., Santa Clara, CA) with Hypersil gold C18 column (50 mm × 4.6 mm, 5 μm) and mobile phase mixture of 0.06% formic acid in water and acetonitrile mixed with the ratio of (1:1) were used (33).
Calibration Curve of TMXC
TMXC standard calibration curve was constructed according to previously reported method (34) with some modifications. RPMI 1640 media was spiked with standard acetonitrile solution of TMXC to obtain a final concentration of 50,150, 250, 500, 1000, 2000, and 4000 ng/ml. To a volume of 0.2 ml of cell culture media samples, 20 μl of internal standard (clomiphene 5 μg/ml) was added; then, samples were vigorously shaken with vortex for 30 s. A liquid-liquid extraction was performed to the samples by addition of 2.5 ml of extracting organic solvent mixture (diethyl ether-dichloromethane) after buffering the samples to pH = 11 and vortex mixing for 2 min at an optimal speed of 2500 rpm. In a cooling centrifuge (Hermle Z 326 K, Hermle Labortechnik GmbH, Wehingen, Germany), samples were centrifuged at 4°C at 3800 rpm for 8 min, and clear supernatant organic layer was transferred and placed in a concentrator (Vacufuge® Plus, Eppendorf, Germany) at 45°C for 20 min to evaporate until dryness. The dry residue resulted from evaporation was reconstituted with 200 μl of reconstitution solvent (water:acetonitrile 50:50 v/v). A volume of 5 μ was injected into an LC Agilent 1200 series LC/MS/MS system and an Agilent 6410 Quadrupole mass spectrometer (Agilent Technologies, Inc., Santa Clara, CA). The calibration curve was constructed by plotting TMXC concentration (ng/ml) versus peak area. All assays were performed in triplicates (34).
Statistical Analysis of Collected Data
Data scrutiny was performed using GraphPad InStat 3 program. Results were expressed as a mean ± standard deviation. Statistically significant difference was determined using one-way ANOVA test and paired and un-paired Student t test with P < 0.05 as a minimal level of significance.
RESULTS AND DISCUSSION
Saturation Solubility of TMXC
Saturation solubility of TMXC in water and solutions of different concentrations of Pluronic polymers (F127, P84, and P123) was examined by UV-visible spectroscopy. The saturation solubility of TMXC in water was 0.45 mg/ml indicating its poor aqueous solubility (35). The solubility of TMXC markedly increased in solutions of different concentrations of Pluronic polymers (Fig. 1). It is obvious that the amount of TMXC solubilized in P123 micelles was higher than that of the other two Pluronic polymers and the solubility modality of TMXC follows the order P123 > P84 > F127. This result is in a good agreement with previous reports (36,37). The higher solubilization effect of P123 compared to that of F127 and P84 could be assigned to its hydrophobic nature (HLB = 8) which permits the transfer of TMXC into the core zone of micelles. However, thickness of the hydrophilic PEO corona region of F127 and P84 (HLB = 14 and 22 respectively) acts as a steric hindrance and retards the solubilization of drug into the core of micelles (38). The results of solubility study revealed a direct relationship between Pluronic polymer concentration and TMXC solubility. According, Pluronic polymer concentration of 10 mg/ml was utilized as an optimal concentration for preparation of TMXC-loaded micelles.
Preparation of TMXC-Loaded Polymeric Micelles
A micellar system that consists of single surfactant poloxamer is known to have many drawbacks such as large particle size, low drug capacity, and low stability, which can be improved by mixing of different poloxamers to produce mixed micellar systems (39). Therefore, the system was formulated by using two types of Pluronic polymers with different HLB values to reach the ideal kinetic and thermodynamic stabilities for the formed micelles. Accordingly, six polymeric micelles formulae (PM1–PM6) composed of P123 and P84 were fabricated by the abovementioned method with different percentages of P123 (20, 40, 50, 60, 80, and 90%) with total Pluronic polymer concentration of 10% (Table I).
In Vitro Characterization of TMXC-Loaded Micelles
TMXC Saturation Solubility in Mixed Micelles
The results of saturation solubility of TMXC in mixed micelles (Table I) revealed that the formulae containing higher percentage of P123 exhibited higher solubilization of TMXC in the mixed micelles. Previous studies (40,41) indicated that mixtures of Pluronic polymers are good solubilizing agents of hydrophobic drugs. Moreover, mixed micelles with higher ratio of hydrophobic Pluronic polymer are expected to increase in the entrapment efficiency of lipophilic drugs into the micelles.
Entrapment Efficiency (EE%)
The EE% for the prepared TMXC-loaded micelles extended between 60.62 ± 3.1 and 89.09 ± 4.22% as represented in Table I. These results indicate that TMXC as a hydrophobic drug was successfully entrapped into the pulp cavity of mixed micelles. The results also revealed that an observed increase in TMXC EE% was associated with increasing concentration of P123 as indication for the higher compatibility of TMXC with the core of the prepared micellar mixture. This harmony is expected due to the existence of high proportion of P123. These results depend on the hydrophobic nature of P123 and presence of long PPO as well as short PEO chains in combination with P84 is a hydrophilic polymer. This mixture provides a reasonable compatibility and stability for the loaded drug inside the core-forming block (42).
Determination of Critical Micelle Concentration (CMC)
The determined values of CMC for pure P123 and P84 were 0.00162% and 0.0407% (Fig. 2, a and b) respectively. The obtained data were in agreement with that previously reported in the literature (43,44). While the CMC values of P123 and P84 mixtures were ranged from 0.0354 to 0.00295% wt for PMS 1 to PMS 6 respectively. The obtained results indicated that the values of CMC decreased gradually as the percentage of P123 in the mixture increased. The lowest value of CMC (0.00295% wt) was obtained by using a mixture of P123:P84 (90:10 w/w, PM6) (Fig. 2c). The results showed that CMC value of PM6 was closely similar to CMC value of pure P123 due to the higher content (90%) of P123 in PM6 formula which is convenient with the previous reported results by Fares et al. (45). The relatively low CMC values of the mixed micelles could be a reason for maintaining the integrity of micelles at very low polymer concentrations, bringing to these mixtures promising pharmacological applications even upon dilution in the blood (39,46).
Particle Size, Zeta Potential, and Size Distribution
The average size of mixed micelles ranged from 7.66 to 16.63 nm as expressed in Table II. It was well recognized that nanoparticles with size range between 10 and 100 nm could show an ideal cellular uptake in both epithelial and smooth muscle cells (47). Moreover, TMXC-loaded micelle exhibits specific accumulation with tumor tissue through passive targeting and response to the known phenomenon of enhanced permeability and retention (EPR) effect. This phenomenon revealed that the nano-sized particle with diameter exceeding than 6 nm is expected to escape kidney filtration and detection by liver or phagocytosed by RES. This allows for retention of our particles in circulation for longer period with better accumulation in the tumor due to EPR effect (48). The fabricated micelles had PDI values extended from 0.158 to 0.262 (Table II) which indicates narrow size distribution and excellent sample size homogeneity.
All fabricated formulae had zeta potential extended from 4.769 to 15.9 mV (Table II). The relatively low zeta potential values of the fabricated micelle might be assigned to the external rotate slipping plane that initiated from thick absorbance of polymer layers on the surface. It was confirmed that the hydrophobic PPO copolymer triblock reclined on the hydrophobic surface which resulted in extending of hydrophilic PEO chains to the aqueous phase of dispersion. This arrangement leads to formation of a brush modulation. According to Gouy-Chapman theory, the slipping plane exhibits outward shift where the surface potential is smaller than on the surface resulting in lower zeta potential (49).
MORPHOLOGICAL STUDIES
Transmission Electron Microscopy (TEM)
A representative TEM photomicrograph of TMXC-loaded micelles (PM6) is illustrated in Fig. 3 a. The selected micelle formula (PM6) was properly dispersed in aqueous media to give homogeneous small-sized spherical forms with a narrow size distribution.
Atomic Force Microscope (AFM)
The morphology of TMXC-loaded micelles (PM6) was further analyzed using AFM. The 3D topographical image is demonstrated in Fig. 3 b. The obtained micrograph revealed spherical particles with size correlated with TEM, and dynamic light scattering technique.
Fourier Transform Infrared Spectroscopy (FT-IR)
FT-IR spectra of free TMXC, P84, P123, non-medicated mixed micelles, and TMXC-loaded micelles are exemplified in Fig. 4. The spectrum of TMXC demonstrates characteristic absorption bands at 3028 cm−1 representing the C–H stretching and 1732 cm−1 corresponding to C=O the stretching bond of citric acid (50). Aliphatic C–H stretching vibrations of PEO segments in P84 and P123 appeared at 3001 cm−1 and 2870 cm−1, while the peak at 1111 cm−1 represented the characteristic C–O–C stretching vibration. The characteristic peaks of TMXC were disappeared in medicated mixed micelles which may indicate for complete encapsulation of drug (51). The FT-IR results suggest that TMXC was molecularly dispersed in the fabricated polymeric micelles and no chemical reaction occurred.
Differential Scanning Calorimetry (DSC)
The DSC thermogram of pure TMXC (Fig. 5) exhibited a sharp endothermic peak at 144.71°C confirming the crystalline nature of TMXC and corresponding to its melting point, while P123 and P84 showed endothermic peaks at 36.79 and 36.86°C, respectively and were corresponding to their melting point. The micellar thermogram displayed complete absence of the drug and Pluronic polymer peaks. Absence of the distinctive endothermic peak of TMXC possibly implies that TMXC was either located inside polymeric micelles core or completely changed to the molecular state (52). The thermogram TMXC-loaded micelles (PM6) displayed the disappearance of the TMXC and Pluronic polymers endothermic peaks, and appearance of a new sharp endothermic peak appeared at 164.26°C, which expected to be corresponding to the melting point of mannitol (53). Mannitol presents as a cryoprotectant during the lyophilization process.
In Vitro Release of TMXC from Micelles
In vitro release study of TMXC from prepared micelles was studied by dialysis with PBS solution containing 0.1% Tween 80 at 37°C as release medium to cover the sink condition. The rate of TMXC release versus time is shown in Fig. 6. The obtained pattern revealed that TMXC release from mixed polymeric micelles was slower than free TMXC. Furthermore, the drug release from micelle showed initial rapid release at first hour, followed by plateau pattern. This initial burst release could be assigned to presence of small amount of the drug loaded in the hydrophilic shell of micelle, whereas the hydration of the superficial molecules of drug and the passive diffusion caused double effect on release pattern (54). However, the drug that was entrapped in the core of micelle was released by diffusion mechanism as well as swelling and erosion of polymer materials (55). These facts together might be responsible for the sustained release pattern in the following phase. The slow sustained release modality of TMXC from micelles is a beneficial targeted result because efficient chemotherapy is required to maintain the drug level within the therapeutic window for longer periods. This result explained that mixed micelle carriers could increase the solubility of TMXC, as well as sustain its release. Kinetic analysis of release data, based on regression coefficient analysis, indicated that Higuchi model was best fitted and diffusion is the prevalent mechanism behind the TMXC release from the fabricated Pluronic micelles (56,57).
Effect of Storage on TMXC-Loaded Micelles
The optimized formula (PM6) kept its physicochemical properties with no observed change in the particle size, zeta potential, or EE% after 3- and 6-month storage at 25°C (Table III).
In Vitro Cytotoxicity and Cellular Uptake
In Vitro Cytotoxicity Assessment
More investigation of the anticancer capability of micelles as suitable carrier for TMXC was evaluated with SRB assay method. Human breast cancer cells (MCF-7) were grown with different concentrations of TMXC (1–20 μg/ml) and equivalent concentration of TMXC-loaded polymeric micelles (PM6) and blank micelles for 72 h. The results revealed that TMXC-loaded micelles had cytotoxic effect against breast cancer cells and were comparable to free TMXC (Fig. 7). In the applied concentration range, IC50 for free TMXC, PM6, and blank micelles were 6.092, 2.980, and 139.6 μg/ml, respectively.
Free TMXC displayed lower cytotoxicity which may be attributed to the efflux defense mechanism of TMXC by the P-gp pumps’ resistance mechanism. However, the selected micelle formulae were engulfed by the cells via endocytosis and may not a target efflux by the P-gp pumps, allowing the TMXC to remain inside the cancer cells for a longer period resulting in higher cytotoxicity. The obtained results were similar to earlier reported results in literature (58). Further, the higher cytotoxicity of micelle mixture may be due to the presence of Pluronic polymers, which were reported as well-known P-gp efflux inhibitors or reducers (59). Moreover, the better cytotoxic effect of the mixed micelle containing TMXC be due to the enhanced cellular uptake of TMXC from the fabricated polymeric micelles (60).
In Vitro Cellular Uptake
Experiment was carried out to investigate the cellular uptake behavior of TMXC-loaded micelles (PM6) and free TMXC solution at different intervals (61). Cellular uptake efficiency of TMXC micelles was determined after incubation with breast cancer cells MCF-7 (Fig. 8). Result exhibited that the cumulative intracellular uptake efficiency of TMXC solution after 3 and 6 h was 18.98% and 38.82% respectively. On the other hand, cellular uptake of selected TMXC-loaded micelles at the same time intervals was 56.33% and 88.87% respectively. The results suggest that the polymeric micelles interfered with the cancer cells in a significant way as essential and well-known feature of nano-size vesicle. This behavior of interaction between cell lines and nanoparticle was usually correlated with non-specific rapid phagocytosis which is responsible for cell internalization (62). Also micelles carried positive charge which enhances cellular uptake with negatively charged cell membrane (63). Finally, it was clear that the cellular uptake of TMXC-loaded micelles was 2.04 folds higher than that of TMXC solution.
CONCLUSIONS
Polymeric micelles containing TMXC were fabricated by thin-film hydration method. Loaded micelles of TMXC were developed by using very low concentration of a mixture of P123 and P84 (90:10 w/w). The relatively low CMC values of micelles could keep their stability even upon great dilution in the blood. The prepared TMXC-loaded polymeric micelles exhibited nano-sized particles and entrapped high percentage of TMXC along with sustained TMXC release compared to TMXC solution. The TMXC-loaded polymeric micelles successfully improved cellular uptake of TMXC and were associated with higher cytotoxicity against breast cancer cells compared to free TMXC. These results revealed that Pluronic micelles could be a promising potential carrier for chemotherapeutic agents in cancer treatment. Further investigation for animal trials is expected to be accomplished in near future as our next step.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ, et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20(8):1319–29.
Tinoco G, Warsch S, Glück S, Avancha K, Montero AJ. Treating breast cancer in the 21st century: emerging biological therapies. J Cancer. 2013;4(2):117–32.
Barnabas N, Cohen D. Phenotypic and molecular characterization of mcf10dcis and sum breast cancer cell lines. Int J Breast Cancer. 2013;2013:1–16.
Seruga B, Ocana A, Tannock IF. Drug resistance in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol. 2011;8(1):12–23.
Lagadec C, Adriaenssens E, Toillon RA, Chopin V, Romon R, Van Coppenolle F, et al. Tamoxifen and TRAIL synergistically induce apoptosis in breast cancer cells. Oncogene. 2008;27(10):1472–7.
Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–71.
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–60.
Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65(1):71–9.
Pellosi DS, Moret F, Fraix A, Marino N, Maiolino S, Gaio E, et al. Pluronic® P123/F127 mixed micelles delivering sorafenib and its combination with verteporfin in cancer cells. Int J Nanomedicine. 2016;11:4479.
Batrakova EV, Kabanov AV. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release. 2008;130(2):98–106.
Mandal A, Bisht R, Rupenthal ID, Mitra AK. Polymeric micelles for ocular drug delivery: from structural frameworks to recent preclinical studies. J Control Release. 2017;248:96–116.
Li Z, Ye E, Lakshminarayanan R, Loh XJ. Recent advances of using hybrid nanocarriers in remotely controlled therapeutic delivery. Small. 2016;12(35):4782–806.
Kedar U, Phutane P, Shidhaye S, Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine. 2010;6(6):714–29.
Kabanov AV, Batrakova EV, Alakhov VY. Pluronic® block copolymers for overcoming drug resistance in cancer. Adv Drug Deliv Rev. 2002;54(5):759–79.
ten Tije AJ, Verweij J, Loos WJ, Sparreboom A. Pharmacological effects of formulation vehicles: implications for cancer chemotherapy. Clin Pharmacokinet. 2003;42(7):665–85.
Aliabadi HM, Lavasanifar A. Polymeric micelles for drug delivery. Expert Opin Drug Deliv. 2006;3(1):139–62.
Fan Z, Chen C, Pang X, Yu Z, Qi Y, Chen X, et al. Adding vitamin E-TPGS to the formulation of Genexol-PM: specially mixed micelles improve drug-loading ability and cytotoxicity against multidrug-resistant tumors significantly. PloS One. 2015;10(4).
Dutra LM, Ribeiro ME, Cavalcante IM, Brito DH, Semião LD, Silva RF, et al. Binary mixture micellar systems of F127 and P123 for griseofulvin solubilisation. Polímeros. 2015;25(5):433–9.
Saxena V, Hussain MD. Polymeric mixed micelles for delivery of curcumin to multidrug resistant ovarian cancer. J Biomed Nanotechnol. 2013;9(7):1146–54.
Nasr M, Ghorab MK, Abdelazem A. In vitro and in vivo evaluation of cubosomes containing 5-fluorouracil for liver targeting. Acta Pharm Sin B. 2015;5(1):79–88.
Wei Z, Hao J, Yuan S, Li Y, Juan W, Sha X, et al. Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: formulation, optimization and in vitro characterization. Int J Pharm. 2009;376(1–2):176–85.
Patra A, Satpathy S, Shenoy AK, Bush JA, Kazi M, Hussain MD. Formulation and evaluation of mixed polymeric micelles of quercetin for treatment of breast, ovarian, and multidrug resistant cancers. Int J Nanomedicine. 2018;13:2869–81.
Liu JS, Wang JH, Zhou J, Tang XH, Xu L, Shen T, et al. Enhanced brain delivery of lamotrigine with Pluronic® P123-based nanocarrier. Int J Nanomedicine. 2014;9:3923.
Shaker DS, Shaker MA, Hanafy MS. Cellular uptake, cytotoxicity and in-vivo evaluation of Tamoxifen citrate loaded niosomes. Int J Pharm. 2015;493(1–2):285–94.
Higuchi WI. Analysis of data on the medicament release from ointments. J Pharm Sci. 1962;51(8):802–4.
Bao Y, Li S, Liu L, Luan Y, Lin G, Shao W. Carmofur-loaded Pluronic P123 polymeric micelles: preparation and characterization. J Dispers Sci Technol. 2012;33(4):617–21.
Perillo B, Sasso A, Abbondanza C, Palumbo G. 17β-Estradiol inhibits apoptosis in MCF-7 cells, inducing bcl-2 expression via two estrogen-responsive elements present in the coding sequence. Mol Cell Biol. 2000;20(8):2890–901.
Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1(3):1112–6.
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82(13):1107–12.
Cosco D, Paolino D, Cilurzo F, Casale F, Fresta M. Gemcitabine and tamoxifen-loaded liposomes as multidrug carriers for the treatment of breast cancer diseases. Int J Pharm. 2012;422(1–2):229–37.
Ashidi JS, Houghton PJ, Hylands PJ, Efferth T. Ethnobotanical survey and cytotoxicity testing of plants of South-western Nigeria used to treat cancer, with isolation of cytotoxic constituents from Cajanus cajan Millsp. leaves. J Ethnopharmacol. 2010;128(2):501–12.
Dahmane E, Mercier T, Zanolari B, Cruchon S, Guignard N, Buclin T, et al. An ultra performance liquid chromatography–tandem MS assay for tamoxifen metabolites profiling in plasma: first evidence of 4′-hydroxylated metabolites in breast cancer patients. J Chromatogr B. 2010;878(32):3402–14.
Alam MA, Ahmad N, Ahmad R, Talegaonkar S, Ahmad FJ, Iqbal Z, et al. Quantification of tamoxifen polymeric nanoparticles in female rodent breast tissue by UPLC/ESI-Q-TOF MS/MS. J Young Pharm. 2016;8(4):415–23.
SreeHarsha N, Hiremath JG, Chilukuri S, Aitha RK, Al-Dhubiab BE, Venugopala KN, et al. An approach to enhance dissolution rate of tamoxifen citrate. BioMed Res Int. 2019.
Jindal N, Mehta SK. Nevirapine loaded Poloxamer 407/Pluronic P123 mixed micelles: optimization of formulation and in vitro evaluation. Colloids Surf B: Biointerfaces. 2015;129:100–6.
Kadam Y, Yerramilli U, Bahadur A. Solubilization of poorly water-soluble drug carbamezapine in Pluronic® micelles: effect of molecular characteristics, temperature and added salt on the solubilizing capacity. Colloids Surf B: Biointerfaces. 2009;72(1):141–7.
Turco Liveri ML, Licciardi M, Sciascia L, Giammona G, Cavallaro G. Peculiar mechanism of solubilization of a sparingly water soluble drug into polymeric micelles. Kinetic and equilibrium studies. J Phys Chem B. 2012;116(16):5037–46.
Attia AB, Ong ZY, Hedrick JL, Lee PP, Ee PL, Hammond PT, et al. Mixed micelles self-assembled from block copolymers for drug delivery. Curr Opin Colloid Interface Sci. 2011;16(3):182–94.
Lee ES, Oh YT, Youn YS, Nam M, Park B, Yun J, et al. Binary mixing of micelles using Pluronics for a nano-sized drug delivery system. Colloids Surf B: Biointerfaces. 2011;82(1):190–5.
Kulthe SS, Inamdar NN, Choudhari YM, Shirolikar SM, Borde LC, Mourya VK. Mixed micelle formation with hydrophobic and hydrophilic Pluronic block copolymers: implications for controlled and targeted drug delivery. Colloids Surf B: Biointerfaces. 2011;88(2):691–6.
Allen C, Maysinger D, Eisenberg A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf B: Biointerfaces. 1999;16(1–4):3–27.
Zhang M, Djabourov M, Bourgaux C, Bouchemal K. Nanostructured fluids from Pluronic® mixtures. Int J Pharm. 2013;454(2):599–610.
Alexandridis P, Hatton TA. Poly (ethylene oxide)/poly (propylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: thermodynamics, structure, dynamics, and modeling. Colloids Surf A Physicochem Eng Asp. 1995;96(1–2):1–46.
Fares AR, ElMeshad AN, Kassem MA. Enhancement of dissolution and oral bioavailability of lacidipine via Pluronic P123/F127 mixed polymeric micelles: formulation, optimization using central composite design and in vivo bioavailability study. Drug Delivery. 2018;25(1):132–42.
Attwood D, Booth C, Yeates SG, Chaibundit C, Ricardo NM. Block copolymers for drug solubilisation: relative hydrophobicities of polyether and polyester micelle-core-forming blocks. Int J Pharm. 2007;345(1–2):35–41.
Feng SS, Mei L, Anitha P, Gan CW, Zhou W. Poly (lactide)–vitamin E derivative/montmorillonite nanoparticle formulations for the oral delivery of Docetaxel. Biomaterials. 2009;30(19):3297–306.
Longmire MR, Ogawa M, Choyke PL, Kobayashi H. Biologically optimized nanosized molecules and particles: more than just size. Bioconjug Chem. 2011;22(6):993–1000.
Abdelbary AA, Al-Mahallawi AM, Abdelrahim ME, Ali AM. Preparation, optimization, and in vitro simulated inhalation delivery of carvedilol nanoparticles loaded on a coarse carrier intended for pulmonary administration. Int J Nanomedicine. 2015;10:6339.
Kojima T, Kato F, Teraoka R, Matsuda Y, Kitagawa S, Tsuhako M. Physicochemical characterization of tamoxifen citrate pseudopolymorphs, methanolate and ethanolate. Chem Pharm Bull. 2007;55(3):407–11.
Mohamed EA, Hashim II, Yusif RM, Shaaban AA, El-Sheakh AR, Hamed MF, et al. Polymeric micelles for potentiated antiulcer and anticancer activities of naringin. Int J Nanomedicine. 2018;13:1009–27.
Basalious EB, Shamma RN. Novel self-assembled nano-tubular mixed micelles of Pluronics P123, Pluronic F127 and phosphatidylcholine for oral delivery of nimodipine: in vitro characterization, ex vivo transport and in vivo pharmacokinetic studies. Int J Pharm. 2015;493(1–2):347–56.
Elsayed I, Abdelbary AA, Elshafeey AH. Nanosizing of a poorly soluble drug: technique optimization, factorial analysis, and pharmacokinetic study in healthy human volunteers. Int J Nanomedicine. 2014;9:2943.
Gao Y, Li LB, Zhai G. Preparation and characterization of Pluronic/TPGS mixed micelles for solubilization of camptothecin. Colloids Surf B: Biointerfaces. 2008;64(2):194–9.
Kim KS, Park SJ. Effect of porous silica on sustained release behaviors of pH sensitive Pluronic F127/poly (acrylic acid) hydrogels containing tulobuterol. Colloids Surf B: Biointerfaces. 2010;80(2):240–6.
Wei Z, Yuan S, Hao J, Fang X. Mechanism of inhibition of P-glycoprotein mediated efflux by Pluronic P123/F127 block copolymers: relationship between copolymer concentration and inhibitory activity. Eur J Pharm Biopharm. 2013;83(2):266–74.
Sahu A, Kasoju N, Goswami P, Bora U. Encapsulation of curcumin in Pluronic block copolymer micelles for drug delivery applications. J Biomater Appl. 2011;25(6):619–39.
Zhou M, Li L, Li L, Lin X, Wang F, Li Q, et al. Overcoming chemotherapy resistance via simultaneous drug-efflux circumvention and mitochondrial targeting. Acta Pharm Sin B. 2019;9(3):615–25.
Guan Y, Huang J, Zuo L, Xu J, Si L, Qiu J, et al. Effect of Pluronic P123 and F127 block copolymer on P-glycoprotein transport and CYP3A metabolism. Arch Pharm Res. 2011;34(10):1719–28.
Chen LC, Chen YC, Su CY, Hong CS, Ho HO, Sheu MT. Development and characterization of self-assembling lecithin-based mixed polymeric micelles containing quercetin in cancer treatment and an in vivo pharmacokinetic study. Int J Nanomedicine. 2016;11:1557.
Abbasalipourkabir R, Salehzadeh A, Abdullah R. Tamoxifen-loaded solid lipid nanoparticles-induced apoptosis in breast cancer cell lines. J Exp Nanosci. 2016;11(3):161–74.
Wang N, Wang Z, Nie S, Song L, He T, Yang S, et al. Biodegradable polymeric micelles coencapsulating paclitaxel and honokiol: a strategy for breast cancer therapy in vitro and in vivo. Int J Nanomedicine. 2017;12:1499–514.
Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomedicine. 2012;7:5577.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nasr, M., Hashem, F., Abdelmoniem, R. et al. In Vitro Cytotoxicity and Cellular Uptake of Tamoxifen Citrate-Loaded Polymeric Micelles. AAPS PharmSciTech 21, 306 (2020). https://doi.org/10.1208/s12249-020-01850-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01850-6