Abstract
Freeze-dried immunoglobulin G (IgG) incorporating trehalose and human serum albumin (HSA) was statistically evaluated regarding the existence of synergism between additives on the stability profile. The levels of HSA (X1) and trehalose (X2) were independent variables. Aggregation following the process (Y1), after 2 and 3 months at 40°C (Y2) and (Y3), respectively, along with the rate constant of aggregation (Y4) were dependent variables. Aggregation and beta-sheet conformation were quantified through size-exclusion chromatography (SEC-HPLC) and Fourier transform infrared spectroscopy (FTIR). Central composite design (CCD) suggested the best formulation. The integrity and thermodynamic stability of optimized formulation were investigated through sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and differential scanning calorimetry (DSC). The calculated responses were Y1, 0–0.90%; Y2, 0.4–4.3%; Y3, 2.10–13.46%; and Y4, 0.16–0.69 1/month. The optimized formulation had 10 mg IgG, 86 mg trehalose, and 1 mg HSA with observed responses of Y1, 0.01%; Y2, 0.51%; Y3, 3.08%; and Y4, 0.33 1/month. The models were statistically well-fitted. The optimized formulation was amorphous during freeze-drying (FD), and no fragmentation was observed. Trehalose and HSA demonstrated statistical synergism. CCD was successfully employed to recommend the best ratio of stabilizers and achieve the maximum stabilization of IgG as a model freeze-dried antibody.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The noticeable specificity of therapeutic antibodies has successfully made them as one of the most promising categories of human therapeutic agents. Although significant improvements have been achieved in terms of antibody manufacturing and characterization, some challenges should be still overcome with a main focus on stability issues (1). Liquid formulations of antibodies, such as other macromolecular proteins, are more sensitive to physicochemical degradations compared with solid-dosage forms (2).
To achieve an acceptable shelf-life, proteins are frequently lyophilized through freeze-drying (FD), as the most common drying technology of biopharmaceuticals (3). In spite of process advantages, antibodies may experience different stresses within FD. Formation of ice crystals, cold denaturation, condensation of the antibody in the unfrozen phase, and changes in the pH of the solution may occur during the freezing step (4). On the other hand, dehydration of the protein and loss of hydrogen bonds, which can lead to aggregation, mainly happen via the drying stage (5). Incorporation of suitable lyoprotectants or cryoprotectants is therefore a prerequisite to protect the antibody against process-related tensions (6,7).
Sugars are the most applicable group of stabilizers in freeze-dried formulations of biopharmaceuticals (8). Water substitution theory and glassy immobilization are the basic mechanisms through which proteins would be preserved by sugars within FD and following high temperature of storage conditions (9). Belimumab, vedolizumab, infliximab, pembrolizumab, mepolizumab, and blinatumomab are some examples of numerous existing freeze-dried therapeutic antibodies in the market which are composed of sucrose or trehalose as stabilizer (8).
HSA is the 66-kDa, amphiphilic globular protein and also the most typical one in human plasma. HSA is frequently applied as a stabilizer in liquid and solid formulations of biopharmaceuticals to prevent protein adsorption and/or aggregation through a competitive mechanism (10,11). High glass transition temperature (Tg) and surfactant-like effect of albumin have made this protein a favorably stabilizing candidate in freeze-dried preparations (12,13). The role of HSA as lyoprotectant and cryoprotectant has been confirmed in freeze-dried formulations in many instances. In a study, synergistically induced cryoprotection and surface protection of HSA in freeze-dried lactate dehydrogenase (LDH) caused more stabilization than through disaccharides (14). Additionally, improvement in the solid reconstitution due to enhanced solubility has been reported in the presence of HSA (15,16). On the other hand, increasing the concentration of HSA above its optimum level did not positively influence protein stability (17). Further, minimizing the amount of totally applied additives should be carefully examined in terms of formulation aspects. Therefore, the best ratio of HSA should be selected according to the parameters of the FD process as well as the specific type of proteins.
Our previous experience employed a combination of polymers (PEGs) and trehalose as excipients in freeze-dried antibody formulation. A co-stabilizing effect was observed leading to lower required amounts of each excipient as compared with their separate application (18). Although co-administration of HSA and sugars like sucrose has been examined in some freeze-dried protein formulations (14), there has been no all-inclusive research in which the effect of the proper HSA ratio has been evaluated in the presence of trehalose within FD of the IgG antibody, which has been the subject of the current research.
The main goal of the present study was to reduce the amount of HSA and trehalose in freeze-dried IgG formulation and also to achieve the best stability profile. Further, preparation of the stable lyophilized product at room temperature would be our final strategic objective. Design of experiment was utilized at the optimization phase following the screening stage, where the approximate ranges of independent parameters were estimated. Response surface methodologies (RSM), as multivariate techniques, were used to determine the mathematical relationship between dependent variables and independent parameters (19). Central composite design (CCD) is one of the most common designs usually used in RSM and characterized with center and axial points to estimate curvatures (20). Here, the optimized levels of trehalose and HSA with regard to the minimum rate of antibody aggregation were selected with the aid of CCD.
MATERIALS AND METHODS
Materials
Human IgG (150 kDa) was purchased from Biotest (Germany). Human serum albumin (66 kDa) was obtained from Kedrion (Italy). Trehalose dihydrate, disodium hydrogen phosphate, sodium sulfate, and Coomassie brilliant blue were purchased from Merck (Germany). All SDS-PAGE reagents and potassium bromide (KBr) were purchased from Sigma (Germany).
Preparation of IgG Formulation and FD
IgG and HSA were separately dialyzed against deionized water at 4°C for 24 h to remove stabilizing excipients. The final concentration of the antibody solution was 10 mg/ml. The concentration ranges of the HSA solution were from 100 to 500 μg/ml. Different aqueous solutions of antibody were prepared containing HSA and trehalose. The final solutions were then frozen at − 20°C for 24 h. The lyophilization process was performed on the lab-scale freeze-dryer (Christ, Germany) through 24 h primary drying at − 50°C and 0.01 mbar vacuum and secondary drying at 25°C at the same time and pressure. Lyophilized samples were kept at 4°C for subsequent examinations.
Size-Exclusion Chromatography (SEC-HPLC)
Size-exclusion high-performance liquid chromatography (SEC-HPLC) was performed to determine the percentage of IgG soluble aggregates after FD and after 2 and 3 months of storage at 40°C. The chromatographic system was equipped with a pump (Jasco, USA), a TSK 3000 SWXL column (Tosoh, Biosep, Germany), and a UV detector at 280 nm to separate the monomer from aggregates. The mobile phase consisted of 0.1 M sodium sulfate and 0.1 M disodium hydrogen phosphate with pH adjusted at 6. Approximately 20 μl of filtered solution, containing 2.5 mg/ml of IgG, was injected at a flow rate of 0.5 ml/min.
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectra were prepared at room temperature by a Nicolet Magna spectrometer (Nicolet Magna, USA). About 2 mg of lyophilized powders was mixed with 200 mg KBr. A hydraulic press at 6–7 T was used to produce a clear tablet. Amid I region (1600–1700 cm−1) of the spectrum was analyzed applying Jasco Spectra Manager® software (Japan), to evaluate the secondary structure of IgG. The curve fitting was adjusted by a mixed Gaussian/Lorentzian function.
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
SDS-PAGE analyses were performed to evaluate the fragmentation and/or aggregation of optimized IgG formulation. Samples were prepared under reducing conditions using lysis buffer containing 500 μl dithiothreitol (DTT) 1 M. One hundred milligrams per milliliter of diluted samples as well as pure HSA was boiled for 10 min to complete dissociation of antibody chains. Then the samples were loaded on 10% polyacrylamide gels according to the Laemmli method. The gels were run at 20 mA for 2.5 h at 100 V. Coomassie blue solution, 0.1%, was applied to visualize a sample’s bands. Molecular weight markers included 11, 17, 25, 35, 48, 63, 75, 100, 135, and 180 kDa as standard.
Differential Scanning Calorimetry (DSC)
Analysis of thermal behavior (amorphous/crystalline state) of optimized freeze-dried formulation was conducted by a Mettler calorimeter (Mettler Toledo, Switzerland), calibrated with indium. About 10 mg of powders was transferred in aluminum pans as sealed samples. Then the pans were heated at a rate of 10°C/min from 20 to 220°C.
Stability Assessment and Kinetic of Aggregation
Vacuum-sealed glass bottles of freeze-dried powders were stored at 40°C for 2 and 3 months. The induced soluble aggregates were quantified through SEC-HPLC. The kinetic of aggregation was determined with preliminary assumption of zero-order kinetic (linear regression of % aggregation versus time) as well as a first-order one (linear regression of log % aggregation versus time). According to the higher values of R2, the fitted model was chosen and calculated rate constants of aggregation were reported.
Screening
To investigate the effect of HSA and trehalose on the stability of IgG after FD and storage at 40°C, 8 formulations were prepared which were composed of excipients at various amounts. The SEC-HPLC characterization was carried out to assess the molecular stability of the antibody while the FTIR results were employed to evaluate the thermodynamic features of the powders. Based on the achieved data from screening, the roughly suitable ranges of additives were selected for the subsequent optimization step of the research.
Experimental Design and Optimization by CCD
CCD, 2 independent variables at 3 levels, in Design-Expert version 6.0.10 (Stat-Ease, Inc., Minneapolis, MN 55413, USA) software was utilized to obtain the optimized level of independent variables, (i.e., the amount of trehalose and HSA), evaluate the main effects, and assess interaction effects and possible quadratic impacts of independent parameters on the aggregation of freeze-dried IgG following process and storage at high temperatures. The total number of experiments was calculated accordingly:
K is the number of factors, n0 is the replication of the center point (n0 > 1), and 2 is the number of the axial point. α is defined as the distance between the axial point and the center point (α = 2K/4) (21,22).
The CCD consisted of two main factors involving the amount of HSA (X1) and trehalose (X2), (K=2), five replicates of the center point (n0 = 5), four axial points, and α = 1.414. The range of HSA was from 1 to 5 mg with a center point of 3 mg. Trehalose was from 0 to 100 mg with center point of 50 mg.
Percent of soluble aggregates following the process (Y1), after 2 months of storage at 40°C (Y2) and upon 3 months at 40°C (Y3), and the rate constant of aggregation (Y3) were determined as dependent variables.
The non-linear computer-generated quadratic model was given as
where Y was the calculated response for each factor level combination, b0 to b22 was related to regression coefficients, and X1X2 demonstrated the interaction and quadratic terms. The Variables and constraints are shown in Table I. The models were finally analyzed regarding statistically significant coefficients and p values.
RESULTS AND DISCUSSION
FD is one of the most applicable methods to prepare solid protein products on industrial scale (3). Meanwhile, antibodies are macromolecular proteins with a high degree of sensitivity to various stresses of freezing and drying steps (18). There are many inert excipients/stabilizers such as carbohydrates, amino acids, and proteins to stabilize proteins during FD (16). Human serum albumin (HSA) has been utilized in freeze-dried protein formulations as stabilizer (12). There are many commercial products containing HSA as excipient (15). HSA has played a role as a stabilizer with other excipients, including amino acids, sugars, or salts. The level of HSA varied in different formulations accordingly. Also, a broad range of HSA has been applied in lyophilized formulations. For instance, 20% w/w of HSA was added to freeze-dried Baby BIG (Baby Botulism Immune Globulin) (16). The stability of lyophilized-human interferon-β activity composed of HSA and dextrose was investigated after 2 years at 2–8°C and at multiple isothermal temperatures (25–80°C) (23). According to the results, higher concentrations of HSA revealed enhanced thermal stability at multiple isothermal temperatures of storage condition. Also, the HSA/dextrose concentration ratio was 4:1 in the best formulation.
The current investigation aimed to incorporate HSA (at a different ratio) as a co-stabilizer of freeze-dried IgG in combination with trehalose to both achieve the desired stability and possibly minimize the total employed stabilizers. In the subsequent part of the article, the characterization results of freeze-dried formulations have been described at two stages of screening plus optimization.
Screening the Suitable Range of HSA for Stabilization of IgG During FD and Following Storage
Eight different preparations were lyophilized based on the composition (Table II). The amount of generated soluble aggregates following FD and after 1 month of storage at 40°C was determined by SEC-HPLC. Aggregation varied within 0–9.03% and 0.8–11.6%, immediately following FD and after storage, respectively. Interestingly, the highest extent of aggregation was observed in F1 (containing 40 mg HSA and 100 mg trehalose), while the best stable sample, F5, contained 1 mg HSA and 100 mg trehalose.
Based on the results obtained from the screening phase, HSA above 5 mg could not properly stabilize IgG and the greatest stabilization was obtained within the range of 1 to 5 mg of HSA. The FTIR examination assessed the percentage of beta-sheet conformation in IgG, as a beta-dominant protein. All powders contained more than 66% of beta-sheet structure suggesting the preservation of the secondary structure of IgG across all formulations with trehalose and/or HSA.
Characterization of Dependent Variables and Their Analysis Through CCD
Aggregation Following Process (Y 1)
Aggregation is the most critical phenomenon which can detrimentally influence antibody stability following FD and upon storage (24). Based on the composition proposed by CCD, the formulations were prepared and analyzed through SEC-HPLC (Table III). Soluble aggregates were ranged from 0 to 0.9% following the process. The data were fitted using a first-order regression model which consisted of an intercept and two variables plus their one-way interaction.
The best-fitted model was 2-factor interactions (2FI) based on CCD with a p value < 0.0120, and the following equation:
The statistical analysis of dependent variables is presented in Table IV. It could be recognized that the effect of trehalose plus HSA and their interaction were statistically significant. The results indicated that the presence of HSA and trehalose immediately following the process could decrease the total amount of soluble aggregates. Also, elevation of the amount of each excipient reduced the value of soluble aggregates. The 3D surface plot is presented to follow the trend of interaction between independent variables (Fig. 1).
Bovine serum albumin and HSA could stabilize protein during freezing and drying via different mechanisms such as steric hindrance of protein-protein interaction, prevention of protein unfolding, preferential exclusion, and increasing the solution viscosity which could cause saturation of the ice/water interface by the second protein (15). Excessive amounts of HSA, as a second protein in formulation, could saturate the binding sites of the container and prevent active protein loss during lyophilization of interferon-β (23). Different freeze-dried cytokines have been commonly stabilized by HSA at 0.05–0.1% (25,26). The stabilizing effect of trehalose was greater than that of HSA with respect to short-term stability (− 0.18 versus − 0.16 for HSA). Water substitution mechanism can justify the high efficacy of trehalose in dried-protein formulations (27). In order to add HSA as a stabilizer of IgG formulation, it was dialyzed and therefore HSA was free from any buffering agents. Accordingly, the corresponding tensions correlated with FD including cold denaturation, solute concentration, and pH shifts were unavoidable which might affect both IgG and HSA stability. Such circumstances could bring about albumin partial inactivation and could be responsible for the weaker potency of HSA in stabilization of IgG in comparison to trehalose.
Aggregation After Two Months of Storage at 40°C (Y 2)
The 2-month stored samples at 40°C were injected into the SEC column to compute the aggregates (Table III). The fitted model was 2FI, as indicated in Table IV, with the equation being as follows:
The response surface model 3D plot, which described the interaction effect of HSA and trehalose, is depicted in Fig. 2. The formulations were stabilized containing aggregates ranging from 0.4 to 4.3%. The combination of HSA and trehalose desirably decreased the amount of soluble aggregates. Increasing the ratio of trehalose enhanced protein stability by a coefficient value of − 1.02 which was in agreement with the data available in (28). The combination of sucrose and BSA in freeze-dried enzyme mixtures of nucleoside phosphorylase and xanthine oxidase previously preserved their activity up to 86% (29). The disaccharide to IgG molar ratio of 360:1 was generally approved as an effective ratio for lyophilized formulation of monoclonal antibody (30).
It has been shown that aggregation of BSA was slow at lower temperatures. For instance, in one study, 24 h incubation of BSA at 37°C, 22°C, and 4°C caused the aggregations of 97%, 56%, and 12%, respectively (25). Considering heat-induced aggregation of BSA, it has been shown to be stable up to 40°C. Within the temperature range of 42 to 50°C, BSA exhibited reversible conformational changes. The high degree of BSA unfolding and beta-aggregation began from 60°C (27). Also, the high temperatures and high concentrations of HSA promoted the aggregation of albumin (31).
In the present study, the interaction of HSA and trehalose was significant (coefficient value of − 0.67), following the storage condition.
Aggregation After Three Months of Storage at 40°C (Y 3)
The stability of IgG monomers was detected by chromatography methods, as with the 2 months of storage (Table III). The final equation was reached as stated in the following part:
The amount of IgG aggregates varied from 1.49 to 13.46%. The quadratic model was the best-fitted model with a p value of < 0.0017 (Table IV). Noting the resultant equation, it could be concluded that increasing the ratio of trehalose promoted IgG stability (coefficient value of − 2.91); however, HSA had limitations in providing the stability for freeze-dried IgG at high temperatures of storage, and increasing its concentration would both be positively influential and could enhance the amount of soluble aggregates (coefficient value of 1.21). A significant interaction existed between HSA and trehalose (coefficient value of − 3.24) with the corresponding response surface plot represented in Fig. 3. It was observed that, in the absence of trehalose, 3 mg and 5 mg of HSA, in F10 and F8, produced the highest levels of aggregates, 11.46 and 13.46%, respectively. This suggests that trehalose, as a prerequisite stabilizer, should be incorporated in freeze-dried IgG formulation, especially to provide satisfactory storage stability. It has been reported that sugars like sucrose and trehalose could stabilize recombinant HSA during lyophilization and storage at 35°C (32).
Therefore, trehalose most probably had a dual stabilizing role for both HSA and IgG. Further, trehalose has proved to interfere with the intermolecular disulfide bonds of BSA and decrease the aggregation following 4 months of storage (33). Schematic comparison of aggregates in IgG formulations is shown in Fig. 4 following process as well as upon storage.
Rate Constant of Aggregation (Y 4)
To evaluate the rate constants of IgG aggregation at times 0, 2, and 3 months of storage at 40°C, first-order kinetic of aggregation was observed which was in agreement with previous findings (34). According to Table III, rate constants of aggregation ranged from 0.11 to 0.69 (1/month).
The quadratic model was properly fitted, p value < 0.0067. The achieved equation was
Figure 5 presents the surface plot of interaction between HSA and trehalose concerning the rate constant of aggregation. Significant interaction existed between additives through a coefficient value of − 0.16.
Optimization and Validation of the Results
The optimum formulation of IgG was selected based on the constraints defined in Table I. It is composed of 100 mg IgG, 86 mg trehalose, and 1 mg HSA which was prepared, freeze-dried, and characterized accordingly with respect to determined dependent variables. The percentages of induced aggregation were calculated after the process and following 2 and 3 months of storage at 40°C. The measured responses for Y1, Y2, and Y3 were 0.0105%, 0.52%, and 3.2%, respectively (Table V). Finally, the rate constant of aggregation, Y4, in the optimum formulation was 0.34%. The desirability plot of the optimization process and the overlay plot showing the region of variable settings (human serum albumin (HSA) amount = X1 and trehalose amount = X2) are shown in Figs. 6 and 7, respectively. The yellow space in the overlay plot is demonstrated by the optimum region for variables which resulted in minimized aggregation after the process and following 2 and 3 months of storage at 40°C. The optimum formulation of IgG was composed of 100 mg IgG, 86 mg trehalose, and 1 mg HSA which is comparably presented in the overlay plot.
In order to validate the suggested model, the observed values of each response were compared with the proposed predicted magnitudes (Table V). As can be observed, for all responses, the measured errors were less than 5% confirming the validity of the fitted model. Although it was proved that raising the amount of trehalose promoted the IgG stability after process and storage, in the optimized run, the level of trehalose was significantly lower than that of the defined upper limit (86 mg instead of 100 mg). Also, contrary to some existing publications, higher ratios of HSA were not necessarily better in terms of antibody protection against aggregation and even more aggregates were created at higher values of albumin (16). It could be rationally concluded that co-administration of trehalose and HSA decreased the required amount of trehalose from the routine molar ratio of IgG to trehalose 1:360 to 1:333. The optimized formulation was evaluated further through SDS-PAGE, FTIR, and DCS analyses.
Beta-Sheet Content of Optimized IgG Formulation
FTIR spectroscopy is frequently used to determine the secondary structure of peptides and proteins. Based on previous research, more than 66% of the secondary structure of immunoglobulin G is composed of beta-sheet (35). The FTIR spectrum of the optimized formulation can be observed in Fig. 8. The beta-sheet-related bands were seen at 1613, 1621, 1639, and 1691 cm−1 with a total percentage of 76%, providing the preservation of antibody stability following FD and in the presence of Trehalose and HSA.
SDS-PAGE
SDS-PAGE was carried out to qualitatively visualize the IgG integrity in the optimized formulation following the process and 2 months of storage under reducing conditions (Fig. 9). In comparison with the data from SEC-HPLC, there were not any observable fragmentations of antibody. Two distinct bands at 50 kDa (heavy chain) and 25 kDa (light chain) were indicative of IgG antibody. The intact band at 66 kDa was related to pure HSA, lane 1.
DSC Analysis
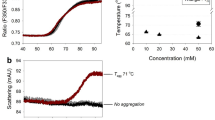
DSC analysis was applied to characterize the thermal behavior of optimum formulation in terms of amorphous/crystalline type (Fig. 10). Sample 1 was pure HSA. Interestingly, the achieved thermogram was completely similar to an existing report in which two peaks at approximately 110°C and 215°C were found and were typical of albumin (36). Unfolding of HSA weak bonds occurred at a lower temperature and the stronger ones happened at higher temperatures. Samples 2 and 3 were composed of 86 mg trehalose and 1 mg HSA as an optimized formulation. Minor differences were observed between samples 2 and 3, as thermal events at about 59°C and 160°C. Sample 2 was analyzed following 3 months of storage at 40°C, while sample 3 was characterized immediately after the processing. Note that both samples 2 and 3 were desirably preserved under the process and storage conditions. Lack of any special thermal appearances up to the storage temperature definitely confirmed the existence of an amorphous state of all excipients and their complete ability for stabilization of IgG via formation of glassy-state amorphous matrix.
CONCLUSIONS
The combination of trehalose and HSA was studied on the molecular and structural stability of freeze-dried IgG as a model antibody. The design of the experiment methodology was employed to statistically investigate the separate role of additives in addition to their possible interactions. It was proved that there was a positive interaction between HSA and trehalose both after the process as well as upon storage at high temperatures. Although increasing the amount of trehalose enhanced the antibody stability against aggregation, the synergistic effect induced by albumin reduced the required amount of trehalose (from its common molar ratio of 1:360 IgG to disaccharide). It could be concluded that the combination of HSA and sugars like trehalose would be a rational approach to obtain desired stability of antibody while adding totally lower levels of excipients. Supplemental studies could be proposed to mechanistically evaluate the role of HSA, as antibody stabilizers, in more detail.
References
Goswami S, Wang W, Arakawa T, Ohtake S. Developments and challenges for mAb-based therapeutics. Antibodies. 2013;2(3):452–500.
Carpenter JF, Pikal MJ, Chang BS, Randolph TW. Rational design of stable lyophilized protein formulations: some practical advice. Pharm Res. 1997;14(8):969–75.
Abdul-Fattah AM, Kalonia DS, Pikal MJ. The challenge of drying method selection for protein pharmaceuticals: product quality implications. J Pharm Sci. 2007;96(8):1886–916.
Bhatnagar BS, Bogner RH, Pikal MJ. Protein stability during freezing: separation of stresses and mechanisms of protein stabilization. Pharm Dev Technol. 2007;12(5):505–23.
Luthra S, Obert JP, Kalonia DS, Pikal MJ. Investigation of drying stresses on proteins during lyophilization: differentiation between primary and secondary-drying stresses on lactate dehydrogenase using a humidity controlled mini freeze-dryer. J Pharm Sci. 2007 Jan;96(1):61–70.
Kamerzell TJ, Esfandiary R, Joshi SB, Middaugh CR, Volkin DB. Protein-excipient interactions: mechanisms and biophysical characterization applied to protein formulation development. Adv Drug Deliv Rev. 2011;63(13):1118–59.
Liao Y-H, Brown MB, Quader A, Martin GP. Protective mechanism of stabilizing excipients against dehydration in the freeze-drying of proteins. Pharm Res. 2002;19(12):1854–61.
Mensink MA, Frijlink HW, van der Voort MK, Hinrichs WLJ. How sugars protect proteins in the solid state and during drying (review): mechanisms of stabilization in relation to stress conditions. Eur J Pharm Biopharmaceutics. 2017;114:288–95.
Chang L, Shepherd D, Sun J, Ouellette D, Grant KL, Tang XC, et al. Mechanism of protein stabilization by sugars during freeze-drying and storage: native structure preservation, specific interaction, and/or immobilization in a glassy matrix? J Pharm Sci. 2005;94(7):1427–44.
Hawe A, Friess W. Stabilization of a hydrophobic recombinant cytokine by human serum albumin. J Pharm Sci. 2007;96(11):2987–99.
Johnston MJ, Nemr K, Hefford MA. Influence of bovine serum albumin on the secondary structure of interferon alpha 2b as determined by far UV circular dichroism spectropolarimetry. Biologicals. 2010;38(2):314–20.
Tarelli E, Mire-Sluis A, Tivnann HA, Bolgiano B, Crane DT, Gee C, et al. Recombinant human albumin as a stabilizer for biological materials and for the preparation of international reference reagents. Biologicals. 1998;26(4):331–46.
Chaubal M. Human serum albumin as a pharmaceutical excipient. Drug Deliv Technol. 2005;5(8).
Liu W. The impact of formulation composition on the stability of freeze-dried proteins. 2000.
Wang W. Lyophilization and development of solid protein pharmaceuticals. Int J Pharm. 2000;203(1–2):1–60.
Hawe A, Friess W. Formulation development for hydrophobic therapeutic proteins. Pharm Dev Technol. 2007;12(3):223–37.
Anchordoquy TJ, Carpenter JF. Polymers protect lactate dehydrogenase during freeze-drying by inhibiting dissociation in the frozen state. Arch Biochem Biophys. 1996;332(2):231–8.
Mohammad Zadeh AH, Rouholamini Najafabadi A, Vatanara A, Faghihi H, Gilani K. Effect of molecular weight and ratio of poly ethylene glycols’ derivatives in combination with trehalose on stability of freeze-dried IgG. Drug Dev Ind Pharm. 2017;43(12):1945–51.
Hanrahan G, Lu K. Application of factorial and response surface methodology in modern experimental design and optimization. Crit Rev Anal Chem. 2006;36(3–4):141–51.
Granato D, de Araújo Calado VM. The use and importance of design of experiments (DOE) in process modelling in food science and technology. Mathematical and Statistical Methods in Food Science and Technology: John Wiley & Sons, Ltd; 2014. p. 1–18.
Walia A, Mehta P, Guleria S, Shirkot CK. Improvement for enhanced xylanase production by Cellulosimicrobium cellulans CKMX1 using central composite design of response surface methodology. 3 Biotech. 2015 06/0312/16/received05/12/accepted;5(6):1053–66.
Gunst RF. Response surface methodology: process and product optimization using designed experiments. Technometrics. 1996;38(3):284–6.
Geigert J, Ziegler DL, Panschar BM, Creasey AA, Vitt CR. Potency stability of recombinant (Serine-17) human interferon-beta. J Interf Res. 1987;7(2):203–11.
Wang W, Singh S, Zeng DL, King K, Nema S. Antibody structure, instability, and formulation. J Pharm Sci. 2007;96(1):1–26.
Felgner PL, Wilson JE. Hexokinase binding to polypropylene test tubes: artifactural activity losses from protein binding to disposable plastics. Anal Biochem. 1976;74(2):631–5.
Dawson PJ. Effect of formulation and freeze-drying on the long-term stability of rDNA-derived cytokines. Dev Biol Stand. 1992;74:273–82 discussion 82-4.
Arakawa T, Kita Y, Carpenter JF. Protein--solvent interactions in pharmaceutical formulations. Pharm Res. 1991;8(3):285–91.
Cleland JL, Lam X, Kendrick B, Yang J, Yang TH, Overcashier D, et al. A specific molar ratio of stabilizer to protein is required for storage stability of a lyophilized monoclonal antibody. J Pharm Sci. 2001;90(3):310–21.
Srirangsan P, Kawai K, Hamada-Sato N, Watanabe M, Suzuki T. Stabilizing effects of sucrose–polymer formulations on the activities of freeze-dried enzyme mixtures of alkaline phosphatase, nucleoside phosphorylase and xanthine oxidase. Food Chem. 2011;125(4):1188–93.
Balcão VM, Vila MMDC. Structural and functional stabilization of protein entities: state-of-the-art. Adv Drug Deliv Rev. 2015;93:25–41.
Wetzel R, Becker M, Behlke J, Billwitz H, Bohm S, Ebert B, et al. Temperature behaviour of human serum albumin. Eur J Biochem. 1980;104(2):469–78.
Han Y, Jin BS, Lee SB, Sohn Y, Joung JW, Lee JH. Effects of sugar additives on protein stability of recombinant human serum albumin during lyophilization and storage. Arch Pharm Res. 2007;30(9):1124–31.
Jain NK, Roy I. Effect of trehalose on protein structure. Protein Sci. 2009;18.
Faghihi H, Vatanara A, Najafabadi AR, Ramezani V, Gilani K. The use of amino acids to prepare physically and conformationally stable spray-dried IgG with enhanced aerosol performance. Int J Pharm. 2014;466:163–71.
Jones LS, Randolph TW, Kohnert U, Papadimitriou A, Winter G, Hagmann ML, et al. The effects of Tween 20 and sucrose on the stability of anti-L-selectin during lyophilization and reconstitution. J Pharm Sci. 2001;90:1466–77.
Wan X, Zheng X, Pang X, Pang Z, Zhao J, Zhang Z, et al. Lapatinib-loaded human serum albumin nanoparticles for the prevention and treatment of triple-negative breast cancer metastasis to the brain. Oncotarget. 2016 04/1210/27/received03/18/accepted;7(23):34038–51.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nouraei, M.S., Faghihi, H., Najafabadi, A.R. et al. Human Serum Albumin, a Suitable Candidate to Stabilize Freeze-Dried IgG in Combination with Trehalose: Central Composite Design. AAPS PharmSciTech 20, 327 (2019). https://doi.org/10.1208/s12249-019-1509-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1509-6