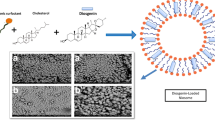
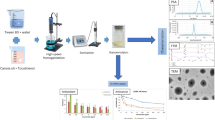
Abstract
3,3′-Diindolylmethane (DIM) is a phytochemical that presents health benefits (antitumor, antioxidant, and anti-inflammatory effects). However, it is water insoluble and thermo- and photolabile, restraining its pharmaceutical applications. As a strategy to overcome such limitations, this study aimed the development and characterization of DIM-loaded nanocapsules (NCs) prepared with different compositions as well as the in vitro assessment of scavenging activity and cytotoxicity. The formulations were obtained using the interfacial deposition of preformed polymer method and were composed by Eudragit® RS100 or ethylcellulose as polymeric wall and primula or apricot oil as the core. All the formulations had adequate physicochemical characteristics: nanometric size (around 190 nm), low polydispersity index (< 0.2), pH value at acid range, high values of zeta potential, drug content, and encapsulation efficiency (~ 100%). Besides, nanoencapsulation protected DIM against UVC-induced degradation and increased the scavenging activity assessed by the 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) and 1-1-diphenyl-2-picrylhydrazyl methods. The developed DIM-loaded nanocapsules were further evaluated regarding the in vitro release profile and cytotoxicity against a human glioblastoma cell line (U87 cells). The results demonstrated that the nanoencapsulation promoted a sustained release of the bioactive compound (in the range of 58–78% after 84 h) in comparison to its free form (86% after 12 h), as well as provided a superior cytotoxic effect against the U87 cells in the highest concentrations. Therefore, our results suggest that nanoencapsulation could be a promising approach to overcome the DIM physicochemical limitations and potentialize its biological properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
3,3′-Diindolylmethane (DIM) is a bioactive compound originated from the oligomerization of the indole-3-carbinol, after the ingestion of cruciferous vegetables, such as broccoli and cauliflower. The scientific literature highlights many health benefits provided by DIM, for example, its antioxidant, anti-inflammatory, and antitumor properties, and recent studies investigated its application against several pathologies (1,2,3). This phytochemical is a free radical scavenger, lipid peroxidation inhibitor, and modulator of different signaling pathways associated with inflammatory and carcinogenic processes (1,4). Despite its beneficial actions, DIM is highly susceptible to photodegradation and also has poor aqueous solubility and low oral bioavailability (5,6,7). Therefore, developing a pharmaceutical DIM dosage form is a challenging task, reinforcing that technological approaches should be applied to better explore its promising therapeutic application.
In this context, the development of nanoparticles-based drug delivery systems has been recognized as a potential alternative to circumvent physicochemical limitations and maximize the biological properties of drugs (8,9). Several advantages are attributed to nanocarrier systems in comparison to conventional therapy, for instance controlled delivery of active substances, reduction of adverse effects, drug protection against chemical and enzymatic degradation, and improvement of their aqueous apparent solubility (10,11,12). Among the nanocarriers, polymeric nanocapsules (NCs) are colloidal systems composed by a polymeric wall surrounding an oily core. Of particular importance, the investigation of novel oils from natural sources, such as vegetable oils, has been an interesting approach in the formulation of NCs. The oil core is a structural component of the NCs that could provide additional pharmacological properties to the formulation (13,14,15). Primula (Primula veris, L.) and apricot (Prunus armeniaca, L.) oils are of special interest for the presence of unsaturated and poly-unsaturated fatty acids in their composition (16,17,18). Despite their potential, as far as we know, there is no nanocapsule formulation composed by such oils.
Concerning the literature, there are a few investigations about DIM incorporation into polymeric nanocarriers. Kiselev and co-workers (19) developed matrix nanoparticles to improve DIM bioavailability. DIM-loaded nanoparticles of zein/carboxymethyl chitosan were developed to increase drug stability (20). Isabella and Mirunalini (21,22) also formulated DIM-matrix nanoparticles containing chitosan and showed the antitumor effect of the formulation against a rat mama tumor. In addition, a recent report showed that a formulation of PLGA matrix nanoparticles containing DIM demonstrated antitumor effects (23). However, there are neither studies about DIM incorporation into NCs composed by primula and apricot oils nor the investigation of the potential impact of such association in DIM biological effects.
Therefore, the current study aimed the development and characterization of novel NC formulations to DIM encapsulation. Different polymers, ethylcellulose (EC) or Eudragit® RS (ERS), and oils, primula or apricot, were used to prepare the NC suspension. The photoprotective potential and scavenging properties of the formulations were assessed. Besides, these formulations were further studied regarding the in vitro DIM release profile from the nanostructures and cytotoxic effect against a malignant glioblastoma cell line (U87 cells).
MATERIALS AND METHODS
Materials
DIM (99.2% purity) was obtained from Active Pharmaceutica (Brazil). Ethylcellulose (EC) was a gift from Colorcon (Brazil). Tween® 80 (polysorbate 80), Span® 80 (sorbitan monooleate), 1-1-diphenyl-2-picrylhydrazyl (DPPH) radical, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), and 3(4,5-dimethyl)-2,5diphenyl tetrazolium bromide (MTT) were purchased from Sigma-Aldrich Co. (USA). Primula oil (PO) and apricot kernel oil (AO) were supplied by Mundo dos Óleos (Brazil). Eudragit® RS100 (ERS) was donated by Almapal (Brazil). Dulbecco’s modified Eagle’s medium (DMEM), fungizone, penicillin/streptomycin, 0.25% trypsin/EDTA solution, and fetal bovine serum (FBS) were obtained from Gibco (USA). All other chemicals and solvents were analytical grade and used as received.
Analytical Procedures
The analytical method for DIM quantification in the NC suspensions was validated according to the ICH guidelines. A LC-10A HPLC system (Shimadzu, Japan), equipped with a LC-20AT pump, an UV–vis SPD-M20A detector, a CBM-20A system controller, and a SIL-20A HT valve sample automatic injector was used to perform DIM quantification. The separation was performed at room temperature using a Kinetex C18 Phenomenex column (250 mm × 4.60 mm, 5 μm; 110 Å) coupled to a C18 guard column. The DIM was detected at 288 nm, using an isocratic mobile phase composed by acetonitrile and water (60:40, v/v) at 1 mL/min flow rate. For the calibration curve, DIM was properly dissolved in methanol (DIM solutions in a concentration range of 0.75–20.0 μg/mL). The method was considered specific, linear (r = 0.9996), accurate, and precise (relative standard deviation ≤ 2.0%) in the above-mentioned concentration range.
DIM Solubility in the Vegetable Oils
The DIM solubility evaluation in the oils was carried out adding an excess amount of the compound in 2 mL of each vegetable oil. The systems were kept under moderate magnetic stirring overnight to assure maximum DIM solubilization. Following, each sample was centrifuged at 3000 rpm for 10 min and supernatant’s aliquot was diluted with methanol, filtered, and injected into the HPLC system to DIM detection, using the chromatographic conditions mentioned in the previous section.
Dissolution/Swelling Test of Polymer Films
Polymer films of EC or ERS were prepared by previous dissolution of each polymer in acetone. After solvent evaporation, the resulting films were accurately weighted (approximately 40 mg to EC and 90 mg to ERS) and separately immersed in an aliquot of each oil at room temperature (n = 6). Over a period of 60 days, at predetermined time intervals, the films were removed from the oil and carefully dried with an absorbing paper. Weight variation was measured in an analytical balance in order to determine if the polymers have swollen and/or dissolved during the contact with the oil.
Preparation of Nanocapsule Suspensions
NCs were prepared (n = 6) by the interfacial deposition of preformed polymers method (24). Briefly, an organic phase containing acetone, vegetable oil, Span® 80, polymer, and DIM was kept under magnetic stirring for 60 min at 40°C. This phase was injected into an aqueous dispersion of Tween® 80 and kept under moderate magnetic stirring for 10 min longer. Then, the acetone and water were eliminated under reduced pressure to achieve 10 mL of NC suspension (final DIM concentration at 1 mg/mL). Blank NCs were prepared using the same method, without the active. All formulations were packaged in amber glass flasks, stored at 4°C, and used in the assays within a period of 2 days. The qualitative-quantitative composition of each formulation is available in Table I.
Physicochemical Characterization of Nanocapsule Suspensions
Mean Particle Diameter, Zeta Potential, and pH value
The average particle size, polydispersity index, and zeta potential of the NCs were measured using the ZetaSizer NanoSeries (Malvern Instruments, UK). This equipment employs the photon correlation spectroscopy methodology to determine particle size distribution (samples diluted in ultrapure water 1:500), while zeta potential is determined by microelectrophoresis after diluting the samples in 10 mM NaCl (1:500). The autocorrelation treatment function method for size distribution analysis was the general purpose and at least 10 runs were performed to each evaluation. For zeta potential, Smoluchowski’s approximation was used for the measurement. The pH value of the formulations was measured by directly immersing the electrode of a calibrated potentiometer in the suspensions (Model pH21, Hanna Instruments, Brazil). All these evaluations were made with 6 (six) independent experiments in triplicate for each batch and at room temperature.
DIM Quantification in the Nanocapsule Suspensions
For total DIM content in the NC suspensions (n = 6), an aliquot of 100 μL of each formulation (NC-PEC-D, NC-PERS-D, NC-AEC-D, NC-AERS-D, see Table I) was diluted in 10 mL of methanol, filtered through a 0.45-μm cellulose membrane and injected into the HPLC system. The DIM encapsulation efficiency was assayed as follows: 300 μL of the NC suspension was transferred to a centrifugal device containing an ultrafilter (Amicon® Ultra, 10,000 MW, Millipore), which was subjected to the ultrafiltration/centrifugation technique (2200×g for 10 min). Free DIM was determined in the ultrafiltrate, while the entrapped active compound was calculated according to the following equation:
In Vitro Assessment of Biological Properties of DIM Nanoformulations
Photostability Evaluation of DIM-Loaded Nanocapsule Suspensions
For the photostability evaluation, an aliquot of each formulation containing DIM (700 μL) was placed in plastic cuvettes equidistantly organized inside a mirrored chamber (1 m × 25 cm × 25 cm) containing an UVC source of light (Phillips TUV lamp, 30 W). At predetermined intervals, an aliquot of the formulations was withdrawn and the DIM content was determined by the previously described HPLC method. For comparison purposes, a methanolic DIM solution (free compound) was simultaneously evaluated. In addition, a dark control (cuvettes protected from light) was carried out to discard possible degradation caused by temperature or any other influence of experimental conditions. The evaluation was performed with 6 (six) independent experiments in triplicate for each batch.
Determination of ABTS and DPPH Radical Scavenging Capacity
The ABTS and DPPH radical scavenging capacity of the formulations was assessed as described by Re et al. (25) and by Sharma and Bhat (26), respectively. DIM-loaded NCs and blank NCs were diluted in distilled water, pure DIM in ethanol, pure PO, and pure AO in DMSO. The samples were evaluated at concentrations of 2.0, 4.0, and 6.0 μg/mL. The ABTS radical cation solution (0.3763 mM) was prepared by mixing ABTS stock solution (7 mM) with potassium persulfate (140 mM), 12 h before the assay (final ABTS concentration 42.7 μM). The DPPH was dissolved in methanol and used as obtained (50 μM). The samples were incubated with ABTS or DPPH solution during 30 min under light protection. Following, each sample was mixed with sodium lauryl sulfate (10% w/v) and the absorbance was measured using a UV/Vis spectrophotometer (Shimadzu, Japan) at 734 nm (ABTS assay) or 517 nm (DPPH assay). Pure DPPH or ABTS and ascorbic acid solutions were used as negative and positive controls, respectively. The radical scavenging activity for both tests was expressed as percentage of scavenging capacity, as follows:
where SC% is the scavenging capacity in percentage, Abs is the absorbance of the incubated sample with DPPH, Abb is the blank sample absorbance, and Abc is the negative control absorbance. These experiments were carried out with 6 (six) independent experiments in triplicate for each batch.
In Vitro Release Studies
The evaluation of DIM release from the formulations NC-PEC-D, NC-PERS-D, NC-AEC-D, and NC-AERS-D was performed using the dialysis bag diffusion method. An aliquot of 2 mL of the formulation was placed inside the dialysis membrane (molecular weight cut-off 10,000 Da, Sigma-Aldrich) and immersed in 200 mL of the release medium (phosphate buffer pH 7.4/ethanol 70:30). This system was kept at 37 ± 2°C under magnetic stirring, and sink condition was maintained over the experiment. At predetermined periods, 1 mL of the external medium was collected and the same volume of fresh medium was replaced. The quantity of DIM released from the NC suspensions was assessed by the HPLC method previously described. For comparison purposes, a methanolic solution of DIM (free compound) was simultaneously evaluated. The experiment was conducted with 6 (six) independent experiments in triplicate for each batch. The results were expressed as percentage of DIM released over time.
The mathematical modeling was performed to determine the kinetic and mechanism of DIM release from the NCs. The experimental data fitted to first order equation (Eq. 3) and Korsmeyer-Peppas model (27) (Eq. 5), using the Scientist software 2.0 (MicroMath®, USA).
where C is the concentration of DIM at the time t, C0 is the initial concentration of the DIM, k is the first order rate constant, ft is the fraction of drug released at the time t, a is the constant incorporating structural and geometric characteristics of the nanostructured system, and n is the exponent related to the release mechanism (n = 0.43 for drug diffusion; n = 0.85 for polymer degradation; 0.43 < n < 0.85 for anomalous transport).
General Cell Culture Procedures
The human malignant glioblastoma cell line (U87MG) was obtained from the American Type Culture Collection (Rockville, MD, USA). Cells were grown and maintained in low-glucose DMEM containing fungizone (0.1%) and penicillin/streptomycin (100 U/L) and supplemented with FBS (10%). After the cultures reached confluency, glioma cells were seeded in 96-well plates at 5 × 103 cells/well in DMEM/FBS (10%) and were kept at 37°C in a humidified atmosphere with 5% CO2 for 24 h.
Cell Viability Assay
The plate containing U87 cells was incubated with free DIM, free oils (PO and AO), NC-PEC-D, NC-PERS-D, NC-AEC-D, and NC-AERS as well as with the respective blank formulations, at concentrations of 3.0, 6.0, 12, and 24 μg/mL. Control cells were treated with vehicle, e.g., 1% of DMSO. Following 72 h of treatment, cell viability was determined by 3(4,5-dimethyl)-2,5diphenyltetrazolium bromide (MTT) assay. This method is based on the ability of viable cells to reduce MTT to blue formazan products. The MTT solution was added to the incubation medium in the wells at a final concentration 0.5 mg/mL, under light protection until the formation of violet formazan crystals (around 90 min). Later, the solution was then removed and an amount of DMSO was added to each well. The optical density of each plate was measured at 492 nm and the results were expressed by cell viability (%). Six independent experiments were performed using triplicates.
Statistical Analysis
The results were expressed as mean ± standard deviation. Data normality was evaluated by the Kolmogorov-Smirnov normality test. The statistically significant difference was calculated by means of one-way ANOVA of ordinary or repeated measures followed by Tukey’s test. The GraphPad Prism software version 7 (San Diego, CA, USA) was used to perform these analyses. Values of p < 0.05 were considered statistically significant.
RESULTS
Preformulation Studies
The DIM solubility in both PO and AO was investigated. Values obtained were 4.3 ± 0.7 mg/mL and 7.1 ± 1.2 mg/mL, respectively. Regarding a possible undesirable interaction between the vegetable oils and polymers used to develop DIM NCs, the film masses modified less than 5% after 60 days of experiment (p < 0.05), suggesting no polymer solubilization in the tested oils.
Preparation and Characterization of NC Suspensions
Macroscopically, all formulations presented turbid aspect and opalescent bluish reflection, which are characteristics of the chaotic motion presented by the colloidal particles. Table II depicts the physicochemical characterization of the NC suspensions. The mean diameter was not significantly influenced by the different oils and polymers used in the preparation of the nanostructures (p > 0.05). The polydispersity indexes were less than 0.23 to all the formulations (p > 0.05). The zeta potential values were negative for NCs composed by EC, while suspensions prepared with ERS presented positive values. The pH values were slightly more acidic for the DIM-loaded NCs containing ERS than those formulated with EC (p < 0.05). Concerning DIM content, all formulations presented values close to the theoretical concentration (1 mg/mL), regardless the type of polymer and oil used to prepare the formulations, indicating minimal losses and discarding a possible compound degradation throughout the formulation preparation process. Encapsulation efficiency was 97% for NC-PEC-D and NC-PERS-D, 94% for NC-AEC-D, and about 92% for NC-AERS-D.
Photostability Evaluation
The results of photostability studies are depicted in Fig. 1. After 150 min of experiment, the DIM content reduced to 40.0 ± 1.8% in the methanolic solution while the formulations had around 90% of DIM content. At the end of the experiment, only 28 ± 0.6% of DIM remained in the methanolic solution, while the final DIM content was 81 ± 7.1%, 76 ± 5.0%, 80 ± 2.5%, and 84 ± 5.4% to NC-PEC-D, NC-PERS-D, NC-AEC-D, and NC-AERS-D, respectively, demonstrating a significant decrease in DIM content in comparison to the DIM-loaded NC suspensions (p < 0.001). These results showed that the nanoencapsulation increased around 3-folds the photostability of DIM independent of the type of oil and polymer used to prepare the nanostructure. The dark control exhibited 100% of DIM, which indicates that the degradation occurred only because of the radiation exposure (data not shown).
DIM remaining proportion after the NC suspension and the methanolic solution exposure to UVC radiation. Each bar represents the mean ± SD of six independent experiments. The asterisks denote the significant levels when compared to DIM methanolic solution (DIM) (one-way ANOVA of repeated measures, followed by Tukey’s test). ***p < 0.001
Determination of DPPH Scavenging Capacity
Figure 2 displays the results of DPPH assay. Except the NC-PERS-D formulation, the nanoencapsulation provided an increase in the DIM DPPH scavenging activity in comparison to its free form. At 2 μg/mL, the formulation NC-AERS-D presented DPPH scavenging capacity of 41.0 ± 2.5%, which was significantly higher than that of the pure DIM (25.0 ± 6.0%) (p < 0.05). At 6 μg/mL, NC-AERS-D and NC-PEC-D improved the antiradical capacity (65.0 ± 8.2% and 71.0 ± 6.6%, respectively) in comparison to DIM methanolic solution (47.0 ± 1.6%; p < 0.05). Besides, at this concentration, the formulation NC-PEC-D demonstrated no significant difference for scavenger property with ascorbic acid solution, a powerful antioxidant used as reference (95.0 ± 0.4%) (p > 0.05). Both vegetable oil solutions showed low scavenger activity, about 6.0 ± 0.5% and 3.0 ± 0.8% for PO and AO, respectively. In contrast, the blank NCs containing the oils had superior scavenging capacity in comparison to their pure forms (p < 0.05).
DPPH radical scavenging capacity of pure DIM, pure oils, NCs with DIM, and the respective blank NCs. Each bar represents the mean ± SEM of six independent experiments (ordinary one-way ANOVA, followed by Tukey’s test). *p < 0.05. Significant difference between samples and positive control. #p < 0.05. Significant difference between pure DIM and DIM-loaded NCs. @p < 0.05. Significant difference between NCs with and without DIM. $p < 0.05. Significant difference between blank NCs and pure oils
Determination of ABTS Scavenging Capacity
The results displayed in Fig. 3 show that regardless the NC composition and concentration tested, all the formulations containing DIM had scavenging capacity (63–100%) superior than free DIM (50–63%; p < 0.05). In addition, the blank NCs containing the oils increased their scavenger capacity in comparison to the pure oils (p < 0.05).
ABTS radical scavenging capacity of pure DIM, pure oils, NCs with DIM, and the respective blank NCs. Each bar represents the mean ± SEM of six independent experiments (ordinary one-way ANOVA, followed by Tukey’s test). *p < 0.05. Significant difference between samples and ascorbic acid (positive control). #p < 0.05. Significant difference between pure DIM and DIM-loaded NCs. @p < 0.05. Significant difference between NCs with and without DIM. $p < 0.05. Significant difference between blank NCs and pure oils
In Vitro Release Studies
The results of DIM release profile are shown in Fig. 4. The nanoencapsulation controlled the DIM release in comparison to the methanolic solution, regardless the nanoformulation, after the first 30 min of experiment (p < 0.05). The free DIM (methanolic solution) released around 100% of its content after 12 h of experiment, while NC-PEC-D released 78.0 ± 4.0% and NC-AEC-D, NC-PERS-D, and NC-AERS-D released 68 ± 2.9%, 66 ± 3.5%, and 58 ± 5.3%, respectively, over a period of 84 h. Besides, NC-PERS-D release profile was not significantly different from NC-PEC-D and NC-AEC-D, regardless the time of experiment (p > 0.05). NC-AERS-D showed a significant slower release (p < 0.01) than NC-PEC-D after 12 h of experiment and differed from NC-PERS-D and NC-AEC-D after 24 h (p < 0.001). NC-PEC-D and NC-AEC release profiles only showed significant difference at 72 h and 84 h (p < 0.01 and p < 0.001, respectively).
DIM release profiles from methanolic solution (DIM) and DIM-loaded nanocapsules (NC-PEC-D, NC-AEC-D, NC-PERS-D, NC-AERS-D). Each point represents the mean ± SD of six independent experiments. %p < 0.05. Significant difference between NC-PEC-D and control (DIM). #p < 0.05. Significant difference between NC-AEC-D and control (DIM). $p < 0.05. Significant difference between NC-PERS-D and control (DIM). *p < 0.05. Significant difference between NC-AERS-D and control (DIM). (One-way ANOVA of repeated measures, followed by Tukey’s test)
The mathematical modeling of the experimental data showed that free DIM and DIM-loaded NCs fitted to the first order equation (r > 0.989 for DIM and r > 0.992 for DIM-loaded NCs). The dissolution rate constant (k) was approximately 0.01 h−1 for NC-AERS-D and 0.02 h−1 for other formulations, while for pure DIM it was 0.28 h−1, showing slower DIM dissolution from the NCs than from the pure drug. In addition, the release exponent calculated according to the Korsmeyer-Peppas equation suggested anomalous transport as a release mechanism of the NC suspension (Table III).
Evaluation of Nanocapsule Cytotoxicity Against Glioma Cells
The in vitro antiglioma effect of all the nanocapsule suspensions was investigated in U87MG cell line employing MTT test (Fig. 5). Free DIM reduced the U87MG cell viability only at higher concentrations (12 and 24 μg/mL, 78.0 ± 1.5% and 38.0 ± 3.0%, respectively; p < 0.01; p < 0.001), while the DIM-loaded NCs had antiglioma effect in all concentrations tested. Importantly, NC-PEC-D and NC-AERS-D at the concentration 3 μg/mL presented significantly higher cytotoxic effect (77.0 ± 0.5% and 85.3 ± 0.5%, respectively) in comparison to free DIM (100.0%) (p < 0.001). At 6 μg/mL, besides NC-PEC-D and NC-AERS-D, NC-PERS-D also presented higher cytotoxic effect (83.6 ± 1.0%) than free DIM (96.0 ± 3.4%) (p < 0.01). At 24 μg/mL, free DIM and all the NCs showed significant lower cell viability than the control and it was possible to observe that NC-PEC-D (38.0 ± 2.0%) showed similar cytotoxic potential to free DIM. Overall, the incubation with blank formulations also reduced the U87MG cell viability, but the pure oils had no significant antitumor effect independent of the concentration tested (p > 0.05). Nevertheless, it is important to mention that the highest concentration tested (24 μg/mL) in the formulation NC-PEC-D caused a significant higher cytotoxic effect (p < 0.05) in comparison to the respective blank formulation, NC-PEC-B. Regardless the concentration tested, the statistical analysis showed that NC-PEC-D presented the most cytotoxic effect among all the DIM-loaded NCs.
In vitro cell viability of human glioblastoma cells (U87MG) after 72 h of incubation with crescent concentrations of pure DIM, PO, AO, DIM-loaded nanocapsules (NC-PEC-D, NC-PERS-D, NC-AEC-D, NC-AERS-D), and blank nanocapsules (NCPEC-B, NC-PERS-B, NC-AEC-B, NC-AERS-B) by the MTT reduction assay. Each bar represents the mean ± SEM of six independent experiments. (Ordinary one-way ANOVA, followed by Tukey’s test). (*) p :< 0.05, (**) p < 0.01, (***) p < 0.001: significant difference between treatments and control. (#) p < 0.05, (##) p < 0.01, (###) p < 0.001: significant difference between pure DIM and DIM-loaded NCs. (@) p < 0.05: significant difference between NCs with and without DIM. (&&) p < 0.01, (&&&) p < 0.001: significant difference in comparison with NC-PEC-D at the same concentration. ($ ) p < 0.05, ($$) p < 0.01, ($$$) p < 0.001: significant difference between blank NCs and pure oils
DISCUSSION
Preformulation evaluations are very important for this study, since there are no reports in the literature about the preparation of DIM-loaded polymeric NCs as well as the association of EC or ERS with the proposed oils (PO or AO). To ensure the formation and maintenance of this type of supramolecular structure, the chosen polymer must be insoluble in both oily and aqueous phases. Besides, the absence of polymer swelling by the oil is also required (8,28). Our results indicate that there was no modification in the weight of the polymeric films over time, suggesting that both PO and AO were suitable materials for the NC preparation. Furthermore, due to the high DIM lipophilicity, its solubilization in both oils was already expected, which reinforces the feasibility of preparation of DIM-loaded NCs with primula or apricot oily core. Thus, these data demonstrate that the materials used to prepare the NC formulation are compatible.
Several scientific studies report that the nanoprecipitation methods usually generate particles in the range of 100–500 nm (13,14,15,29,29,30,31,33), which are in accordance with the present study. NCs are instantaneously obtained by rapid solvent diffusion with slow injection of an organic solution (formed by a semi-polar solvent, an aqueous insoluble polymer, oil, and drug) into an aqueous phase, in the presence of surfactants, which allows the encapsulation of poorly soluble drugs. Besides, this method is suitable to deliver compounds that are intended for pharmaceutical or dietary application, because the organic solvent can be completely removed from the formulation (8,11,34). In our study, colloidal particles were obtained in the nanoscale (154–200 nm), regardless the oil or polymer used in the NC composition and the presence of DIM. All formulations presented low polydispersity indexes (< 0.25), indicating narrowed particle size distribution and appropriate system homogeneity.
The zeta potential of colloidal systems depends on the composition of the particle as well as on its characteristics in the external phase of the suspension. Such parameter is used as an indirect measurement of the nanoparticle’s surface charge nature and intensity (8,11,35). In our study, the oil type did not influence this parameter, but the formulations composed of ERS showed positive values probably due to the presence of quaternary ammonium groups in the polymer chains (13,29,30), while EC formulations were negatively charged, reflecting the polymer anionic nature. Besides, in an attempt to minimize the in vivo opsonization process and to improve the NC stabilization, the polysorbate 80, a biodegradable copolymer with hydrophilic segments, was used as a non-ionic stabilizing agent. Hence, the values found in this study are in accordance with the properties presented by the raw materials employed in the constitution of the NCs and predicted adequate system stabilization. As observed for zeta potential, pH value results depended on the polymer used in the NC preparation. The formulations containing ERS showed pH values in the range of 5.0–5.9, while EC NCs exhibited higher values (5.9–6.2), which were similar to previous studies that used the same polymers (13,15,36).
The results of drug content indicate minimal active leakage during the NC preparation. Also, all formulations presented high values of entrapment efficiency, which could be explained considering that (i) the interfacial deposition of the preformed polymer method generally ensures adequate active encapsulation; (ii) DIM has high solubility in both vegetable oils tested; and (iii) DIM is a water-insoluble compound, with a high log P value (4.05). Considering such characteristics, it is possible to suggest that DIM presented a superior affinity with the oily core and/or with the hydrophobic polymeric wall than to the external aqueous phase of the colloidal suspension, which prevented its partitioning to the aqueous phase.
In addition, the physicochemical properties found in this study are in agreement with previous studies that reported the development of DIM-loaded nanostructures. Luo and collaborators (20) developed zein and zein/carboxymethyl chitosan nanoparticles containing DIM, presenting mean size in the range 89–250 nm, polydispersity indexes lower than 0.20, and negative zeta potentials. However, it is important to mention that the NCs developed in our study showed higher encapsulation efficiency as well as a DIM content efficiency 20-folds greater than that of the nanoparticles obtained by Luo and collaborators (encapsulation efficiency and content efficiency of 69–78% and 50 μg/mL, respectively). These findings could be explained by a better solubilization/dispersion of DIM promoted by the vegetable oil contained in the inner core of the NCs. Indeed, another study supports the hypothesis of improvement of DIM encapsulation in the presence of oily substances. Boakye and col. (37) also reported high entrapment efficiency values for a DIM-derivative compound encapsulated in cationic liposomes. The authors developed a formulation containing a mixture of phospholipids, cholesterol, and polysorbate 80 by the ethanolic injection method and obtained liposomes presenting DIM-derivative encapsulation rate of approximately 91% and drug content of 50 μg/mL.
Considering that it was already defined that DIM photolability was due to the presence of aromatic rings in its structure (20), we evaluated if its nanoencapsulation could prevent its degradation in an accelerated condition (under UVC light). The results demonstrated that independent of the NC composition, all formulations enhanced DIM photostability. This improvement may be due to the structural characteristics of the NCs, which can absorb or scatter the incident radiation, acting as a physical barrier against the UV light (13,30,38,39). Luo and collaborators (20) also observed that DIM nanoencapsulation improved its photostability, which was attributed to the double coating of zein and carboxymethyl chitosan nanoparticles.
It is well known that compounds with high radical scavenging capacity are promising candidates to the management of several diseases. In our study, the scavenger potential of the formulations was determined in vitro using the DPPH and ABTS radical assays. Both methods are simple, precise, and useful to evaluate the antioxidant potential of vegetable oils and extracts, pharmaceutical formulations, and pure or nanoencapsulated compounds (25,40,41).
The DPPH assay is based on a reaction that reduces DPPH to diphenyl-picrylhydrazine, which changes the reaction medium from deep-violet to light-yellow color that can be measured on a UV/visible light spectrophotometer at 517 nm (41,42). However, this technique lacks selectivity because of spectral interferences. Hence, the association of DPPH assay with another method is recommended. In this context, ABTS cation solution presents a blue/green color and its reduction by a hydrogen-donating antioxidant is measured by the extent of decolorization and consequent decrease of its characteristic absorption at 734 nm (40). This method is considered more versatile, because the measure is not interfered by the spectra of complex products and it is useful to evaluate the antiradical activity of compounds in several media, including phosphate-buffered solution pH 7.4, which simulates a physiological environment (25,40). In our study, ABTS findings corroborated the results obtained for DPPH assay.
The DIM scavenging capacity could be explained by the presence of two N–H groups in its structure, which can act as H-donating groups and neutralizes free radicals (40,43). Overall, the results of our study showed that the nanoencapsulation increased the radical scavenging activity in comparison to pure DIM and pure oils. Previous studies suggested that the nanometric size of the particles in suspension could provide superior contact surface area between the H donator and DPPH or ABTS molecules, facilitating the access of the hydrogen atom to the radical site (14,15,44,45). Besides, blank NCs also showed scavenging capacity. Therefore, the antioxidant substances present in both PO and AO could contribute for the improved effect verified for DIM-loaded NCs. This finding is in accordance with a previous study of our group that reported superior scavenger capacity of pomegranate oil NCs than the pure oil (15).
The in vitro release profiles demonstrated that DIM release from all the DIM-loaded NCs fitted to first order equation, indicating that release rate depends on the concentration and occurs in a single step. Furthermore, the absence of burst release reinforces the total DIM encapsulation in the oil core, corroborating the values of DIM encapsulation efficiency. It should be noted that even pure DIM showed a slow release, which can be attributed to its high lipophilicity. In the NCs, DIM may be dissolved in the oily core and/or adsorbed onto the hydrophobic polymeric wall. The presence of the oil and the polymer could act as additional barriers to DIM release, slowing down its diffusion from the nanostructures to the medium. Regarding the release mechanism, n values of 0.73–0.80 were found for NCs, which suggests that drug release is driven by anomalous transport, where the drug diffuses from the oil to the particle/water interface, followed by the polymer chain relaxation. These results are in accordance with the characteristic of the polymers used in the NC preparation, because both EC and ERS are water-insoluble polymers used to promote sustained-release profiles (13,15,29,46).
Considering the promising results showed by the developed NCs, their cytotoxicity was tested against a human U87MG glioma cell line to evaluate the biological performance of formulations. This study demonstrated interesting results even with lowest concentrations because the DIM-loaded NCs caused a significant increase in the cytotoxicity in comparison with free DIM. Observing the highest concentration, both compound forms as well as the blank formulation reduced the U87MG cell viability. Such results could be explained by the DIM slow and incomplete release from the nanocapsules within the incubation time (72 h), whereas the solubilized DIM could be more available to interact with the cells, as shown in previous studies (47,48,49,50). In addition, the cytotoxicity observed for blank nanocapsules can be possibly explained by two reasons: (i) the presence of polysorbate in the constitution of the formulation. This component may disrupt the cell membranes and increase cell permeability; (ii) the nanoparticles can adhere to the cell membrane causing the release of cytotoxic products or uptake triggering cell death (51,52,53,54). In comparison with the blank nanocapsules, the improvement of the cytotoxic effect by the DIM nanoencapsulation could be evidenced in the highest concentration (24 μg/mL). At this concentration, the formulation NC-PEC-D was remarkably more cytotoxic than NC-PEC-B, highlighting the role of DIM and reinforcing the positive impact of its nanoencapsulation in biological properties of DIM.
The DIM antitumor property was previously reported, but its physicochemical restrictions could represent a limitation to further studies aiming at its therapeutic application (5,6,7). It is important to highlight that the DIM nanoencapsulation enhanced the antiglioma effect because in concentrations where the free compound had no action, the NC-PEC-D caused a reduction in U87MG cells viability. These results are in accordance with other authors that reported an improvement of in vitro and in vivo biological effects by associating active substances in nanocarrier systems (14,30,30,32,55).
Taking into account the above-mentioned results, together with the other in vitro experiments (determination of DPPH and ABTS scavenging capacity), the present study clearly showed the advantages of the DIM-loaded NCs, especially NC-PEC-D. The superior photostability, antioxidant effect, and controlled drug release presented by the developed NCs reinforce the positive impact of DIM nanoencapsulation in enhancing its biological properties.
CONCLUSION
In conclusion, this study showed the feasibility of preparing DIM-loaded NCs based on two different vegetable oils and polymers. All formulations presented adequate physicochemical characteristics and were able to enhance DIM photostability and radical scavenger property. The NC suspension composed by PO and EC seemed to be the most promising system because it provided a prolonged DIM release as well as increased its cytotoxic effect. Overall, the nanoencapsulation promoted a general improvement in the physicochemical characteristics and biological properties of DIM, indicating that such approach is an interesting alternative for future studies regarding the management of different pathological conditions.
References
Banerjee S, Kong D, Wang Z, Bao B, Hillman GG, Sarkar FH. Attenuation of multi-targeted proliferation-linked signaling by 3,3′-diindolylmethane (DIM): from bench to clinic. Mutat Res. 2011;728(1–2):47–66. https://doi.org/10.1016/j.mrrev.2011.06.001.
Li Y, Kong D, Ahmad A, Bao B, Sarkar FH. Antioxidant function of isoflavone and 3,3′-diindolylmethane: are they important for cancer prevention and therapy? Antioxid Redox Signal. 2013;19(2):139–50 Available from: http://online.liebertpub.com/doi/abs/10.1089/ars.2013.5233.
Maruthanila VL, Poornima J, Mirunalini S. Attenuation of carcinogenesis and the mechanism underlying by the influence of indole-3-carbinol and its metabolite 3,3???-diindolylmethane: a therapeutic marvel. Adv Pharmacol Sci. 2014;2014.
Kim EJ, Park H, Kim J, Park JHY. 3,3′-Diindolylmethane suppresses 12-O-tetradecanoylphorbol-13- acetate-induced inflammation and tumor promotion in mouse skin via the downregulation of inflammatory mediators. Mol Carcinog. 2010;49(7):672–83.
Roy S, Mandal M, Pal C, Giri P, Kumar GS, Mukherjee J, et al. Studies on aqueous solubility of 3,3′-diindolylmethane derivatives using cyclodextrin inclusion complexes. J Mol Struct. 2013;1036:1–6.
Vallejo F, Tomás-Barberán FA, Garcia-Viguera C. Glucosinolates and vitamin C content in edible parts of broccoli florets after domestic cooking. Eur Food Res Technol. 2002;215(4):310–6.
Wu T, Huang Y, Zhang C. Pharmacokinetics and pharmacodynamics of 3 , 3 0 -diindolylmethane ( DIM ) in regulating gene expression of phase II drug metabolizing enzymes. J Pharmacokinet Pharmacodyn. 2015;42(4):401–8.
Mora-Huertas CE, Fessi H, Elaissari A. Polymer-based nanocapsules for drug delivery. Int J Pharm. 2010;385(1–2):113–42.
Nicolas J, Mura S, Brambilla D, MacKiewicz N, Couvreur P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem Soc Rev. 2013;42:1147–235.
Dimer FA, Friedrich RB, Beck RCR, Guterres SS, Pohlmann AR. Impact of nanotechnology on public health: production of medicines. Quim Nova. 2013;36(10):1520–6.
Bhokare SG, Marathe RP, Gaikwad MT, Salunke PB. Biodegradable polymer based nanoparticles: a novel approach. Int J Pharm Sci Rev Res. 2015;35(1):43–52 Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84949635537&partnerID=40&md5=038578252a233be9c5e020874bec2613.
Frank LA, Contri RV, Beck RCR, Pohlmann AR, Guterres SS. Improving drug biological effects by encapsulation into polymeric nanocapsules. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(5):623–39.
Santos SS, Lorenzoni A, Pegoraro NS, Denardi LB, Alves SH, Schaffazick SR, et al. Formulation and in vitro evaluation of coconut oil-core cationic nanocapsules intended for vaginal delivery of clotrimazole. Colloids Surf B: Biointerfaces. 2014;116:270–6. https://doi.org/10.1016/j.colsurfb.2014.01.011.
Gehrcke M, Giuliani LM, Ferreira LM, Barbieri AV, Sari MHM, da Silveira EF, et al. Enhanced photostability, radical scavenging and antitumor activity of indole-3-carbinol-loaded rose hip oil nanocapsules. Mater Sci Eng C. 2017;74:279–86. https://doi.org/10.1016/j.msec.2016.12.006.
Marchiori MCL, Rigon C, Copetti PM, Sagrillo MR, Cruz L. Nanoencapsulation improves scavenging capacity and decreases cytotoxicity of silibinin and pomegranate oil association. AAPS PharmSciTech. 2017;18(8):3236–46 Available from: http://springerlink.bibliotecabuap.elogim.com/10.1208/s12249-017-0810-5.
Sayanova O, Napier JA, Shewry PR. Δ6-Unsaturated fatty acids in species and tissues of the Primulaceae. Phytochemistry. 1999;52(3):419–22.
Femenia A, Rossell C, Mulet A, Caiiellas J. Chemical composition of bitter and sweet apricot kernels. J Agric Food Chem. 1995;(1978):356–61.
E-aal MHA, Khalil MKM, Rahma EH. Apricot kernel oil : characterization , chemical composition and utilization in some baked products. Food Chem. 1986;19:287–98.
Paltsev M, Kiselev V, Muyzhnek E, Drukh V, Kuznetsov I, Pchelintseva O. Comparative preclinical pharmacokinetics study of 3,3′-diindolylmethane formulations: is personalized treatment and targeted chemoprevention in the horizon? EPMA J. 2013;4:25 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4029298&tool=pmcentrez&rendertype=abstract.
Luo Y, Wang TTY, Teng Z, Chen P, Sun J, Wang Q. Encapsulation of indole-3-carbinol and 3,3'-diindolylmethane in zein/carboxymethyl chitosan nanoparticles with controlled release property and improved stability. Food Chem. 2013;139(1–4):224–30. https://doi.org/10.1016/j.foodchem.2013.01.113.
Isabella S, Mirunalini S. Chemotherapeutic effect of 3, 3′-diindolylmethane encapsulated chitosan nanoparticles on 7, 12-dimethylbenz (a) anthracene induced mammary cancer - a dose dependent study. 2016;3(1):1–8.
Isabella S, Mirunalini S. Protective effect of 3, 3′-diindolylmethane encapsulated chitosan nanoparticles prop up with lipid metabolism and biotransformation enzymes against possible mammary cancer. J Appl Pharm Sci. 2017;7(3):194–201.
Bhowmik A, Chakravarti S, Ghosh A, Shaw R, Bhandary S, Bhattacharyya S, et al. Anti-SSTR2 peptide based targeted delivery of potent PLGA encapsulated 3,3′-diindolylmethane nanoparticles through blood brain barrier prevents glioma progression. Oncotarget. 2017;8(39):65339–58.
Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989 [cited 2016 Jul 30];55(1):R1–4. Available from: http://linkinghub.elsevier.com/retrieve/pii/0378517389902810
Re R, Pellegrini N, Proteggente A, Pannala A, Min Yang A, Catherine R-E. Antioxidant activity appliying an improved ABTS radical. Free Radic Biol Med. 1999;26(9/10):1231–7.
Sharma OP, Bhat TK. DPPH antioxidant assay revisited. Food Chem. 2009;113:1202–5.
Korsmqer RW, Gumy R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. 1983;15:25–35.
Guterres SS, Weiss V, De Lucca Freitas L, Pohlmann AR. Influence of benzyl benzoate as oil core on the physicochemical properties of spray-dried powders from polymeric nanocapsules containing indomethacin. Drug Deliv. 2000 [cited 2016 Aug 1];7(4):195–9. Available from: http://www.tandfonline.com/doi/full/10.1080/107175400455119.
Santos SS, Lorenzoni A, Ferreira LM, Mattiazzi J, Adams AIH, Denardi LB, et al. Clotrimazole-loaded Eudragit® RS100 nanocapsules: preparation, characterization and in vitro evaluation of antifungal activity against Candida species. Mater Sci Eng C. 2013;33(3).
Gehrcke M, Sari MHM, Ferreira LM, Barbieri AV, Giuliani LM, Prado VC, et al. Nanocapsules improve indole-3-carbinol photostability and prolong its antinociceptive action in acute pain animal models. Eur J Pharm Sci. 2018;111(May 2017):133–41. https://doi.org/10.1016/j.ejps.2017.09.050.
Pegoraro NS, Mattiazzi J, da Silveira EF, Azambuja JH, Braganhol E, Cruz L. Improved photostability and cytotoxic effect of coenzyme Q10 by its association with vitamin E acetate in polymeric nanocapsules. Pharm Dev Technol. 2017:1–19 Available from: https://www.tandfonline.com/doi/full/10.1080/10837450.2017.1332641.
Pegoraro NS, Barbieri AV, Camponogara C, Mattiazzi J, Brum ES, Marchiori MCL, et al. Nanoencapsulation of coenzyme Q10 and vitamin E acetate protects against UVB radiation-induced skin injury in mice. Colloids Surf B: Biointerfaces. 2017;150:32–40.
Rigon C, Giuliani LM, Fabiele M, Stangarlin L, Mattiazzi J, Gomes FP, et al. Sistemas nanoestruturados contendo óleo de linhaça: desenvolvimento tecnológico e caracterização físico-química de nanoemulsões e nanocápsulas poliméricas. Saúde (Santa Maria). 2017;43(1):153–61.
dos Santos PP, Flôres SH, de Oliveira Rios A, Chisté RC. Biodegradable polymers as wall materials to the synthesis of bioactive compound nanocapsules. Trends Food Sci Technol. 2016;53:23–33.
Mohanraj VJ, Chen Y. Nanoparticles – a review. Trop J Pharm Res. 2006;5(June):561–73.
Schaffazick SR, Pohlmann AR, Mezzaliraa G, Guterres SS. Development of nanocapsule suspensions and nanocapsule spray-dried powders containing melatonin. J Braz Chem Soc. 2006;17(3):562–9.
Boakye CHA, Patel K, Doddapaneni R, Bagde A, Chowdhury N, Safe S, et al. Ultra-flexible nanocarriers for enhanced topical delivery of a highly lipophilic antioxidative molecule for skin cancer chemoprevention. Colloids Surf B: Biointerfaces. 2016;143:156–67. https://doi.org/10.1016/j.colsurfb.2016.03.036.
Ourique AF, Pohlmann AR, Guterres SS, Beck RCR. Tretinoin-loaded nanocapsules: preparation, physicochemical characterization, and photostability study. Int J Pharm. 2008;352:1–2):1–4.
Detoni CB, Souto GD, Da Silva ALM, Pohlmann AR, Guterres SS. Photostability and skin penetration of different E-resveratrol-loaded supramolecular structures. Photochem Photobiol. 2012;88(4):913–21.
Shalaby EA, Shanab SMM. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J Mar Sci. 2013;42(September):556–64.
Miliauskas G, Venskutonis PR, Van Beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85(2):231–7.
Alves CQ, David JM, David JP, Bahia MV, Aguiar RM. Métodos para determinação de atividade antioxidante in vitro em substratos orgânicos. Quím Nova. 2010;33(10):2202–10.
Benabadji SH, Wen R, Zheng J, Dong X, Yuan S. Anticarcinogenic and antioxidant activity of diindolylmethane derivatives. Acta Pharmacol Sin. 2004;25(5):666–71 Available from: http://www.ncbi.nlm.nih.gov/pubmed/15132835.
Li F, Jin H, Xiao J, Yin X, Liu X, Li D, et al. The simultaneous loading of catechin and quercetin on chitosan-based nanoparticles as effective antioxidant and antibacterial agent. Food Res Int. 2018;111(May):351–60. https://doi.org/10.1016/j.foodres.2018.05.038.
Kosaraju SL, D’ath L, Lawrence A. Preparation and characterisation of chitosan microspheres for antioxidant delivery. Carbohydr Polym. 2006;64(2):163–7.
Chassot JM, Ribas D, Silveira EF, Grünspan LD, Pires CC, Farago PV, et al. Beclomethasone dipropionate-loaded polymeric nanocapsules: development, in vitro cytotoxicity and in vivo evaluation of acute lung injury. J Nanosci Nanotechnol. 2015;15:855–64.
Anuchapreeda, S., et al. Preparation of Lipid Nanoemulsions Incorporating Curcumin for Cancer Therapy. Journal of Nanotechnology. 2012;1.
Liang, N. et al. ALFA-Tocopherol succinate-modified chitosan as a micellar delivery system for paclitaxel: Preparation, characterization and in vitro/in vivo evaluations. International Journal of Pharmaceutics. 2012;423:480– 488.
Schultze, E., et al. Drug-loaded nanoemulsion as positive control is an alternative to DMSO solutions for in vitro evaluation of curcumin delivery to MCF-7 cells. Pharmacological Reports. 2017;1408–1412.
Chittasupho, C. et al. Nanoparticles of Combretum quadrangulare leaf extract induce cytotoxicity, apoptosis, cell cycle arrest and anti-migration in lung cancer cells. Jornal of drug delivery science and technology. 2018;45:378.
Li, Y., et al. Emulsion-Based Delivery Systems for Tributyrin, a Potential Colon Cancer Preventative Agent. J Agric Food Chem. 2009;57:9243–9249.
Mendes, L.P., et al. Biodegradable nanoparticles designed for drug delivery: The number of nanoparticles impacts on cytotoxicity. Toxicology in Vitro. 2015;29:1268–1274.
Krai, J., et al. Doxazosin nanoencapsulation improves its in vitro antiproliferative and anticlonogenic effects on breast cancer cells. Biomedicine & Pharmacotherapy. 2017;94:10–20.
Rocha, V., et al. In vitro cytotoxicity evaluation of resveratrol-loaded nanoparticles: Focus on the challenges of in vitro methodologies. Food and Chemical Toxicology. 2017;103:214-222.
Sari MHM, Ferreira LM, AngonesiZborowski V, Araujo PCO, Nadal JM, Farago PV. p,p’-Methoxyl-diphenyl diselenideincorporation into polymeric nanocapsules improves its antinociceptive action: physicochemical and behavioral studies. Colloids Surf B: Biointerfaces. 2017;157(et al):464–72. https://doi.org/10.1016/j.colsurfb.2017.06.016.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mattiazzi, J., Sari, M.H.M., Lautenchleger, R. et al. Incorporation of 3,3′-Diindolylmethane into Nanocapsules Improves Its Photostability, Radical Scavenging Capacity, and Cytotoxicity Against Glioma Cells. AAPS PharmSciTech 20, 49 (2019). https://doi.org/10.1208/s12249-018-1240-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-018-1240-8