Abstract
The application of the nanotechnology in medicine and pharmaceutics opens new horizons in therapeutics. Several nanomedicines are in the market and an increasing number is in clinical trials. But which is the advantage of the medicines in nanoscale? The scientists and the regulatory authorities agree that the size and consequently the physiochemical/biological properties of nanomaterials play a key role in their safety and effectiveness. Additionally, all of them agree that a new scientific-based regulatory landscape is required for the establishment of nanomedicines in the market. The aim of this review is to investigate the parameters that the scientists and the regulatory authorities should take into account in order to build up a dynamic regulatory landscape for nanomedicines. For this reason, we propose an “astrolabe-like system” as the guide for establishing the regulatory approval process. Its function is based on the different physicochemical/biological properties in comparison to low molecular weight drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
According to European Medicines Agency (EMA), nanotechnology is “the use of tiny structures less than 1000 nanometres across, which are designed to have specific properties. In medicine, nanotechnology has the potential to open up new possibilities for the improvement of the properties of medicines, such as their solubility or stability, and the development of more efficient ways to deliver medicines and target them accurately in the body.” Furthermore, according to EMA nanotechnology is “the production and application of structures, devices and systems by controlling the shape and size of materials at nanometre scale (range from atomic level at 0.2nm up to around 100nm)” (EMA, 2006).Footnote 1 According to Food and Drug Administration (FDA): “Nanoscale materials often have chemical, physical, or biological properties that are different from those of their larger counterparts. Such differences may include altered magnetic properties, altered electrical or optical activity, increased structural integrity, or altered chemical or biological activity. Because of these properties, nanoscale materials have great potential for use in a vast array of products. Of interest to the Food and Drug Administration (FDA, the agency), nanoscale materials may enable new developments in products to advance public health. Also because of some of their special properties, nanoscale materials may pose different safety issues than their larger or smaller (i.e., molecular) counterparts.” Non-biological complex drugs (NBCDs) are medical compounds that cannot be defined as small molecule active pharmaceutical ingredients and are not biologicals (i.e., highly complex biomacromolecules). For this reason, they cannot be defined as biologicals or Active Pharmaceutical Ingredients (APIs). NBCDs are synthetic complex biomaterials and they contain nanoparticulate systems.
THE DEFINITION OF NANOMEDICINE
Moreover, EMA defines nanomedicine as “the application of nanotechnology in view of making a medical diagnosis or treating or preventing diseases” (EMA, 2006). In other words, nanomedicine is applied to medicinal products, which use nanomaterial and/or nanotechnology for their development and manufacturing (1). Nanomedicines are designed to provide biological and physicochemical properties attributed to their size and surface morphology. Their main difference from low molecular weight drugs is the improvement of drug delivery by controlling the drug release on a specific site for better efficacy and safety and improving the drug transport across biological barriers (uptake) (2).
According to the preliminary risk analysis on the basis of a workshop organized by European commission (in Brussels on 1–2 March 2004 by the health and consumer protection directorate general of the European Commission), the scientists highlighted the potentially hazardous nature of some free, engineered NPs—the most significant one relating to nanotechnologies. In that document, the main concerns regarding the development of nanoproducts and nanomaterials are the toxicology, the ecotogicology, the ethics, and the security. Detailed explanation was given during the workshop, which became the basis in EU and globally for the safe design and development of engineered nanoparticles. Additionally, the cost of organizing a new production plant based on nanotechnology-related procedures is another limitation/risk. On the other hand, the benefits of the design and the development of nanoproducts are numerous for the customers. In the field of pharmaceutics, the nanodrugs exhibit several advantages such as lower dose of toxic drugs, ameliorated pharmacokinetics, and controlled release properties.
The most common types of nanomedicines are the liposomes, nanocrystals, emulsions, and iron-carbohydrate complexes as shown in Table I. Here, the launched intravenously administered (known as parenteral) nanomedicines, focusing more on liposomes are summarized and discussed below; for all the other categories, only the nanosimilar nanomedicines were selected (2). These nanomedicines have been used therapeutically for decades since 1949 for applications such as cancer therapy, inflammatory diseases, infections, and anemia. These nanomedicines are also referred to as synthetic NBCDs. NBCDs consist of nanostructures that cannot be fully characterized and quantified by physicochemical analytical techniques; thus, there is a vast need for a well-controlled robust manufacturing process to ensure quality, safety, and efficacy (3). According to Crommelin et al. NBCD is “A medicinal product, not being a biological medicine, where the active substance is not a homo-molecular structure, but consists of different (closely related and often nanoparticulate structures that can’t be isolated and fully quantitated, characterized, and/or described by physicochemical analytical means. It is also unknown which structural elements might impact the therapeutic performance. The composition, quality, and in vivo performance of NBCD are highly dependent on the manufacturing processes of both the active ingredient as well as the formulation. Examples of NBCD are, amongst others, liposomes, iron–carbohydrate (‘iron–sugar’) drugs, and glatiramoids.” The physicochemical properties of both NBCD and BCD are not fully characterized (3,4,5).
THE FIRST APPROVED NANOMEDICINE: LESSONS LEARNED
Liposomes are lipid drug delivery systems structurally similar to biological membranes. Doxil® was the first nanomedicine approved by FDA (4). According to Barenzohz, this liposomal doxorubicin is a NBCD with stealth properties due to PEGylated nano-liposomes that can avoid the complexation with serum proteins; high loading efficiency using the pH gradient protocol which is well established in the literature and “liquid-ordered” phase liposomal bilayer composed of the high-T(m) (53°C) phosphatidylcholine, and cholesterol (4). These physicochemical and technological characteristics play a key role in the efficacy and safety of the liposomal doxorubicin. LipoDox® is the first liposomal generic nanomedicine that has been approved by the FDA and has been classified as the generic version of Doxil® (Doxil®’s patent expired in the USA in 2010). According to Crommelin et al. “Generic Medicinal Product is a drug product that is comparable to a reference-listed drug product in dosage form, strength, route of administration, quality and performance characteristics, and intended use” (5). The main question is if the generic medicinal products can define the generic nanomedicinal products, i.e., nanosimilars. Nanosimilars (called in EU, a term that is not used by the FDA, there are recognized more as follow-on versions) are the next-generation of off-patent nanotechnological products, and their similarity is considered as an emerging issue to be discussed within the scientific community and moreover in the regulatory agencies which are responsible for their approval. There are over 50 nanomedicines in clinical development, and the two major western regulatory bodies (EMA-EU and FDA-US) are addressing the complexity of these products (nanomedicines and their nanosimilars) with different evaluation and authorization approaches. A harmonized regulatory evaluation pathway to evaluate and authorized a nanosimilar is a great challenge among the stakeholders, regulatory bodies, and experts in the field.Footnote 2 The access of the nanosimilars to a larger population with reduced treatment cost (patient benefit) and innovation in drug development to cover unmet therapeutic needs (scientific and technological progress) are the two main challenges. An increasing number of manuscripts is appearing in the literature and request further studies about the equivalence between the reference (nanomedicines) and nanosimilars product (6,7,8,9,10). Special attention is given to the physicochemical characteristics, especially to the size and the size distribution of nanomedicinal products. The experts exploring nanomedicines still find difficulties in identifying the critical quality attributes (CQAs) responsible for the quality, safety, and efficacy of these products for the patient. Attributes such as particle concentration, morphology and size, surface properties (area, charge, hydrophobicity, reactivity), function, coating properties, porosity, in vitro release, impurities, endotoxin levels, and sterility are the properties that affect the pharmacokinetic and pharmacodynamics profile of the products (11–12). Di Francesco and Borchard established a simple and reproducible dynamic light scattering protocol to unequivocally define the size and size distribution of iron sucrose (which is a nano-colloidal solution used in the treatment of iron deficiency anemia) by using size distribution approximation in [13]. Additionally, there are some papers in the literature trying to study comparatively the physicochemical and morphological characteristics of the PEGylated liposomal doxorubicin nanomedicines (14–15). According to Schilt et al., these liposomal products were found to be structural similar when they were investigated by using small-angle X-ray scattering (SAXS) (14). On the other hand, Wibroe et al. proposed that cryo-TEM should be considered and introduced by the regulatory agencies as part of the physicochemical and morphological characterization portfolio of liposomal nanomedicines (15). The differences between the marketed liposomal products are summarized in (15).
Surface charge and zeta potential is also very crucial parameters for the physicochemical characterization of nanomedicines (16). The chemical properties of the product are also an important factor. For instance, the drug undergoes self-degradation without altering the nanoformulation physical properties (size, zeta, appearance). For this reason, the encapsulation of active substances into nanoparticles protects them from oxidation and degradation in the “unfriendly” environment of the human body. At a European level, there are funded projects under the framework of European Union Seventh Framework Programme (FP7/2007-2013) and HORIZON 2020 Programme in risk management approaches for manufactured nanomaterials (MNMs) and products containing MNMs. In particular, NANoREG (NaNoREG is a common European approach to the regulatory testing of manufactured nanomaterials. This project has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 310584.) aimed in establishing a common European approach to the regulatory testing of MNMs; and ProSafe focused in promoting the implementation of Safe by Design and evaluating the results of a wide range of EU projects on Environment, Health and Safety (EHS) research in the field of nanotechnology (under grant agreement no. 646325. The “common ground” in all these projects is the involvement of the three main stakeholders (Regulation, Industry, and Science) to significantly contribute to reducing the risks from MNMs in industrial and consumer products. Furthermore, NANoREG focused on producing a toolbox of relevant instruments for risk assessment, characterization, toxicity testing, and exposure measurements of MNMs. The interface between any type of nanomaterial with the surrounding environment either proteins/cells in a culture medium or bound to a matrix/composite or in a solvent depends on colloidal forces, as well as dynamic bio-physicochemical interactions. The development of predictive relationships between structure of nanomaterials and activity (functionality) is determined by nanomaterial properties. The key parameters of size, shape, composition, surface charge, aggregation, and test medium are the priority parameters affecting all the functionalities, while the rest of parameters are of importance (NANoREG Deliverable 6.06). It was also reported in NANoREG a list of recommendations from the Committee for Medicinal Products for Human Use (CHMP) (expert group on nanomedicines since 2009) leading to the approval of a number of medicines based on nanotechnology. These nanomedicines were the liposomes, such as Caelyx (doxorubicin) with its nanosimilar in the USA, Mepact (mifamurtide) and Myocet (doxorubicin) in the field of cancer therapeutics; and nanoparticles such as Abraxane (paclitaxel) (NANoREG Deliverable 6.03) as shown in Table I. The ProSafe “White Paper” proposes recommendations for policy-makers and regulators to solve or work around the problems and limitations such as the absence of standardized test methods, and differences in hazard potential during the life cycle of nanomaterials (ProSafe White Paper).
THE REGULATORY LANDSCAPE OF NANOSIMILARS
The EMA has published a reflection paper on general issues for consideration regarding the parenteral administration of coated nanomedicines. The surface coating affects the stability of the nanoparticles in vitro (eliminated the aggregation phenomena) and in vivo (alters the interactions with serum proteins and changes the biodistribution and the pharmacokinetics of the encapsulated API).
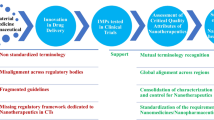
The main queries that have been raised regarding the approval process of nanomedicines and their off-patent copies, i.e., nanosimilars, by the regulatory authorities are presented in Table I. It is our belief that the discovery of an “astrolabe”-like regulatory dynamic system, as the guide for establishing the regulatory approval process, should be taken into account. The astrolabe-like system is a unique tool to investigate the nanoparticles due to their complexity and multi-functionality. Based on the ancient device, astrolabe is a navigator. In other words, the astrolabe is a complex instrument that investigates and discloses the meaning of multicomplex phenomena with precision by using its dynamic and multifunctional abilities. Moreover, the well-known and sustainable scientific approaches (i.e., biophysics, nanothermodynamics of non-equilibrium systems, non-Euclidian geometries, etc.) should be performed in an updated, and adaptable to pharmaceutical industry, proposal. The several “barriers” found throughout the development of a nanomedicine product are listed in Fig. 1. According to Soares et al., another challenge in the pharmaceutical development is the control of the preformulation and manufacturing processes by the identification of the critical parameters/steps and technologies required to analyze them. New analytical and physicochemical techniques are required (17). Reforms and cross-checking implemented scientific and law-relevance tools and should also be adapted to a new and dynamic regulatory framework that should take into consideration all the existing asymmetries between academia, stakeholders, and regulatory agencies. Table III presents the nano-related criteria for selecting nanosimilar products.
Schematic representation of the several “barriers” found throughout the development of a nanomedicine product (adapted from (17))
We have to point out that the risk/benefit ratio in each step of the development and of the evaluation processes should be taken into consideration. Additionally, the ethics, the patient rights, and the market rules should be considered as crucial. The globalization and the implementation of all the above quotes after gaining exhausting debate and approval should be considered as obvious in the development and evaluation process of nanomedicines and nanosimilars. Moreover, the submission processes in regulatory authorities (i.e., EMA, FDA, etc.) including documentation files, both of prototype nanomedicines and of the corresponding nanosimilars, should be considered as similar (the term needs clarification and definition) in terms of their safety and their effectiveness. The terms similar and its linguistic derivative similarity should be used as an intergrade concept in the development and evaluation processes of nanomedicinal products. Namely, similar products are the copies of nanomedicines (off-patented nanomedicines). Similarity is a term which should be used for nanomedicines produced by different excipients or by different types of nanocarriers (i.e., liposomes and micelles, etc.). Similarity highlights the different delivery systems/different carriers.
THE “ASTROLABE-LIKE SYSTEM”
All such above quotes including the common questions are highlighted as emerged and should be considered as crucial challenges in order to exceed the conventional and established ones such as scientific and regulatory paths and practices. The applicability of the above challenges as the consequences of the above remarks needs to be implemented by following the directions below.
First, the already existing and established scale-up and manufacturing processes of nanomedicines as well as the submission process of the documents to the regulatory agencies of new medicinal products should be revised and should include new scientific outcomes that go beyond existing ones. This direction has to be characterized as emerge (Table II). The key phrases and concepts that are presented in Table 2 correspond to proposals that should be checked and evaluated by all the entities involved in the research and development process of nanomedicines and nanosimilars, towards the establishment of a new and dynamic regulatory framework.
Second, the new challenges regarding the new scientific and regulatory environment, i.e., an “astrolabe”-like dynamic regulatory system and the consequences by the new requirements should be considered as a part of the dynamical characteristics of an intergrade approval process by the regulatory authorities (Table II).
Third, the tools that should be used for cross-checking the implementation of the regulatory “astrolabe”-like dynamic regulatory system should be established and should be considered as a part of the Investigational New Drug (IND) application form and of the Common Technical Document (CTD) of new nanomedicines and nanosimilars.
It is well known that nanomedicines are categorized as NBCDs. A great number of NBCD products are available in market while only one nanosimilar has been approved by FDA, via the generic approval procedure. Ιt is important to state at this point that the authorization pathway for nanomedicines either as generic or similar is still under debate in the USA and in the EU as there is no harmonized procedure established. Besides this, the clinical data showed differences to that with the prototype nanomedicine (18). It is obvious that there is an overriding need to discuss in-depth new approaches for establishing a modern and clear regulatory and scientific framework as a consequence of in-depth debate among the regulatory authorities, academia, stakeholders, and patient parties.
This note deals with the constructions and organization of the high priority quotes and questions that should be put on table with well-documented scientific proposals for evaluating new regulatory insights based on Table II and on the “astrolabe”-like system. This approach could be the driving force for going beyond and overcoming the conventional methodologies in the scale-up and manufacturing processes and in the submission and approval processes of new nanomedicinal and nanosimilar products.
NANOCARRIERS AS INNOVATIVE EXCIPIENTS
The important and functional part of a drug formulation are the excipients. They are characterized as non-pharmacological active substances that they efficiently contribute to the therapeutic effect of the drug. Despite obstacles from the regulatory point of view concerning their approval process, excipients can be introduced as new and innovative biomaterials that held the innovation demand of drugs. Specific needs that are disclosed during the formulation process, i.e., poorly water-soluble drugs, new physicochemical demands that have been raised for nanotechnological products, etc., provide opportunities for discovering new and innovative excipients. It is major to point out that the quality by design (effective) is a challenge and an opportunity for the effective formulation development and should be considered during the formulation process of innovative medicinal products (5). The Pharmaceutical Industry should work closely with the excipient manufacturers especially those that are working on self-assembled biomaterials’ structures and on self-assembled nanosystems like liposomes (19). BCC Research [http://BCCResearch|~www.bccresearch.com] specified key implements in the excipients in nanotechnology such as nanosized liposomes. According to the BCC Research study, “The pharmaceutical industry has made overtures toward the use of liposomes (or 60 nanometer-sized emulsion droplets) as delivery systems but has yet to accept them fully,” and “That acceptance has been stalled, in part because of the lack of precise scientific data on the exact role of lipid-based excipients (oleochemicals) and their influence on adsorption of the drug.” The BCC Research noted that the excipients, which are used in liposomal formulation, should be checked for their ability to stabilize liposomal vesicles (Ref. Excipients in Pharmaceuticals, Report No. PHM010E, BCC Research (Norwalk, CT, 2006)). Liposomes are pseudo-spherical vesicles that are composed mainly of phospholipids and cholesterol. There are numerous publications regarding the lipidic vesicles and the properties of their membranes, which are well adapted with the well-known fluid-mosaic membrane model of Singer and Nicolson of the cell membranes. In 2014, the pioneer in describing the cell membranes’ behavior, G. L. Nicolson, published an amazing article by celebrating the 40 years of the fluid-mosaic model of membrane structure (19). He states that new data were added in this model due to the evolution of science and technology and he provides the complexity and hierarchy as crucial parameters that there were not exist in the past when the membrane structural model has been described (19). At the end, G.L. Nicolson states that the composition, functions, and dynamics of membranes, as well as questions that have been raised by thermodynamics and physics on the structural activity and the functionality of membranes should be efficiently approached by the scientific community (19). It is realized that G.L. Nicoloson understood that all that we mentioned at the beginning of our article play a key role in studying biological membranes as well as artificial biomembranes like liposomes or nanoparticulate systems (19).
However, the biological properties of lipidic membrane of liposomes should be functional, stimuli-responsive, and able to control the interfacial phenomena that are taken place within the microenvironment of the liposomal membrane. In our point of view, the complexity of the membrane of the lipidic nanoparticulate systems, such as liposomes, is an obstacle towards the repeatability and the massive production nanosimilars.
Towards finding solutions for the repeatability of nanosimilars, we propose that the adoption of information and entropy principles may be useful. Indeed, elements like self-assembly, shape, morphology, dimension, asymmetry, structural hierarchy, and topology (Table II) could constitute the information, while terms like physical chemistry, energetic topology, structural hierarchy, and interfacial phenomena reflect to the physics and thermodynamics and more in particular to the entropy. Entropy and information are directly related to order or disorder phenomena of natural objects, as pointed out by Shannon on the mathematical theory of information (20,21). More important, the balance between them should be considered as an extremely important variable on the problem. This approach may be proved as important towards developing bio-inspired artificial membranes that could be used as drug delivery nanosystems. More in particular, thermodynamics may be changed by the term “nanothermodynamics,” while the theory of small systems should also be taken into consideration (22). We have also to point out here that the term “dimension” should also be clarified in terms of the approach for small systems published in the past by Hill’s theories of small systems. It is also of great importance to take into consideration that the nanosystems are non-equilibrium systems and their macroscopic characteristics (in nanodimension) affected by their microscopic behavior that should be studied in depth (Table III).
The scientific tools that should be used in our point of view should include the extended Boltzmann-Gibbs theory, which is based on the entropy as the non-extensive statistical mechanism. Such approach incorporated the Shannon and Tsallis theories, which regard the information and the nanothermodynamical profile of small systems (i.e., nanoparticulate systems), respectively, that are able to deliver bioactive compounds to the injury tissues (20,21,22).
The main problem for the in-depth studies to clarify the terms similar and similarity is the complexity of the nanoparticulate system, for example, liposomes, regarding their behavior, which depends not only on their composition, properties, etc. but also on the changes in the biological environment. However, the ordered and non-ordered phases of the self-assembled nanostructures and their structural dynamics need non-extensive statistical mechanics and power-law distributions that are well adapted to their metastability behavior and their dynamical properties.
It is obvious that such approaches are very complicated and should be evaluated by experts in particular scientific fields and to explore the capacity of the stakeholders and pharmaceutical industry to realize such approaches. Moreover, scientists should improve their knowledge by adding new insights that were proved and published or they were applied and tested in other applications like electronics, engineers, and foods.
IS THE REVISION OF THE CURRENT SCIENTIFIC APPROACHES CONSIDERED AS A RATIONAL AND REALISTIC APPROACH THAT CAN CHANGE AND INFLUENCE THE REGULATORY FRAMEWORK?
The main question that should be raised is “should nanosimilars and nanomedicines consider to be nanoplatforms that by compiling their endogenous and even undisclosed properties, provide reliable clinical data and even effective therapeutic outcomes and is this the endpoint of their contribution to human health? Or should the scientific community spend more time and should fund specific research approaches in order to disclose their encrypted properties by adopting a more reliable and realistic strategic research and by developing ways away from the conventional paths?” The conceptual revision of the scientific and regulatory fingerprint of nanomedicines and nanosimilars through the trends of a more reliable and realistic strategic research. In recent years, due to the amazing evolution of science and technology, scientists looking back recognize the revisitation of well-established scientific ideas, laws, and mathematical equations. This glow back process can reconstitute the phenomena and natural processes that could be very helpful for establishing new road maps in order to develop innovative nanomedicines and consequently nanosimilars. The concept of the spherical shape structure could not be adapted to spherical-like (i.e., liposomes) nanomedicinal and nanosimilars due to the conventional approach based on hypothetical assumptions that nanoparticles are spherical (23,24,25,26,27). We have recently proposed that there is a balance between the shape and the morphology, via fractal analysis (23,24). This proceed (i.e., spherical shape structure of nanoparticulate drug delivery systems like liposomes) belongs to past beliefs and nowadays the morphology must be taken into account because of the lack of precise definition of “what drug delivery nanosystems are?”, in terms of their structural characteristics and rearrangements due to the/their interactions with biological components (27,28). We can promote an approach based on the basic laws of physics and mathematics in order to evaluate and disclose the sub-molecular and molecular properties of drug delivery nanosystems and consequently of nanosimilar products. However, in order to contribute with our thoughts in the already published works and efforts of the scientific community in opening a more realistic avenue on what nanomedicines are, we can propose three scientific directions for their studying and for their evaluation, which will enforce the quality, safety, and efficacy of nanomedicines and nanosimilars. The first direction is the thermodynamics approach for studying nanoparticulate systems and their similar copies, which is considered to be an important tool for their design and development and which contributes to the basic laboratory research (preformulation studies) and to the scaling-up process in the pharmaceutical industry, respectively (26,27). The interactions of materials in bulk solution, the metastable phases, and the interfaces of phases provide insight into the behavior of nanomedicines concerning the cellular uptake and the endosomal escape processes. The formed template strategy for nanomedicine formulations is in line with the second direction for the design of multifunctional nanodevices, which should be considered, i.e., the shape/morphology balance. This balance is in line with the fractal geometry, which is the tool to describe systems and devices in the nanoworld, the kinetics of physical phenomena like aggregation, as well as the determination of the morphology of colloidal nanostructures. The last but not the least direction, i.e., the encrypted code, which is synonymous with quantum necessities and could be characterized as encrypted due to the unfamiliarity of scientists working in the field of drug delivery in terms of quantum mechanics, still remains undisclosed and it could be correlated with the cryptic properties and behavior of biomaterials during their journey into the human body (28). The mesoscopic level of bionanomaterials, which are used as the structural elements of nanomedicines, plays a key role in their behavior. It is important to point out that at the nanoscale level, the effects and the properties of bionanomaterials become very significant due to their surface-to-volume ratio, which is larger than those in bulk systems, as well as due to the quantum effects and the quantum entanglement phenomena occurring in living colloidal systems. In other words, the properties of materials are size-dependent and for this reason, the matter at the nanoscale no longer follows the principles and laws of Newtonian Physics but rather quantum mechanics. The latter is in line with the electronic structure of nanoparticles, which is very discrete and not overlapping as in bulk materials (29). At the mesoscopic level, quantum confinement effects dominate the optical and electromagnetic properties of the nanosystems. They also render new opportunities for manipulating (nanomanufacturing) the response of such systems. The mesoscopic character of nanomedicines, as well as their open nature, in the sense of thermal losses due to the interaction with the human body, implies the quantum nature of the system. Indeed, the quantum biology is a scientific field that scientists should take into consideration in order to create bio-inspired nanosystems for drug delivery, diagnosis, and imaging in line with the new outcomes from systems biology and pharmacology and gather the scientific instruments for their reproducibility in terms of nanosimilars (30,31).
The proposed three directions may be the driving forces for recalling basic principles and laws that govern the behavior of drug delivery nanosystems in biological media, which is quite different—and sometimes extremely different—from their behavior “trapped” in the final formulation. The release of them in biological media provokes morphological and physicochemical alterations that should be determined through high-level diagnostic techniques. It should be emphasized that, to date, there is lack of full understanding of their behavior. This lack is trapped in the encrypted algorithm, which is encoded in a quantum language. Quantum phenomena are therefore crucial to design and develop nanomedicines and nanosimilars with complete knowledge of their physical behavior (31,32,33). Our proposal overcomes or even smoothens the uncertainty of the experimental and manufacturing procedures during the development process of nanomedicines and facilitates the way to understand the term similarity.
THE INTEGRATION PROCESS TO OVERCOME THE REGULATORY ABNORMALITIES
To reconstruct the currently existing regulatory framework, we should provide new challenges regarding the evaluation of nanoparticulate platforms for drug delivery (34,35,36,37,38). Such direction needs to overcome the classic laws of physics and the current and well established and applicable Euclidean geometry. In terms of nanosized particles, we should consider the nanosized dimension as too small to follow classic physics and too big to follow the principles of quantum mechanics (26). However, nanosystems should be categorized as mesoscale systems and further scientific tools are required to balance among classical physics and mathematics and quantum approaches.
CONCLUSIONS
After decades of research, nanotechnology has been used in a broad array of biomedical products including medical devices, drug products, drug substances, and pharmaceutical-grade excipients. The application of nanotechnology in cosmeceuticals, nutraceuticals, and herbal medicines exhibit several advantages for the cosmetic ingredients and food supplements, too. For these reasons, other routes of administration (i.e., ocular and topical) except for parental (that is presented in Table I), are used from the consumers (39–40). The types of nanoparticles in approved drugs available for clinical use are liposome (10), polymer (15), nanocrystal (15), inorganic (5), micelle (10), and proteins (2), while those in investigational drugs are liposome (33), polymer (11), nanocrystal (2), inorganic (2), micelles (9), proteins (1), and dendrimers (2) (39–40).
According to the recent literature, nanomedicines and copies of NBCD require special attention regarding their regulations (41–42). There is a starting point of discussion in the scientific community about the best approach that the regulatory authorities should follow about follow-on nanomedicinal products. The aim of this mini-review is to underline the significance of some parameters, i.e., thermodynamics, shape, morphology, etc., that play a key role in the effectiveness of nanomedicinal products and should be taken into account from the regulatory agencies for their copies. The tools for enriching the regulatory landscape are presented in Table II. The self-assembly, the interfacial phenomena, the nanothermodynamics, the nonlinear dynamics, and the fractal dimensions are the key concepts for the deeper understanding and fully characterizing of nanomedicines and their follow-on.
From our point of view, always by showing respect to past efforts in the field, the dilemma with regard to nanosimilars and in reality to nanomedicines should be considered and should be discussed in depth. In our point of view, biophysics and thermodynamics could be efficiently involved in new regulatory approaches. It is very serious regarding nanomedicines and their off-patent copies, i.e., nanosimilars, to apply instruments that are able to disclose their “silent functionality” which is related with their biophysical and thermodynamical profile. This “silent functionality” is encoded in metastable phases that constitute a dynamic multi-dimensional phenomenon that dictates the stability, the release profile, and the macroscopic fingerprint of the nanomedicinal products (26). This approach should be involved in a new New Drug Application (NDA) descriptive document which could be a revised NDA, coined as “r-nano NDA” for nanomedicines and “r-nanosimilar NDA” for nanosimilars, contrary to the conventional NDA folder used for the starting of the approval process.
Notes
European Medicines Agency (EMA), 2006. EMEA/CHMP/79769/2006. Reflection paper on nanotechnology-based medicinal products for human use (29 June). http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_ guideline/2010/01/WC500069728.pdf, Accessed date: 20 August 2018.
This “White paper” states the situation of nanomedicines and nanosimilars and suggests new scientific directions that should be considered towards a harmonized regulatory pathway.
References
Mühlebach S. Regulatory challenges of nanomedicines and their follow-on versions: a generic or similar approach? Adv Drug Deliv Rev. 2018;131:122–31. https://doi.org/10.1016/j.addr.2018.06.024.
Flühmann B, Ntai I, Borchard G, Simoens S, Mühlebach S. Nanomedicines: the magic bullets reaching their target? Eur J Pharm Sci. 2019;12:73–80. https://doi.org/10.1016/j.ejps.2018.11.019.
Crommelin DJA, Shah VP, Klebovich I, McNeil SE, Weinstein V, Flühmann B, et al. The similarity question for biologicals and non-biological complex drugs. Eur J Pharm Sci. 2015;76:10–7. https://doi.org/10.1016/j.ejps.2015.04.010.
Barenholz Y. Doxil®--the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–34. https://doi.org/10.1016/j.jconrel.2012.03.020.
Crommelin DJ, de Vliger JS, Weinstein V, Mühlebach S, Shah VP, Schellekens H. Different pharmaceutical products need similar terminology. AAPS J. 2014;16(1):11–4. https://doi.org/10.1208/s12248-013-9532-0.
Sainz V, Conniot J, Matos AI, Reres C, Zupancic E, Moura L, et al. Regulatory aspects on nanomedicines. Biochem Biophys Res Commun. 2015;468(3):504–10. https://doi.org/10.1016/j.bbrc.2015.08.023.
Schellekens H, Stegemann S, Weinstein V, de Vlieger JS, Flühmann B, Mühlebach S, et al. How to regulate non biological complex drugs (NBCD) and their follow-on versions: points to consider. AAPS J. 2014;16(1):15–21. https://doi.org/10.1208/s12248-013-9533-z.
Mühlebach S, Borchard G, Yildiz S. Regulatory challenges and approaches to characterize nanomedicines and their follow-on similar. Nanomedicine (London). 2015;10:659–74.
Hussaarts L, Mühlebach S, Shah VP, McNeil S, Borchard G, Flühmann B, et al. Equivalence of complex drug products: advances in and challenges for current regulatory frameworks. Ann N Y Acad Sci. 2017;1407(1):39–49. https://doi.org/10.1111/nyas.13347.
Ehmann F, Sakai-Kato K, Duncan R, Hernán Pérez de la Ossa D, Pita R, Vidal JM, et al. Next-generation nanomedicines and nanosimilars: EU regulators’ initiatives relating to the development and evaluation of nanomedicines. Nanomedicine (London). 2013;8(5):849–56. https://doi.org/10.2217/nnm.13.68.
Di Francesco T, Philipp E, Borchard G. Iron sucrose: assessing the similarity between the originator drug and its intended copies. Ann N Y Acad Sci. 2017;1407(1):63–74. https://doi.org/10.1111/nyas.13517.
Hafner A, Lovrić J, Lakoš GP, Pepić I. Nanotherapeutics in EU: an overview on current state and future directions. Int J Nanomedicine. 2014;9:1005–23. https://doi.org/10.2147/IJN.S55359.
Di Francesco T, Borchard G. A robust and easily reproducible protocol for the determination of size and size distribution of iron sucrose using dynamic light scattering. J Pharm Biomed Anal. 2018;152:89–93. https://doi.org/10.1016/j.jpba.2018.01.029.
Schilt Y, Berman T, Wei X, Barenholz Y, Raviv U. Using solution X-ray scattering to determine the high-resolution structure and morphology of PEGylated liposomal doxorubicin nanodrugs. Biochim Biophys Acta. 1860;2016:108–19. https://doi.org/10.1016/j.bbagen.2015.09.012.
Wibroe PP, Ahmadvand D, Oghanian MA, Yaghmur A, Mofhimi SM. An integrated assessment of morphology, size, and complement activation of the PEGylated liposomal doxorubicin products Doxil®, Caelyx®, DOXOrubicin, and SinaDoxosome. J Control Release. 2016;221:1–8. https://doi.org/10.1016/j.jconrel.2015.11.021.
Bhattacherjee S. DLS and zeta potential-what they are and what they are not? J Control Release. 2016;235:337–51.
Soares S, Souza J, Pais A, Vitorino C. Nanomedicine: principles, properties and regulatory Issues. Front Chem. 2018;6:360. https://doi.org/10.3389/fchem.2018.00360.
Astier A, Barton Pai A, Bissig M, Crommelin DJA, Flühmann B, Hecq JD, et al. How to select a nanosimilar. Ann N Y Acad Sci. 2017;1407(1):50–62. https://doi.org/10.1111/nyas.13382.
Nicolson GL. The fluid-mosaic model of membrane structure: still relevant to understanding the structure, function and dynamics of biological membranes after more than 40years. Biochim Biophys Acta. 1838;2014:1451–66.
Shannon CE. A mathematical theory of communication. Bell Syst Tech J. 1948;327:27.
Naziris Ν, Pippa N, Pispas S, Demetzos C. The role of the information/entropy balance in self-assembly. The structural hierarchy of chimeric drug delivery nanosystems. Pharmakeftiki. 2017;29:77–82.
Tsallis C. Introduction to nonextensive statistical mechanisms. Approaching a complex world, Springer Science Business Media, LLC; 2010,
Pippa N, Dokoumetzidis A, Demetzos C, Macheras P. On the ubiquitous presence of fractals and fractal concepts in pharmaceutical sciences: a review. Int J Pharm. 2013;456(2):340–52. https://doi.org/10.1016/j.ijpharm.2013.08.087.
Pippa N, Psarommati F, Pispas S, Demetzos C. The shape/morphology balance: a study of stealth liposomes via fractal analysis and drug encapsulation. Pharm Res. 2013;30(9):2385–95. https://doi.org/10.1007/s11095-013-1082-8.
Demetzos C, Pippa N. Fractal analysis as a complementary approach to predict the stability of drug delivery nano systems in aqueous and biological media: a regulatory proposal or a dream? Int J Pharm. 2014;473:213–8. https://doi.org/10.1007/978-3-319-08927-0_27.
Demetzos C, Pippa N. Fractal geometry as a new approach for proving nanosimilarity: a reflection note. Int J Pharm. 2015;483(1–2):1–5. https://doi.org/10.1016/j.ijpharm.2015.02.008.
Demetzos C. Biophysics and thermodynamics: the scientific building blocks of bio-inspired drug delivery systems. AAPS PharmSciTech. 2015;16(3):491–5. https://doi.org/10.1208/s12249-015-0321-1.
Demetzos C. Pharmaceutical nanotechnology. Fundamentals and practical application. Springer, 2016.
Hubbell JA, Chilkoti A. Chemistry. Nanomaterials for drug delivery. Science. 2012;337:303–5.
Baars BJ, Edelman DB. Consciousness, biology and quantum hypotheses. Phys Life Rev. 2012;9:285–94.
Foffi G, Pastore A, Piazza F, Temussi PA. Macromolecular crowding: chemistry and physics meet biology. Phys Biol. 2013;10(4):040301.
Guisbiers G. Size-dependent materials properties toward a universal equation. Nanoscale Res Lett. 2010;5(7):1132–6. https://doi.org/10.1007/s11671-010-9614-1.
Mershin A, Nanopoulos DV, Skoulakis EMC. Quantum brain? Proc Acad Athens. 1999;74(A).
Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33(10):2373–87. https://doi.org/10.1007/s11095-016-1958-5.
Knoeff J, Flühmann B, Mühlebach S. Medication practice in hospitals: are nanosimilars evaluated and substituted correctly? Eur J Hosp Pharm Sci Pract. 2018;25(2):79–84. https://doi.org/10.1136/ejhpharm-2016-001059.
Rosenmayr-Templeton L. Industry update: the latest developments in therapeutic delivery. Ther Deliv. 2013;4(5):1347–52. https://doi.org/10.4155/tde.13.32.
Trikle S, McNeil SE, Mühlebach S, Bawa R, Borchard G, Barenholz YC, et al. Nanomedicines: addressing the scientific and regulatory gap. Ann N Y Acad Sci. 2014;1313:35–56. https://doi.org/10.1111/nyas.12403.
Wolfram J, Zhu M, Yang Y, Shen J, Gentile E, Paolino D, et al. Safety of nanoparticles in medicine. Curr Drug Targets. 2015;16:1671–81.
Lee VC. Progress in nanomedicine: approved and investigational nanodrugs. PT. 2017;42(12):742–55.
Marques MRC, Choo Q, Ashtikar M, Rocha TC, Bremer-Hoffmann S, Wacker MG. Nanomedicines-tiny particles and big challenges. Adv Drug Deliv Rev. 2019;In press.https://doi.org/10.1016/j.addr.2019.06.003.
Klein K, Stolk P, De Bruin ML, Leufkens HGM, Crommelin DJA, De Vlieger JSB. The EU regulatory landscape of non-biological complex drugs (NBCDs) follow-on products: observations and recommendations. Eur J Pharm Sci. 2019;133:228–35.
Rocco P, Musazzi UM, Franzè S, Minghetti P. Copies of nonbiological complex drugs: generic, hybrid or biosimilar? Drug Discov Today. 2019;24:250–5.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
“Astrolabe” was an ancient device that has been used as navigator. The Astrolabe is a complex instrument that investigates and discloses the meaning of multicomplex phenomena with precision by using its dynamic and multifunctional abilities (Scheme 1).
Rights and permissions
About this article
Cite this article
Demetzos, C., Kavatzikidou, P., Pippa, N. et al. Nanomedicines and Nanosimilars: Looking for a New and Dynamic Regulatory “Astrolabe” Inspired System. AAPS PharmSciTech 21, 65 (2020). https://doi.org/10.1208/s12249-019-1573-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1573-y