Abstract
The current investigation aimed at formulating self-microemulsifying drug delivery system (SMEDDS) to ameliorate oral bioavailability of a hydrophobic functional ingredient, limonene. Solubility test, compatibility test, and pseudo-ternary phase diagrams (PTPD) were adopted to screen the optimal compositions of limonene-SMEDDS (L-SMEDDS). The characteristics of this system assessed in vitro, mainly included determination of particle size distribution, observation of morphology via transmission electron microscopy (TEM), testing of drug release in different dissolution media, and evaluation of stability. The oral bioavailability study in vivo of the formulated limonene was performed in rats with the free limonene as the reference. Compared with the free limonene, the distribution study of L-SMEDDS was conducted in Kunming mice after oral administration. The optimized SMEDDS (ethyl oleate, 14.2%; Cremophor EL, 28.6%; isopropanol, 28.6%; and loaded limonene, 28.6%) under the TEM (about 100 nm) was spherical with no significant variations in size/appearance for 30 days at 4, 25, and 60°C. In comparison with free limonene, higher than 89.0% of limonene was released from SMEDDS within 10 min in different dissolution media. An in vivo study showed a 3.71-fold improved oral bioavailability of the formulated limonene compared to the free limonene. The tissue distribution results showed that limonene predominantly accumulated in the various tissues for the L-SMEDDS compared with the free limonene. Hence, L-SMEDDS could remarkably improve the concentration of limonene in the various organs. These findings hinted that the oral bioavailability of limonene could be improved via an effectual delivery system like SMEDDS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Extensive research is ongoing to investigate the functional ingredients obtained from edible plants in eastern countries, especially in China, for their potential applications in food engineering and medicinal use. The beneficial health effects of the functional components exhibited relatively moderate toxicity and secondary side effect than drugs used to eventually treat similar illnesses. Among them, limonene seems to be a typical representative.
Limonene (1-methyl-4-isopropenyl-1-cyclohexene, Fig. 1), a major aromatic compound in lemon essential oils and the most commonly encountered terpenes in nature, has been isolated from oranges, grapefruits, and lemons (1). For the past two decades, limonene has been broadly applied as an adjuvant in perfumes, soaps, and beverages for its pleasant citric fragrance. Apart from its utility possessing attributes like flavoring and scented agents, it is also associated with various biofunctional and pharmacological activities, such as anti-oxidation (2), anti-microbial (3), anti-depressant-like (4), and anxiolytic-like activities (5). Moreover, limonene is effective in dissolving cholesterol-containing gallstones, promoting secretion of gastric acid and treating cancers including lung neoplasms, pulmonary adenoma, colon (6), and liver cancer (7).
Limonene, as a hydrophobic functional constituent, has gained worldwide attention owing to its safety when used as the food additive as well as active pharmacological component. However, its poor dissolution attribute is the main obstruction in the development of effectual oral formulations. Previous study on the oral formulation of limonene was mostly in capsule form (8). The product named Limonene Capsules (Sichuan Changwei Pharmaceutical Co., Ltd.) is already on the market in China, with its associated low therapeutic effect and high dose requirement (9–15 capsules per day) against gastroesophageal reflux disorder, which render limonene inappropriate for clinical applications. More importantly, this oral product has been withdrawn from the market due to its limited bioavailability and clinical efficacy. Therefore, in order to serve as clinical drug or part of daily diet, the absorption and bioavailability of limonene should be improved with sustained meaningful therapeutic level when ingested orally. At present, various formulations such as liposome, nanoemulsion (9), and β-cyclodextrin (10) have been explored to improve the solubility and the oral absorption of limonene.
In the last decade, great attention in pharmaceutics, nutraceutics, and food sciences has been centered on self-microemulsifying drug delivery system (SMEDDS) as a promising approach for delivery of hydrophobic functional ingredients or compounds. SMEDDS is composed of isotropic mixtures of water-insoluble compounds, oils, surfactants, plus cosurfactants, which form microsized emulsion with particle diameter smaller than 200 nm when exposed to aqueous phase after oral administration under gastrointestinal fluid and gastrointestinal motility conditions (11). In this system, the bioactive compounds are dispersed mainly in the oil phase, to keep bioactive compounds soluble, thus enhancing their solubility, avoiding the slow and rate-limiting dissolution process (12), improving the dissolution rate, and promoting oral absorption in gastrointestinal tract (13). Furthermore, SMEDDS is a novel drug delivery system that has attracted increasing interests because of its promising properties of hydrotropy (14), low dose of formulation needed, thermodynamic stability, and ease of manufacturing. Extensive previous studies have confirmed that SMEDDS could be widely used to increase the solubility, absorption, and bioavailability of hydrophobic functional ingredients (15), such as puerarin found in roots of Pueraria lobata, curcumin from Curcuma longa L., and Coenzyme Q10 from meat or fish. Specifically, several hydrophobic compounds, like cyclosporin A, ritonavir, and saquinavir (16), formulated in SMEDDS have been successfully commercialized. However, to the best of our knowledge, L-SMEDDS is yet to be evaluated.
Therefore, in this study, the disadvantage of limonene was surmounted via preparation of a fine L-SMEDDS to enhance the oral bioavailability of limonene. The solubility of limonene was tested in different vehicles, while an in vitro release study was performed to assess the dissolution properties of the formulated limonene. Then, the pharmacokinetics of formulated and unformulated limonene after oral administration in rats was evaluated to determine whether SMEDDS formulations could have the capacity to enhance oral bioavailability. In addition, the evaluation of the biodistribution of free limonene and L-SMEDDS in Kunming mice was also conducted following oral administration.
MATERIALS AND METHODS
Materials
Limonene (95% purity) was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Shanghai Feixiang Chemical Co., Ltd. (Shanghai, China) provided ethyl oleate. Isopropanol, oleic acid, PEG 400, Tween 80, Span 80, glycerol, 1,2-propanediol, and isopropyl myristate (IPM) were bought from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Cremophor EL was supplied from BASF (Ludwigshafen, Germany). Soybean oil and medium chain triglycerides (MCT) were obtained from Tieling Beiya Medicinal Oil Co., Ltd. (Tieling, China). Hanbon Science and Technology (Jiangsu, China) provided pure chromatographic methanol. Chromatographically pure acetonitrile was bought from Honeywell Burdick & Jackson (Muskegon, USA). All other chemicals and reagents involved in this study were of analytical grade and were obtained commercially. All animals used fully complied with local and national ethics, as well as licensing requirements of Laboratory Animal Management Assessment and Accreditation (AAALAC) International.
In vitro HPLC Analysis
The Shimadzu HPLC consisting of an LC-20AT pump and SPD-20A UV-Vis detector (Shimadzu, Kyoto, Japan) and a Symmetry C18 column (4.6 mm × 150 mm, 5 μm, Waters, Milford, Massachusetts, USA) with temperature of 30°C was employed for the quantitative analysis of limonene. The mobile phase composing of acetonitrile and H2O (85:15, v/v) was pumped isocratically into the HPLC equipment at 1.0 mL/min. An aliquot (20 μL) of the sample was injected into HPLC system and detected at 205 nm. The retention time for limonene was about 7.5 min. The in vitro assay exhibited a linear relationship (r2 = 0.9999) in the concentration range of 1 to 100 μg/mL. The relative standard deviations (RSDs) of inter- and intra-day precision as well as accuracy of the HPLC method all ranged below 1.6% (at low, medium, and high concentrations). Finally, the average recoveries of the analytes were above 96.1%.
Solubility Studies
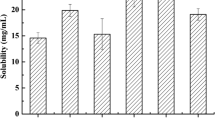
As reported in Table I, limonene was formulated using different vehicles, and the solubility of the formulated limonene was determined accordingly. Briefly, excessive limonene was added into certain amount of different dispersion medium (1 mL), including oils, surfactants, cosurfactants, and water. The accurate weights of the mixtures were recorded for further calculation. Then, the mixtures were continuously shaken in a Water-bathing Constant Temperature Vibrator (TH2-82A, Jintan Zhongda Instrument Corporation, Jintan, Jiangsu, China) at 25°C for 72 h after being vortexed for 3 min. After achieving equilibrium, the different resultant mixtures were subjected to a centrifugation (1237×g for 10 min) to observe the appearance while insoluble limonene was separated. Then, the residual sample (20 μL) was suitably diluted with acetonitrile and analyzed via HPLC method (as mentioned in the in vitro HPLC analysis section) to quantify limonene.
Compatibility Tests
In order to assess the compatibility between the oil and surfactant solution, the ethyl oleate as the oil phase as well as the Cremophor EL and Span 80 as the surfactants were selected based on the solubility results of limonene. Briefly, ethyl oleate (1 mL) was appropriately added to Cremophor EL (1 mL) and Span 80 (1 mL) via mixing (for 3 min). Then, the mixtures were centrifuged (1237×g for 10 min) to observe the appearance of the solutions. In microemulsion system, the clearance, transparency and no phase separation of the resultant solution are used as indicators for evaluating the compatibility of the surfactant solution and oil (17). Similarly, based on its compatibility with the mixed system of the selected oil and surfactant solutions, isopropanol was chosen as a cosurfactant.
Construction of Pseudo-Ternary Phase Diagrams
The pseudo-ternary phase diagram (PTPD) is a tool for identifying the well-suited ratios of constituents in the SMEDDS in order to choose the optimal formulation (18). As described in the previous study (19), water titration method was applied in constructing PTPD of SMEDDS containing oils, surfactants, cosurfactants, and water at various ratios. In this study, surfactant and cosurfactant were considered the components of SMEDDS, and the weight ratio between them (Km) was varied as 8:2, 7:3, 6:4, 5:5, 4:6, and 3:7. Then, the oil phase was added and mixed with each mixture of surfactant and cosurfactant (Smix) thoroughly in different oil-Smix ratios of 0:10, 0.5:9.5, 1:9, 1.5:8.5, 2:8, 2.5:7.5, 3:7, 3.5:6.5, 4:6, 4.5:5.5, and 5:5, respectively. After that, each oil-Smix mixture (2.0 g) was titrated with double-distilled water (DDW) in a drop-wise manner under gentle magnetic stirring at room temperature. The end-point of titration was determined by sudden change of the solution to transpicuous and flowable liquid. The mass fraction of different group was recorded to construct the PTPD.
Preparation and Optimization of L-SMEDDS
Different SMEDDS formulations (F1 to F9 compositions) presented in Table II were maintained at the same Km (5:5). The oil and Smix were mixed at the weight ratios of 0:10, 5:5, 4:6, 3:7, and 2:8. To prepare L-SMEDDS, surfactant and cosurfactant were mixed via magnetic stirring at 25°C, and then the oil phase was added to the mixture. Afterwards, limonene was added with continuous stirring until the mixture became clear and transparent. An aliquot of L-SMEDDS (50 μL) was dispersed in DDW (100 mL). The time of self-microemulsification was observed following the protocol reported by Bachynsky et al. (20). Meanwhile, the dispersibility and appearance were also evaluated visually in accordance with five grading system (21) shown in Table III to screen for the optimal formulation.
Characterization of the Optimized L-SMEDDS
Particle Diameter and Zeta Potential Analysis
Before the particle diameter measurement, the optimized L-SMEDDS (100 μL) was diluted and mixed thoroughly DDW (100 mL) with gentle stirring. The particle diameter/distribution of the diluted SMEDDS was detected by dynamic light Malvern Zetasizer (Malvern Instruments, UK) at 25°C for three times. Each reported data was the average of triplicate measurements. The same instrument was used to measure zeta potential of the particles, which operated under the similar conditions for the diluted sample.
Morphology Observation
The transmission electron microscopy (TEM, JEM-2100, JEOL, Tokyo, Japan) was applied for observing the morphology of the optimized limonene-loaded SMEDDS. In order to prepare suitable sample for the TEM observation, the SMEDDS solution was dropped on a copper grid, and dyed with phosphotungstic acid (2%) prior to drying at 25°C.
Stability Study
The stability of the optimized L-SMEDDS sealed in a 10-mL centrifugal tube was assessed via centrifugation and temperature tests, wherein samples were stored at 4°C, 25°C, and 60°C for one month. In the centrifugation test, the samples were exposed to a centrifuge at 13750×g for 10 min after day 1, day 15, and day 30. This was followed by the observation of their physical appearance. In addition, the particle size and limonene content were monitored to assess the comprehensive stability of L-SMEDDS.
In vitro Release Study
To evaluate the releasing profile of optimized L-SMEDDS, the formulated limonene containing 250 mg limonene or 250 mg of free limonene was loaded into hard gelatin capsules. The L-SMEDDS capsule and free limonene capsule were fixed on the paddles of a dissolution apparatus (ZRS-8G, Tianjin University Radio Factory, China) before their immersion into 200 mL HCl buffer (pH = 1.2), phosphate buffer (pH = 6.8), and DDW. The test was carried out in triplicate at 37°C with a constant speed of 100 rpm. Aliquot (2 mL) of sample was withdrawn and replaced with fresh medium at 10, 20, 30, 45, 60, and 90 min. Then, all the sample solutions were centrifuged at 1684×g for 5 min. Finally, the supernatant (20 μL) was used to ascertain limonene content using HPLC method described previously.
In vivo Pharmacokinetic Study
Experimental protocol for this study was approved and conformed to the guide spelt out by Jiangsu University’s Ethics Committee for the Care and Use of Laboratory Animals. Male Sprague Dawley (SD, n = 12) rats, weighing 250 ± 20 g, were supplied by the Center for Laboratory Animals at Jiangsu University (Zhenjiang, China). All animals were adjusted to environmentally standard conditions and fed with a rodent diet and water ad libitum for at least three days.
All the rats were fasted for 12 h, but allowed free access to water before the experiment. The rats were randomly and equally divided into two groups followed by an oral administration of free limonene or L-SMEDDS with the same dose of limonene (75 mg/kg), respectively. To obtain the plasma containing limonene, blood was collected from the eye-orbit region (about 0.75 mL) at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 24h after the limonene administration. Then, the plasma was obtained by centrifugation at 13750×g for 10 min and stored at − 20°C for further analysis. Limonene in plasma were quantified by the same HPLC system and chromatographic conditions for analyzing in vitro samples, except for the mobile phase, which consisted of 70% acetonitrile containing 0.5% phosphoric acid for the analysis of the plasma samples.
Fifty microliters of betulinic acid solution (50 μg/mL in tetrahydrofuran), an internal standard solution, was vortex-mixed with plasma (200 μL) for 1 min. Afterwards, limonene in plasma was extracted through addition of acetonitrile (450 μL) to the plasma samples with vortex mixing for 3 min. The system was separated through centrifugation (13,750×g; 5 min) and the supernatant was quantitatively measured with HPLC method described above. The calibration curve of internal standard substance was constructed over a range of 0.0125~2.00 μg/mL in plasma (r2 = 0.9971) and it was validated for inter- and intra-day differences within acceptable range.
Herein, the pharmacokinetic parameters were calculated using BAPP 2.3 pharmacokinetic software (supplied by the center of drug metabolism of China Pharmaceutical University, China).
The relative bioavailability (Fr) of the drug was calculated using the Eq. (1):
where AUCT and AUCR were the areas under the concentration-time curve of the L-SMEDDS and free limonene, respectively.
Tissue Distribution Study
In the biodistribution study, 60 male Kunming mice (20 ± 2 g, n = 5) were randomly and equally divided into two groups. Each group was received oral administration of free limonene or L-SMEDDS at a same dosage (30 mg/kg) respectively. Various tissues were collected at 0.5, 1, 2, 4, 6, and 8 h after the administration and rinsed with physiological saline. Tissues were blotted dry and kept frozen (− 20°C) until sample pretreatment.
The tissues were homogenized in standard saline using high-speed shearing homogenizer (FA25, Fluko, Germany) to obtain tissue homogenates. Then, 50 μL of the tetrahydrofuran solution of betulinic acid (50 μg/mL) and acetonitrile (400 μL) were added successively into tissue homogenates (200 μL). After a brief vortexing and centrifugation (10,000 rpm; 10 min), supernatant was collected in a fresh tube and tissue samples (20 μL) were used for HPLC analysis.
Statistical Analysis
All values were expressed as mean ± SD. The statistical differences between two different groups or two related parameters were assessed using student’s t test and one-way analysis of variance with a least significant difference post hoc test. SPSS statistic software (SPSS version 15.0, SPSS Inc., Chicago, IL, USA) was applied for the analysis with p < 0.05 considered the statistical significance level for the differences between means.
RESULTS
Solubility Studies
The solubility of limonene in different vehicles is presented in Table I. The hydrophobicity of limonene resulted in poor aqueous solubility (0.047 mg/g). However, limonene was more soluble in all the oily phases, with highest solubility (628.04 mg/g) in ethyl oleate. Therefore, the ethyl oleate served as oil phase of limonene in L-SMEDDS. Among the three tested surfactants, Span 80 (165.54 mg/g) and Cremophor EL (161.66 mg/g) showed higher limonene solubility but was not statistically significant. Therefore, investigation of the compatibility between oil and surfactant solutions for selection of the best surfactant became paramount. The mixture of Cremophor EL and ethyl oleate was transparent with no phase separation, while the mixture of Span 80 and ethyl oleate was turbid. For this reason, the Cremophor EL was chosen as the desirable surfactant rather than Span 80. In the selection of cosurfactant, isopropanol showed the best solubility for limonene (Table I) and demonstrated better compatibility with ethyl oleate and Cremophor EL. Thus, isopropanol was chosen as a suitable cosurfactant for developing L-SMEDDS.
Pseudo-Ternary Phase Diagrams
A series of blank SMEDDS with manifold component ratios were prepared, and their self-microemulsifying properties observed visually to investigate the phase behavior. The PTPD containing ethyl oleate (oil phase), the mixture of Cremophor EL and isopropanol, and DDW (aqueous phase) with different Km values are depicted in Fig. 2. It was worth noting that the area of the black part (self-microemulsification region) decreased with a peak at the ratio of 5:5 as Km value increased. More importantly, a significant decrease in the self-microemulsification region at a ratio of 4:6 or 3:7 was observed.
Preparation and Optimization of L-SMEDDS
Self-microemulsification was used to optimize the component ratios of the L-SMEDDS based on the same Km value. All the prepared formulations upon dilution with DDW were observed and graded from I to V (Table III). Limonene could also act as oil phase in SMEDDS because it is a volatile oil similar to the edible oil. Nevertheless, as shown in Table II, formulation F1 with only limonene as oil phase was classified visually as grade III with a bright white appearance, suggesting that limonene was not suitable as the sole oil phase in preparing SMEDDS. It was obvious in Table II that formulations F5, F6, F7, and F8 exhibited the same grade (grade I). However, because high limonene loading and less surfactant might lead to less irritation in gastrointestinal tract (22), formulation F8 was selected as the optimal formulation.
Characterization of the Optimized L-SMEDDS
The average particle diameter of the optimized L-SMEDDS was determined using dynamic light scattering measurements. The size distribution was obviously within a narrow range with mean particle diameter of 104.3 ± 0.6 nm. The zeta potential of L-SMEDDS was − 6.51 ± 0.31 mv. The morphological characterization of the optimized L-SMEDDS is shown in Fig. 3. The TEM image that displayed the individual particles was almost spherical in shape with moderate uniformed particle size.
As described in Table IV, the optimized L-SMEDDS exhibited desirable stability with its particle size maintained within 100~120 nm after storage at 4, 25, and 60°C for a month. The optimized L-SMEDDS solution containing limonene was kept clear and transparent without any phase separation at the end of 30 days. However, significant difference was found in limonene content. At three storage temperatures (4, 25, and 60°C) for 30 days, the contents of limonene were respectively 89%, 83%, and 42%, compared with 0 day, which indicate that limonene degraded more at higher temperatures (23). L-SMEDDS ought to be kept in low-temperature condition.
In vitro Release Study
The in vitro releasing profiles of the optimized L-SMEDDS in HCl buffers (pH = 1.2), phosphate buffer (pH = 6.8), and DDW are described in Fig. 4. Obviously, more limonene could be released from the SMEDDS than that of the free limonene in these three media within the same time point. More than 89.0% of limonene was released from SMEDDS formulation within the initial 10 min. Meanwhile, only 1.4%, 0.6%, and 3.9% of limonene were released from the free limonene in the respective media (HCl solution, DDW, and phosphate buffer solution) within 90 min. A negligible release even after 90 min was displayed from the release profile of free limonene since the free drug was practically aqueous insoluble as supported by the results of solubility tests presented in Table I.
In vivo Oral Bioavailability Study
Figure 5 presents the plasma concentration-time profiles of limonene after oral administration of free and formulated limonene. L-SMEDDS exhibited higher concentration of limonene in plasma than that of the unformulated drug at any given time point, particularly from 3 to 8 h. The plasma concentrations of limonene reached the peak at 6 h after oral administration of L-SMEDDS, while the free limonene also showed a slow absorption with the same Tmax (6 h). Evidently, the kinetic curves of the formulated and unformulated limonene exhibited double peaks after oral administration.
The mean pharmacokinetic parameters for unformulated and formulated limonene are listed in Table V. The L-SMEDDS showed a higher mean Cmax and AUC0–24h of 985.0 ± 28.5 ng/mL and 8678.7 ± 473.6 h·ng/mL respectively as compared to the free limonene (330.4± 19.7 ng/mL and 2335.9 ± 237.5 h·ng/mL). This demonstrates that the formulated limonene could be absorbed more and in greater concentration than free limonene. SMEDDS formulation showed almost similar MRT (7.2 h) as compared to the free limonene (7.1 h). No prolonged behavior was observed in this SMEDDS formulation. In the previous study, Chan et al. (24) reported that the plasma level of d-limonene was very low in the conventional formulation and the oral bioavailability of d-limonene was 43%. However, in this research, the relative oral bioavailability of formulated limonene was 371% compared with unformulated limonene, indicating that the SMEDDS formulation could significantly improve its oral bioavailability.
Tissue Distribution Study
Generally, limonene was predominantly distributed in the different tissues for the formulation compared with the free drug. Following oral administration of free limonene and L-SMEDDS (30 mg/kg each), the biodistribution of accumulated limonene was analyzed within 8 h using HPLC. The weights of accumulated limonene from free drug and L-SMEDDS in vital organs are shown in Fig. 6. Limonene was widely distributed among most organs, but the distribution trend was different in various organs. The highest concentration of limonene was in the liver and kidney with a Cmax of 74.1 and 60.3 μg/g respectively after orally administration of L-SMEDDS at 6 h. In comparison with the free limonene, L-SMEDDS could improve the limonene distribution in all the organs. However, the concentration of limonene declined quickly in the kidneys from 6 h (60.7 μg/g) to 8 h (17.1 μg/g), which suggested that most of the drug were eliminated gradually at the end of 8 h. The concentration of limonene in brain for L-SMEDDS was 26.7 μg/g at 8 h. The addition of oil phase and the surfactant in microemulsion might have increased the lipophilicity of the formulation so that L-SMEDDS could improve the biodistribution of limonene in brain.
DISCUSSION
The therapeutic efficiency or physiological benefits of limonene are greatly limited by its insolubility and low oral absorption. SMEDDS in recent times has become a promising approach in solving the problem of solubility and absorption for hydrophobic drugs. Hence, this is a first study attempted to formulate limonene in SMEDDS since it is an efficient and effective delivery system to improve solubility, oral absorption, and bioavailability of limonene. In the current investigation, the following important points were considered in the screening for suitable compositions for L-SMEDDS: (a) a high solubilizing capacity for limonene to achieve optimum drug loading (10) in various oils, surfactants, and cosurfactants; (b) the compatibility and safety of selected formulation compositions; and (c) the self-microemulsifying efficiency of the aforementioned excipients.
Oil is a vital vehicle of the SMEDDS formulation with better capacity to solubilize large quantities of hydrophobic drugs. Thus, the presence of oil phase kept insoluble drugs solubilized during the entrapment of these drugs in the SMEDDS system. Therefore, it has the ability to improve the releasing rate and oral bioavailability. The choice of oil to prepare SMEDDS mainly depends on its drug loading capability. Among the tested oils, ethyl oleate was selected based on its ability to solubilize limonene and was therefore employed to develop SMEDDS in the present study.
A surfactant is required to provide the essential microemulsifying characteristics of SMEDDS. The three issues that govern the selection of a suitable surfactant encompass its safety, better ability to solubilize drug, and hydrophile-lipophilic balance (HLB). The higher HLB values showed better microemulsification ability, which enabled rapid and clear dispersion of the oil in the aqueous phase as very fine and uniform droplets (25). Thus, for attaining high microemulsifying performance, Cremophor EL with the high HLB value (12,13,14) was employed in the preparation of SMEDDS. Moreover, compared to ionic surfactants, Cremophor EL, a member of nonionic surfactants with higher safety than the others, is usually applied for oral administration.
SMEDDS is well recognized to spontaneously produce smaller microemulsion under conditions of water or gastrointestinal fluids. The optimal weight ratio of oil, surfactant, and cosurfactant for preparing SMEDDS was obtained using effective self-microemulsification regions in PTPD. Generally, wider self-microemulsification region indicates efficient self-microemulsification capacity of different formulations. Hence, in this study, the formulated SMEDDS composed of ethyl oleate (oil) coupled with ratio of Cremophor EL (surfactant) to isopropanol (cosurfactant) {5:5} and exhibited broadest self-microemulsification region with the best self-microemulsifying efficiency. The variations in self-microemulsification regions could be observed at different Km values, which might be attributed to the different interaction between the oils and the tail regions of the surfactant, which is known to affect the degree of curvature of the interfacial layer (26). Usually, surfactants might not sufficiently diminish the water/oil interfacial tension in order to form a barrier against coalescence in low cosurfactant concentration (27), thereby resulting in a smaller self-microemulsification region. In addition, system containing less surfactant (or more cosurfactant) was found to demonstrate a smaller self-microemulsification region, suggesting the significance of surfactant in the ease of microemulsification, especially in systems with higher oil content. In general, a high ratio of surfactants is essential for achieving rapid and efficient self-microemulsification (28). However, the large amounts of surfactants present in the SMEDDS formulation could lead to gastrointestinal adverse effects after daily intake. Therefore, a novel supersaturable system containing a reduced amount of surfactant (formulation F8) was developed to improve oral absorption of limonene.
Particle size was a critical element for evaluating a self-microemulsifying system. Previous study indicated that the particle size determines the drug-releasing rate in vitro and influences the oral absorption in vivo (29). In the current study, the optimized formulation was observed to be nano-sized. SMEDDS containing limonene was dispersed in the aqueous phase in the nano-droplet form, which improved the solubility of limonene. Particle diameter was also one of the most important factors for the improvement of dissolution in all the three media (30). In addition, because of the small particle size, SMEDDS formulation was more thermodynamically stable against aggregation, flocculation, and coalescence (31). Consequently, the prepared SMEDDS solution was clarified and limpid with no phase separation at the end of the 30 days with statistical non-significant variation in particle size. For colloidal dispersions, the zeta potential parameter is highly correlated with stability. It is well recognized that a higher zeta potential value (above 30 mV) indicates a higher stability of a nano-system. However, previous studies have shown that SMEDDS with low zeta potential value could also augment its uptake and exert its stability and efficacy (32). The reasons mentioned above were of importance since a negative value of zeta potential was also observed for the prepared SMEDDS formulation, which could be attributed to the fatty acid components of the ethyl oleate (33).
Recently, quite a few investigations have elucidated the cause of the secondary peak due to its commonly occurring phenomenon. From previous reported mechanisms, the occurrence of double peaks might be ascribed to different rates of gastric emptying and short half-life of plasma eliminations as well as poor gastric absorption (34), extensive conjugation metabolism occurring during the first pass at the gut and liver, and the enterohepatic circulation (35). However, the bimodal appearance of limonene has not yet been reported. Therefore, the functional mechanisms about the phenomenon of double peaks in formulated and unformulated limonene should be investigated further.
For hydrophobic functional ingredients, the poor absorption was often due to its poor solubility in the gastrointestinal tract. Therefore, the enhanced oral bioavailability of limonene, which was loaded in SMEDDS, might be due to the higher solubilization, improved release rate, and advantages in spontaneous dispersing and forming small microemulsion in gastrointestinal tract of SMEDDS. These nano-sized droplets with larger surface area for absorption could improve the digestion rate, releasing rate, mucus layer diffusion, epithelium cell permeability, and absorption of limonene (36). Moreover, the improvement in lymphatic transport through the transcellular pathway could probably enhance the oral delivery by SMEDDS as well (37). Besides, as a composition in SMEDDS, surfactants might also affect the bioavailability of the limonene. The oral absorption could be enhanced due to the existence of surfactants in SMEDDS. Emerging reports have illustrated the potential effect of surfactants on cell membrane. For high surfactant concentrations in the cell membrane, surfactant-surfactant contact occurs, and the membrane could be dissolved into surfactant-membrane mixed micelles, which might result in enhanced intestinal permeability (38). Furthermore, surfactant demonstrated a reversible effect on the opening of tight junction, which could increase the permeability of limonene (39).
L-SMEDDS could significantly improve the biodistribution of limonene in the liver, spleen, kidney, and brain compared with free limonene. One reason for this phenomenon is that the nano-sized droplets could easily penetrate the wall of the gastrointestinal hydration layer, thereby improving the oral bioavailability (40). Another reason for the improvement of SMEDDS in the tissue distribution was the improved lymphatic transport through the transcellular pathway so that the drug could be transported into the lymph, which largely existed in the liver, spleen, and lung. In addition, the uptake of the hydrophobic limonene by macrophages of reticuloendothelial system (RES) was enhanced due to nano-sized droplets of L-SMEDDS. This resulted in an accumulated distribution of limonene in the liver and spleen, which exhibited a passive targeting function (41,42,43). The emulsifier and oil phase incorporated in L-SMEDDS might have significant impact on potential clinical application of limonene upon brain delivery.
CONCLUSION
In this study, self-microemulsifying drug delivery system (SMEDDS) was designed and prepared successfully to enhance the aqueous solubility and oral bioavailability of limonene for the first time. L-SMEDDS was stable during the one-month storage with statistical non-significant variation in the particle size and appearance. SMEDDS offers an alternative formulation for oral bioavailability enhancement of low therapeutic effect and commercially available capsules of limonene. The tissue distribution analysis further suggested that the L-SMEDDS could significantly improve the drug concentration in various tissues, particularly the liver, kidney, and brain. The present study further indicates that SMEDDS could act as a promising platform for improving oral bioavailability of functional but poorly soluble ingredients.
References
Lopresto CG, Petrillo F, Casazza AA, Aliakbarian B, Perego P, Calabrò V. A non-conventional method to extract d-limonene from waste lemon peels and comparison with traditional soxhlet extraction. Sep Purif Technol. 2014;137:13–20. https://doi.org/10.1016/j.seppur.2014.09.015.
Chiaradia DSM, Júlia VA, Pereira BF, Helena PC, Henrique NR, Leite RA. Gastroprotective effect of limonene in rats: influence on oxidative stress, inflammation and gene expression. Phytomedicine. 2019;53:37–42. https://doi.org/10.1016/j.phymed.2018.09.027.
Jiaqi S, Guo Q, Mao L, Gao Y, Yuan F. Effect of gum arabic on the storage stability and antibacterial ability of β-lactoglobulin stabilized d-limonene emulsion. Food Hydrocoll. 2018;84:75–83. https://doi.org/10.1016/j.foodhyd.2018.05.041.
Hao C-W, Lai W-S, Ho C-T, Sheen L-Y. Antidepressant-like effect of lemon essential oil is through a modulation in the levels of norepinephrine, dopamine, and serotonin in mice: use of the tail suspension test. J Funct Foods. 2013;5:370–9. https://doi.org/10.1016/j.jff.2012.11.008.
Lima NGPB, De Sousa DP, Pimenta FCF, Alves MF, De Souza FS, Macedo RO, et al. Anxiolytic-like activity and GC–MS analysis of (R)-(+)-limonene fragrance, a natural compound found in foods and plants. Pharmacol Biochem Behav. 2013;103:450–4. https://doi.org/10.1016/j.pbb.2012.09.005.
Kaur J, Kaur G. An insight into the role of citrus bioactives in modulation of colon cancer. J Funct Foods. 2015;13:239–61. https://doi.org/10.1016/j.jff.2014.12.043.
Moraes TM, Kushima H, Moleiro FC, Santos RC, Machado Rocha LR, Marques MO, et al. Effects of limonene and essential oil from Citrus aurantium on gastric mucosa: role of prostaglandins and gastric mucus secretion. Chem Biol Interact. 2009;180:499–505. https://doi.org/10.1016/j.cbi.2009.04.006.
Hu G, Yuan X, Zhang S, Wang R, Yang M, Wu C, et al. Research on choleretic effect of menthol, menthone, pluegone, isomenthone, and limonene in DanShu capsule. Int Immunopharmacol. 2015;24(2):191–7. https://doi.org/10.1016/j.intimp.2014.12.001.
Li PH, Lu WC. Effects of storage conditions on the physical stability of d-limonene nanoemulsion. Food Hydrocoll. 2016;53:218–24. https://doi.org/10.1016/j.foodhyd.2015.01.031.
Costa MDS, Rocha JE, Campina FF, Silva ARP, Da Cruz RP, Pereira RLS, et al. Comparative analysis of the antibacterial and drug-modulatory effect of Dlimonene alone and complexed with β-cyclodextrin. Eur J Pharm Sci. 2019;128:158–61. https://doi.org/10.1016/j.ejps.2018.11.036.
Kalhapure RS, Akamanchi KG. Oleic acid based heterolipid synthesis, characterization and application in self-microemulsifying drug delivery system. Int J Pharm. 2012;425:9–18. https://doi.org/10.1016/j.ijpharm.2012.01.004.
Mukherjee T, Plakogiannis FM. Development and oral bioavailability assessment of a supersaturated self-microemulsifying drug delivery system (SMEDDS) of albendazole. J Pharm Pharmacol. 2010;62:1112–20. https://doi.org/10.1111/j.2042-7158.2010.01149.x.
Yeom DW, Son HY, Kim JH, Kim SR, Lee SG, Song SH, et al. Development of a solidified self-microemulsifying drug delivery system (S-SMEDDS) for atorvastatin calcium with improved dissolution and bioavailability. Int J Pharm. 2016;506(1–2):302–11. https://doi.org/10.1016/j.ijpharm.2016.04.059.
Sprunk A, Strachan CJ, Graf A. Rational formulation development and in vitro assessment of SMEDDS for oral delivery of poorly water soluble drugs. Eur J Pharm Sci. 2012;46:508–15. https://doi.org/10.1016/j.ejps.2012.04.001.
Ting Y, Jiang Y, Ho C-T, Huang Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J Funct Foods. 2014;7:112–28. https://doi.org/10.1016/j.jff.2013.12.010.
Neslihan Gursoy R, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58:173–82. https://doi.org/10.1016/j.biopha.2004.02.001.
Cui J, Yu B, Yu Z, Zhu W, Li H, Lou H, et al. Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. Int J Pharm. 2009;371:148–55. https://doi.org/10.1016/j.ijpharm.2008.12.009.
Yeom DW, Song YS, Kim SR. Development and optimization of a self-microemulsifying drug delivery system for atorvastatin calcium by using D-optimal mixture design. Int J Nanomedicine. 2017;10(6):3865–77. https://doi.org/10.2147/IJN.S83520.
Zhu Y, Zhang J, Zheng Q, Wang M, Deng W, Qiang L, et al. in vitro and in vivo evaluation of capsaicin-loaded microemulsion for enhanced oral bioavailability. J Sci Food Agric. 2015;95(13):2678–85. https://doi.org/10.1002/jsfa.7002.
Bachynsky MO, Shah NH, Patel CI, Malick AW. Factors affecting the efficiency of a self-emulsifying Oral delivery system. Drug Dev Ind Pharm. 1997;23:809–16. https://doi.org/10.3109/03639049709150551.
Parveen R, Baboota S, Ali J, Ahuja A, Vasudev SS, Ahmad S. Oil based nanocarrier for improved oral delivery of silymarin: in vitro and in vivo studies. Int J Pharm. 2011;413:245–53. https://doi.org/10.1016/j.ijpharm.2011.04.041.
Sermkaew N, Ketjinda W, Boonme P, Phadoongsombut N, Wiwattanapatapee R. Liquid and solid self-microemulsifying drug delivery systems for improving the oral bioavailability of andrographolide from a crude extract of Andrographis paniculata. Eur J Pharm Sci. 2013;50:459–66. https://doi.org/10.1016/j.ejps.2013.08.006.
Nilsson U, Magnusson K, Karlberg O, Karlberg A-T. Are contact allergens stable in patch test preparations? Investigation of the degradation of d-limonene hydroperoxides in petrolatum. Contact Dermatitis. 1999;40:127–32. https://doi.org/10.1111/j.1600-0536.1999.tb06009.x.
Chen H, Chan KK, Budd T. Pharmacokinetics of d-limonene in the rat by GC–MS assay. J Pharmaceut Biomed. 1998;17:631–40. https://doi.org/10.1016/s0731-7085(97)00243-4.
Gupta S, Chavhan S, Sawant KK. Self-nanoemulsifying drug delivery system for adefovir dipivoxil: design, characterization, in vitro and ex vivo evaluation. Colloid Surface A. 2011;392:145–55. https://doi.org/10.1016/j.colsurfa.2011.09.048.
Malcolmson C, Satra C, Kantaria S, Sidhu A, Lawrence MJ. Effect of oil on the level of solubilization of testosterone propionate into nonionic oil-in-water microemulsions. J Pharm Sci. 1998;87:109–16. https://doi.org/10.1021/js9700863.
Cirri M, Mura P, Mora PC. Liquid spray formulations of xibornol by using self-microemulsifying drug delivery systems. Int J Pharm. 2007;340:84–91. https://doi.org/10.1016/j.ijpharm.2007.03.021.
Singh A, Chaurasiya A, Awasthi A, Mishra G, Asati D, Khar R, et al. Oral bioavailability enhancement of exemestane from self-microemulsifying drug delivery system (SMEDDS). AAPS PharmSciTech. 2009;10:906–16. https://doi.org/10.1208/s12249-009-9281-7.
Kang JH, Oh DH, Oh Y-K, Yong CS, Choi H-G. Effects of solid carriers on the crystalline properties, dissolution and bioavailability of flurbiprofen in solid self-nanoemulsifying drug delivery system (solid SNEDDS). Eur J Pharm Biopharm. 2012;80:289–97. https://doi.org/10.1016/j.ejpb.2011.11.005.
Mcconville C, Friend D. Development and characterisation of a self-microemulsifying drug delivery systems (SMEDDSs) for the vaginal administration of the antiretroviral UC-781. Eur J Pharm Biopharm. 2013;83:322–9. https://doi.org/10.1016/j.ejpb.2012.10.007.
Lu W-C, Chiang B-H, Huang D-W, Li P-H. Skin permeation of d-limonene-based nanoemulsions as a transdermal carrier prepared by ultrasonic emulsification. Ultrason Sonochem. 2014;21:826–32. https://doi.org/10.1016/j.ultsonch.2013.10.013.
Choudhury H, Gorain B, Karmakar S, Biswas E, Dey G, Barik R, et al. Improvement of cellular uptake, in vitro antitumor activity and sustained release profile with increased bioavailability from a nanoemulsion platform. Int J Pharm. 2014;460:131–43. https://doi.org/10.1016/j.ijpharm.2013.10.055.
Zhao Y, Wang C, Chow AHL, Ren K, Gong T, Zhang Z, et al. Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of Zedoary essential oil: formulation and bioavailability studies. Int J Pharm. 2009;383(1–2):170–7. https://doi.org/10.1016/j.ijpharm.2009.08.035.
Oberle R, Amidon G. The influence of variable gastric emptying and intestinal transit rates on the plasma level curve of cimetidine; an explanation for the double peak phenomenon. J Pharmacokinet Biop. 1987;15:529–44. https://doi.org/10.1007/BF01061761.
Liu W, Tian R, Hu W, Jia Y, Jiang H, Zhang J, et al. Preparation and evaluation of self-microemulsifying drug delivery system of baicalein. Fitoterapia. 2012;83:1532–9. https://doi.org/10.1016/j.fitote.2012.08.021.
Sun Y, Xia Z, Zheng J, Qiu P, Zhang L, Mcclements DJ, et al. Nanoemulsion-based delivery systems for nutraceuticals: influence of carrier oil type on bioavailability of pterostilbene. J Funct Foods. 2015;13:61–70. https://doi.org/10.1016/j.jff.2014.12.030.
Li W, Yi S, Wang Z, Chen S, Xin S, Xie J, et al. Self-nanoemulsifying drug delivery system of persimmon leaf extract: optimization and bioavailability studies. Int J Pharm. 2011;420:161–71. https://doi.org/10.1016/j.ijpharm.2011.08.024.
Scott Swenson E, Curatolo WJ. (C) Means to enhance penetration: (2) intestinal permeability enhancement for proteins, peptides and other polar drugs: mechanisms and potential toxicity. Adv Drug Deliver Rev. 1992;8:39–92. https://doi.org/10.1016/0169-409X(92)90015-I.
Dixit AR, Rajput SJ, Patel SG. Preparation and bioavailability assessment of SMEDDS containing valsartan. AAPS PharmSciTech. 2010;11(1):314–21. https://doi.org/10.1208/s12249-010-9385-0.
Xu WK, Jiang H, Yang K, Wang YQ, Zhang Q, Zuo J. Development and in vivo evaluation of self-microemulsion as delivery system for α-mangostin. Kaohsiung J Med Sci. 2017;33(3):116–23. https://doi.org/10.1016/j.kjms.2016.12.003.
Elgart A, Cherniakov I, Aldouby Y, Domb AJ, Hoffman A. Improved oral bioavailability of BCS class 2 compounds by self nano-emulsifying drug delivery systems (SNEDDS): the underlying mechanisms for amiodarone and talinolol. Pharm Res. 2013;30:3029–44. https://doi.org/10.1007/s11095-013-1063-y.
Joshi RP, Negi G, Kumar A, Pawar YB, Munjal B, Bansal AK, et al. SNEDDS curcumin formulation leads to enhanced protection from pain and functional deficits associated with diabetic neuropathy: an insight into its mechanism for neuroprotection. Nanomed-Nanotechnol. 2013;9:776–85. https://doi.org/10.1016/j.nano.2013.01.001.
Seo YG, Kim DH, Ramasamy T, Kim JH, Marasini N, Oh YK, et al. Development of docetaxel-loaded solid self-nanoemulsifying drug delivery system (SNEDDS) for enhanced chemotherapeutic effect. Int J Pharm. 2013;452:412–20. https://doi.org/10.1016/j.ijpharm.2013.05.034.
Acknowledgements
The authors also thank the Jiangsu University Ethics Committee for the kind guidance in the animal experiments.
Funding
This work was supported by the National Natural Science Foundation of China (grants 81720108030 and 31871810), China Postdoctoral Science Foundation funded project (2015M571700), Research Foundation for Distinguished Scholars of Jiangsu University (15JDG074), and Key Laboratory financial support of Zhenjiang (SS2018004).
Author information
Authors and Affiliations
Contributions
Xi-Ming Xu and Jiang-nan Yu conceived and designed the research. Yuan Zhu and Jia-Jia Zhang performed the majority of the experiments and analyzed the data; Wen Xu wrote the manuscript. Caleb-Kesse Firempong, Youwu Liao, Huiyun Zhang, Michael Adu-Frimpong, and Wenwen Deng modified the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, Y., Xu, W., Zhang, J. et al. Self-microemulsifying Drug Delivery System for Improved Oral Delivery of Limonene: Preparation, Characterization, in vitro and in vivo Evaluation. AAPS PharmSciTech 20, 153 (2019). https://doi.org/10.1208/s12249-019-1361-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1361-8