Abstract
In this paper, a novel self-nanoemulsifying drug delivery system (SNEDDS) was used to improve the oral bioavailability in fasted state and diminish the food effect for rivaroxaban. Oil, surfactant, and co-surfactant were selected by saturated solubility study. IPM, Tween80, and 1,2-propanediol were finally selected as oil, surfactant, and co-surfactant, respectively. The pseudo-ternary-phase diagram was utilized to optimize the preliminary composition of SNEDDS formulation. The optimized rivaroxaban-SNEDDS formulation was selected by central composite design (CCD) of response surface methodology. Optimized SNEDDS formulation was evaluated for drug content, self-emulsifying time, droplet size, zeta potential, polydispersity index, Fourier transform-infrared (FTIR) spectroscopy, and transmission electron microscope (TEM). The drug dissolution profile compared to the commercial formulation Xarelto® (20 mg rivaroxaban) was determined in four different media (pH 1.2HCl, pH 4.5NaAc-HAc, pH 6.8PBS, and water). The result indicated that the SNEDDS formulation had successfully increased the drug solubility in four different media. A HPLC-MS method that indicated a high sensitivity, strong attribute, and high accuracy characteristic was built to measure the drug concentration in plasma. The fast/fed in vivo pharmacokinetics studies of SNEDDS formulation and Xarelto® were carried out in adult beagle dog, rivaroxaban with no food effect was achieved in SNEDDS formulation compared with Xarelto® in fed state. The result suggested that SNEDDS formulation in this study is useful to increase the oral bioavailability and diminish the food effect in fasted state.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In recent years, oral administration is still one of the major route of drug delivery systems; however, food has an obvious effect on the oral bioavailability of some commercial drugs (1). Relevant clinical studies have shown that most of BCSII drugs which are poorly soluble highly permeable have an apparent food effect owing to enhanced drug solubility in the fed state (2,3). Three mechanisms can explain food effect; retention time of poorly soluble drug is improved clearly in stomach because of delayed gastric emptying after meal (4). Food boost the secretion of bile, a function as a surfactant, which can enhance the solubility of poorly aqueous drugs in vivo. A change in gastrointestinal pH profile after food intake may has an evidently impact on the absorption of drug with pH-dependent dissolution (5).
Rivaroxaban, anticoagulant agent, the active ingredient in Xarelto® Tablets, is used in the treatments of deep vein thrombosis (DVT) and pulmonary embolism (PE). Traditional anticoagulant drugs such as vitamin K, unfractionated heparin, and fawarin are not widely used in the course of clinical due to unsatisfactory effectiveness of treatment and toxic effect. Rivaroxaban is an efficient factor Xa (FXa) inhibitor. It does not require a cofactor for activity, which directly inhibits free FXa and prothrombinase activity. These advantages make people notice that rivaroxaban has a broad application foreground in clinical medicine (6). However, the poor solubility of rivaroxaban in aqueous media indirectly results in a difference between fasted and fed state (7). Various techniques have been utilized to solve the problem of poor aqueous solubility of BCSII drugs, such as liposomes (8), nanosuspensions (9), solid dispersions (10), and cyclodextrin inclusions (11).
Self-nanoemulsifying drug delivery system (SNEDDS) is a novel solubilization technique that has gained wide attention due to its excellent properties in improvement of the solubility and oral absorption rate of BCSII drugs (12). SNEDDS is a homogeneous, transparent, and stable liquid formulation, consisting of oils, surfactant, and co-surfactant. Under mild gastrointestinal peristalsis, the formulation can spontaneously form a O/W type emulsion with a droplet size less than 100 nm in digestive juice. SNEDDS provides a lipophilic environment to bad water-solubility drugs, which can remarkably improve solubility and oral bioavailability of these drugs (13). Today, lots of related studies both domestic and overseas are gradually increasing, such as oleanolic acid (14), telmisartan (15), lurasidone (16), and berberine hydrochloride (17), all of them have a significant improvement in oral bioavailability.
Many of theoretical researches can explain formation mechanism of SNEDDS, which including hybrid interface film-liquid crystal theory, negative interfacial tension theory, thermodynamic argument, and solubilization effect mechanism. Negative interfacial tension theory is identified by most scholars (18,19).
The descriptions of negative interfacial tension theory are as follows: self-emulsification occurs when the entropy change that favors dispersion is greater than the energy required to increase the surface area of the dispersion (20). The free energy of normal emulsion is a direct-acting energy required to build a new surface between the oil and aqueous media, which can be summarized by Eq. (1):
Where DG is the free energy related to the process, N is the number of droplets of radius r, p is the interface pressure, and S represents the interface free energy.
A stable SNEDDS first has an emulsifier, which forms a monolayer on the emulsion droplets to minish the interface free energy (21). Just as important, a homogeneous droplet size and the polarity of emulsion droplets help to bring about a rapid drug release in the aqueous media. The polarity of emulsion droplets is determined by the percentage of emulsifying agent, hydrophilic-lipophilic balance, and the chain. In brief, a minute droplets with the appropriate polarity promote an appropriate drug release rate (22).
In this investigation, the components of oil, surfactant, and co-surfactant were screened based on the solubilizing capacity. The preliminary composition of SNEDDS formulation was determined by structuring the pseudo-ternary-phase diagram (23). Then, the central composite design of response surface methodology was used to obtain the optimized SNEDDS formulation. Physical characteristics were investigated by self-emulsifying time, droplet size, zeta potential, polydispersity index, and transmission electron microscope. The in vitro dissolution studies carried out in four different media were used to evaluate the drug release profiles. The ability of rivaroxaban-SNEDDS formulation to diminish food effect on the oral bioavailability was estimated in fast/fed beagle dogs and compared with the commercial formulation (24).
MATERIALS AND METHODS
Materials
Rivaroxaban drug was supplied as a gift from Hausen pharmaceutical industry (Jiangsu, China). Dabigatran reference was purchased from China Pharmaceutical biological products verification Institute (NICPBP). Isopropyl myristate (IPM) was obtined from Xie Tai Chemical Co., Ltd. (Shanghai, China). Ethyl oleate, Tween20, and Tween80 were donated by Shen Yu Pharmaceutical Chemical Co., Ltd. (Shanghai, China). Cremophor, Cremophor HEL, and Transutol were provided from BASF Co., Ltd. (Ludwigshafen, Germany). All other materials were of reagent grade.
METHODS
HPLC Condition
Concentration of rivaroxaban was determined by HPLC method. The HPLC consisted of Waters 1525 HPLC pump, Waters 2489 UV detector, and Waters 2707autosampler. A Phenomenex C18 column (250 mm × 4.6, 5 μm) was used as stationary phase, and its temperature was 30°C. The mobile phase was acetonitrile and 0.01 M phosphate buffer at the ratio of 40:60 (v/v). The velocity of flow, sample load, and wavelength of the UV detector were set at 1.0 ml min−1, 20 μl, and 250 nm, respectively.
Solubility Studies
The important base for preparing a successful rivaroxaban self-emulsion was screened suitable oil, surfactants, and co-surfactants (25). The oil include isopropyl myristate (IPM), olive oil, octyl and decyl glycerate (ODO), ethyl oleate, and oleic acid. Surfactants contain Tween80, Tween40, Span80, and OP-10. Co-surfactant consists of ethanol, PEG200, PEG400, 1,2-propanediol, and glycerinum.
An excess amount of rivaroxaban raw material was added to a 10 ml centrifuge tube, which containing 3 ml of various oils, surfactants, and co-surfactants. Then, the samples were vortexed by vortex mixer for 5 min in order to accelerate the solubilization of rivaroxaban, followed by equilibration in an air-bath oscillator for 48 h at 37 ± 0.5°C. Three copies of each sample were measured in parallel. The samples were removed from the air-bath oscillator and centrifuged at 5000 rpm for 10 min. After that, the supernatant was transferred from centrifuge tube and filtrated by a membrane filter (0.45 μm, PALL, Washington, NY). The filtrates were diluted by mobile phase, and its drug concentration was analyzed by HPLC method which mentioned in the section “HPLC condition.”
Preparation of Pseudo-Ternary-Phase Diagram
The preparation of pseudo-ternary-phase diagram was to determine the selection of rivaroxaban SNEDDS components. According to the result of solubility studies, the pseudo-ternary-phase diagrams of oils (IPM), surfactants (Tween 80), and co-surfactants (1,2-propanediol) were drawn with water titration method to construct the range which self-microemulsifying region is existence (26).
To obtain optimal solubility of rivaroxaban and the stability of the self-emulsion, a series of ratios of oil to surfactant and co-surfactant (Smix) were selected to draw a self-emulsion region by pseudo-ternary-phase diagram. The Smix ratios were 1:3, 1:2, 1:1, and 2:1, respectively, the mixture of oil and Smix ratios were 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 7:3, 8:2, and 1:9. Each of mixture was added in round-bottom flask under powerful stirring by magnetic stirrers at 150 rpm for 5 min, then it was titrated with distilled water until mixture system formed a clear and transparent self-emulsion with a faint blue light through visual observation. The concentration of components was recorded to draw the pseudo-ternary-phase diagram which respectively chose oil, Smix, and water as its three vertices. The software OriginPro 8.6 was used to construct the pseudo-ternary-phase diagram of rivaroxaban-SNEDDS.
Optimized Formulation by Central Composite Design
According to the previous studies, the ratio of Smix also known as Km was roughly determined by the pseudo-ternary-phase diagram. Furthermore, the oil percentage in the formulation and the Km directly affected the properties of rivaroxaban-SNEDDS, which contained self-emulsification time, droplet size, and polydispersity index (PDI). Therefore, the oil percentage (X1) and the Km (X2) were chosen as experimental factors which could be optimized by central composite design (CCD) of RSM.
As shown in Table I, a CCD with five levels (−α, − 1, 0, 1, α) and two factors formed 13 experiments, the ranges of X1 were 35–65% and X2 was 1–2 ratio. Three responses had included self-emulsification time (Y1), droplet size (Y2), and PDI (Y3) since they were regarded as important indicator for evaluating the properties of rivaroxaban-SNEDDS (27,28,29,30). The experimental range of each variable was set based on the result of previous experiments. Design-Expert.V8.0.6.1 software was utilized to design and analyze the experimental data.
Preparation of Rivaroxaban-SNEDDS
The desired component ratio of rivaroxaban-SNEDDS was screened by the pseudo-ternary-phase diagram and response surface methodology. The optimized rivaroxaban-SNEDDS samples were prepared by mixing the oil, Smix, and requisite amount of drug into a solanum bottle, then the mixture was stirred by vortex mixer at room temperature until appearing a transparent yellow oily system.
Characterization of Rivaroxaban-SNEDDS
Drug Content
The drug content in rivaroxaban-SNEDDS was measured as follows: a certain amount of rivaroxaban-SNEDDS (theoretical drug content 20 mg) was added to 100 ml volumetric flask and fixed volume after dissolution with methanol. The same content of reference solution was prepared accurately at the same time. Two solutions were separately filtered through 0.45 μm membrance filter to remove undissolved materials, and their drug content was detected by HPLC method referring to HPLC condition.
Self-Emulsification Time
Self-emulsification time is one of the important indexes evaluating the property of SNEDDS (31). On the basis of the 0931 method of dissolution and release degree in 2015 edition of Chinese Pharmacopeia, the type II dissolution apparatus was selected to measure emulsifying efficiency of SNEDDS. One milliliter rivaroxaban-SNEDDS was added into 900 ml of water medium with a 50 rpm at a temperature of 37 ± 0.5°C, timekeeping started with the SNEDDS came into contact with the water, until it completed emulsification, recording the self-emulsification time through visual observation.
Droplet Size, PDI, and Zeta Potential
Admirable SNEDDS should also possess a suitable droplet size, a small enough PDI, and a proper zeta potential in diluted medium (32). One milliliter rivaroxaban-SNEDDS was transferred to 100 ml volumetric flask and fixed volume with purified water to became a 100 times diluent. The droplet size and PDI of rivaroxaban-SNEDDS diluent were tested by Malvern Zetasizer nano instruments (Nano ZS 90, Worcestershire, UK). Meanwhile, the zeta potential was determined by this instrument.
Effects of pH and Dilution Ratio
It is necessary to keep a stable property of SNEDDS with various fold dilutions at different pH conditions. The optimized rivaroxaban-SNEDDS formulation was diluted 50, 100, and 1000 times with distilled water, pH 1.2 HCl media, pH 4.5 acetic acid buffer media, and pH 6.8 PBS media, respectively. Then, the diluted samples were stored at room temperature for 24 h. Any changes of physical property should be recorded, such as flocculation, precipitation, and phase transition. In addition, the changes in mean droplet size and PDI were tested to evaluate the physical stability.
Fourier Transform-Infrared Spectroscopy
The aim of Fourier transform-infrared (FTIR) spectroscopy was used to test the compatibility of the rivaroxaban active substance with other excipients. The FTIR spectra of rivaroxaban, blank SNEDDS, and rivaroxaban-SNEDDS were obtained using a NICOLET iS 10 FTIR spectrometer. The samples were prepared by potassium bromide disks with hydraulic tablet press and then scanned in the region of 500–4000/cm−1.
Transmission Electron Microscope
The transmission electron microscope (TEM) was used to observe the morphology of rivaroxaban-SNEDDS. 100 folds water medium dilution of sample was added into the copper grid and dyed with 2% mass fraction of Na3O40PW12 for 40 s. After the sample is natural dried at room temperature (25°C) and then observed the particle morphology via TEM (Philips Tecnai 12, Eindhoven, the Netherlands). Finally taking the photo under the appropriate magnification.
Dissolution Study
The optimized rivaroxaban-SNEDDS formulation was filled into size “0” hard gelatin capsule in advance (all of formulations contained 20 mg rivaroxaban) and commercial formulation Xarelto® (20 mg rivaroxaban) was selected as a reference. The dissolution study of rivaroxaban-SNEDDS and Xarelto® was conducted based on the Chinese Pharmacopeia type II apparatus, using paddle method, containing 900 ml of dissolution medium, at a medium temperature of 37 ± 0.5°C with a speed of 50 rpm. pH 1.2 HCl media, pH 4.5 acetic acid buffer media, pH 6.8 PBS media, and distilled water were chosen as the dissolution media to study the drug release profile. At the specified times (2, 5, 10, 15, 20, 30, 45, 60, and 90 min), 1 ml of aliquot was withdrawn, and same volume of fresh dissolution medium was injected to maintain the the total volume. The sample was filtered using a 0.45-μm membrane filter and then transferred into glass vials, 20 μl of subsequent filtrate was determined by HPLC to measure the drug concentration of rivaroxaban. The dissolution profile of homemade SNEDDS formulation was compared with that of commercial formulation Xarelto®.
The similarity factor, f2, can be recommended to evaluate the similarity of release profiles between the test preparation and reference preparation in different dissolution media. The relevant equation is as follows (Eq. (2)):
Where, R and T represent the cumulative dissolution of drug of the reference formulation and the test formulation at the specified time point and n stands for the number of sampling time points. The values of f2 range from 0 to 100. When the f2 > 50, the drug release profiles between reference formulation and test formulation are considered to be similar.
In Vivo Pharmacokinetic Study
Oral Administration and Plasma Collection
The in vivo pharmacokinetic studies described here were approved by Shanghai Jiao Tong University School of Agriculture and Biology. Its use committee (SCXK 2012-0005) adhered to the “Principle of Laboratory Animal Care.” Eight adult beagle dogs were selected to conduct this trial. All of them were maintained in a controlled breeding room for 7 days prior to the beginning of the study. Eight beagle dogs were randomly divided into four groups which adopting a 4 × 4 crossover experimental design. Each group consists of a male and a female (33). Beagle dogs were fasted for 12 h before the experiment began. In the fasted studies, dogs were dosed and then immediately given 60 ml of tap-water by oral gavage. In the fed studies, dogs that have fed high calories fat dog food previously were also dosed and then quickly given the same volume of tap-water by oral gavage.
Four milliliters of blood samples was gathered from the jugular vein of the dogs into 5 ml sodium heparin tubes at 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, and 24 h after oral administration. Plasma was separated from the blood sample immediately by centrifuged at 5000 rpm for 10 min in high speed centrifuge. Then, taking the supernatant plasma to 10 ml centrifuge tube and stored at − 20°C of refrigerator until assayed.
One hundred microliters of plasma extracted was mixed with 100 μl internal standard solution of dabigatran (1 μg/ml) in 800 μl acetonitrile solution and vortexed for 2 min to mix the sample sufficiently, after that, centrifuged at 12000 rpm for 5 min to precipitate the proteins. The supernatant layer was evaporated by Termovap Sample Concentrator until total dryness. The residue was redissolved with 100 μl mobile phase and vortexed for 1 min. Ten microliters of the resulting solution was analyzed by liquid chromatographic and mass spectrometry (LC-MS) method for rivaroxaban concentration.
Liquid Chromatographic and Mass Spectrometry Condition
The analytical column used to detect the drug concentration was a Waters C18 purchased from Waters Science Inc. (Massachusetts, USA). The mobile phase was consisted of 5 mmol/L ammonium acetate and acetonitrile at a 30/70 (v/v) ratio, its velocity of flow was 0.3 ml/min. The column temperature was set to 40°C. Mass spectrum detection conditions were optimized as follows: detection method, NRM; ionic polarity, positive; Ionization style, electrospray ionization (ESI); ion source temperature, 120°C; ion source gas, nitrogen; curtain gas, 40 L/h; capillary voltage, 3000 V; cone voltage, 35 V; desolvent gas flow velocity, 500 L/h; desolvent zone temperature, 250°C. The collision energy for rivaroxaban was optimal at 27 V and its internal standard of dabigatran was set to 29 V. Analytes were detected by monitoring the transitions m/z 436 → 145 and 472 → 289 for rivaroxaban and dabigatran.
RESULTS AND DISCUSSION
Solubility Studies
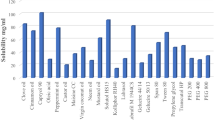
The purpose of drug solubility in various excipients used in SNEDDS was carried out to prevent the drug from settling in the vivo. The solubility of rivaroxaban in various excipients such as oil, surfactant, and co-surfactant was depicted in Fig. 1. In all studied oils, rivaroxaban showed the highest solubility in isopropyl myristate (IPM) (24.1 mg/ml). Among the 11 surfactants, Tween 80 and 1,2-propanediol could dissolve more drug, which was 45.8 and 25.2 mg/ml, respectively. Hence, IPM, Tween 80, and 1,2-propanediol were chosen as oil, surfactant, and co-surfactant respectively to further study the proportion of each composition in rivaroxaban-SNEDDS.
Construction of Pseudo-Ternary-Phase Diagrams
Based on the previous experience of solubility studies, the pseudo-ternary-phase diagrams were constructed in the absence of rivaroxaban to identify the self-emulsifying regions and to demarcate the range for oil and Smix in the rivaroxaban-SNEDDS. Figure 2 shows the systems of pseudo-ternary-phase diagram including IPM, Tween 80, and 1,2-propanediol as oil, surfactant, and co-surfactant, respectively. The self-emulsifying region varies with Smix ratios of Tween 80 and 1,2-propanediol (3:1, 2:1, 1:1, and 1:2). By comparing four graphs, the self-emulsifying region was relatively larger than other ratios when Smix were 1:1 and 1:2. Considering that has a slightly different between two ratios, the further investigation should be carried out to determine the precisely ratio of Smix.
Optimization Formulation of Rivaroxaban-SNEDDS
A total of 13 randomized experiments were designed to research the experimental factors that affected their corresponding response variables. The experimental factors including the oil percentage (X1) and the Km (X2), and the response variables were self-emulsification time (Y1), droplet size (Y2), and PDI (Y3), respectively. Response data of experiment were depicted in Table I.
The BBD experimental design was generated using Design-Expert.V8.0.6.1 software, and its data obtained were analyzed by the ANOVA. On the basis of experimental design, the software generated the relevant polynomial equation, which could be observed to fit the following mathematical model (Eq. (3)):
Where Y is the response variable; β0 is intercept that represents the arithmetic average of all quantitative outcomes of 13 randomized experiments; β1–β5 are coefficients computed from the observed experimental values of Y; X1 and X2 are experimental factors; X1X2 is factor interaction; X12 and X22 are quadratic term (30). The P value of response variables (Y1, Y2, Y3) was less than 0.05, which means that model terms are significant, and their R2 values were 0.9117, 0.7679, and 0.9267, indicting a well correlation between the experimental factors and response variable. The specific polynomial regression equations were as follows (Eqs. (4–6)):
The 3D respond surfaces and contour plots were presented in Figs. 3, 4, and 5. A tendency that approaching the optimized region of response values clearly showed in these graphs. From the response surface of self-emulsifying time, when the value of Km was higher than 1.5 (rough value), a trend could be seen that the higher the value of Km was, the longer self-emulsifying time was. However, when the value of Km was lower than 1.5, self-emulsifying time had a decrease tendency with the increase of the value of Km. Besides, when the value of oil percentage was more than 50% (rough value), oil percentage was higher, the self-emulsifying was longer. Instead, with the increase of oil percentage, the self-emulsifying time descended gradually. The similarity tendency could be observed in Figs. 4 and 5. According to the software calculation, final composition of the optimized formulation was 48.65% (w/w) of IPM, 31.05% (w/w) of Tween 80, and 20.30% (w/w) of 1,2-propanediol.
Study on Evaluation of Self-Nanoemulsion In Vitro
Drug Content
The first condition for further studies was that prescription amount of rivaroxaban-SNEDDS reached the prescribed range of drug content. According to the result which figured out by external standard method, the drug content of optimized rivaroxaban-SNEDDS was 99.16 ± 0.53%, which basically met the requirements of drug content.
Self-Emulsification Time
A success of SNEDDS formulation should be emulsified quickly in aqueous medium. It is reported that a good SNEDDS should spread to the medium within 120 s with a mild stirring (31). The self-emulsification time of rivaroxaban-SNEDDS was 60 s. A rapid self-emulsification time ensured a fast drug release after dilution in vivo.
Droplet Size, PDI, and Zeta Potential
Droplet size and zeta potential of the diluted rivaroxaban-SNEDDS solution in distilled water medium were measured. Particle size distribution diagram of optimized SNEDDS with 100-fold dilution in distilled water was clearly depicted in Fig. 6. Droplet size of optimized SNEDDS was in the range of 50.7 ± 7.3 and 57.4 ± 7.5. PDI of diluted SNEDDS was in the range of 0.180 ± 0.035 and 0.201 ± 0.022. These data indicated a fact that the dilution factor of water medium had a little effect on the droplet size and PDI. Zeta potential of optimized SNEDDS was − 14 ± 1.3 mV, the negative charge of the prepared formulation was maybe owing to the presence of the free fatty acids (34), which could make the nanoemulsion in a stable condition (35).
Effects of pH and Dilution Ratio
To forecast whether the formulation could form a homogeneous system in the gastrointestinal tract. The optimized rivaroxaban-SNEDDS was exposed to different folds of dilution in different pH media to mimic the in vivo conditions where the SNEDDS may be diluted gradually (36). Table II exhibited that the droplet size and polydispersity index have no significant change with different folds of dilution in different media. Even after stored for 24 h in room temperature (25°C), the SNEDDS showed no sign of flocculation, precipitation, or phase transition. This observation indicated that the rivaroxaban emulsion could keep its primary physical characteristics in the vivo.
Fourier Transform-Infrared Spectroscopy
In Fig. 7, the FTIR spectra of samples (rivaroxaban, blank SNEDDS and rivaroxaban-SNEDDS) at 500–4000 cm−1 wavenumber were stacked for visual analysis. Rivaroxaban was identified by characteristic peaks at 3350 cm−1 (N-H stretch), 1715 cm−1 (C=O stretch), and 1590 cm−1 (Ar-Cl stretch). Blank SNEDDS revealed absorption peaks at 3420 cm−1 (O-H stretch), 2900 cm−1 (C-H stretch), and 1700 cm−1 (C=O stretch). On the whole, the spectra of the rivaroxaban-SNEDDS showed no significant differences from the blank SNEDDS, which indicated that no new chemical bonds were created in rivaroxaban-SNEDDS. However, the absorption peaks of blank SNEDDS at 2900 and 1125 cm−1 almost disappeared in the spectra of rivaroxaban SNEDDS. Respectively, some characteristic peaks of rivaroxaban also weaken or vanished from the spectra of rivaroxaban-SNEDDS. These changes suggested that some bonds of rivaroxaban in SNEDDS were probably occurred intermolecular force with other bonds during self-emulsifying process, which proved that there was a good compatibility between drug and excipients.
Transmission Electron Microscope
TEM imaging was done to intuitively confirm the morphology of rivaroxaban-SNEDDS. As presented in Fig. 8, the droplet on the 100 folds of water diluted medium showing a uniform, disperse, and spherical shape with minute size.
In Vitro Dissolution Study
The dissolution of rivaroxaban from SNEDDS was carried out in four different dissolution media previously mentioned to assess the efficiency of the optimized formulation to ameliorate the poor aqueous solubility of rivaroxaban. As shown in Fig. 9, compared to the commercial formulation Xarelto® (20 mg rivaroxaban), SNEDDS formulation displayed a higher dissolution in all of four different media. Commercial formulation showed a relatively low dissolution in hydrochloric acid media with about 54% of the dose dissolved after 60 min, while SNEDDS formulation reached about 98% after the same time. In other three media (acetic acid buffer solution, phosphate buffer solution, and distilled water), drug dissolution rate of commercial formulation was no reached about 66% after 60 min; however, SNEDDS formulation showed about 98% dissolved after the same time. This indicated that the optimized SNEDDS formulation had successfully improved the solubility of rivaroxaban no matter what media. At the same time, the similarity factor f2 of dissolution curves of rivaroxaban between water and hydrochloric acid media was 64, and the f2 values between water and acetic acid buffer were 81. A comparison between the water and PBS media, its f2 values was 89. Therefore, in terms of overall dissolution performance, what could be predicted is that the dissolution of SNEDDS formulation would not be affected by the pH variation in vivo.
In Vivo Pharmacokinetic Study
The calibration equation for rivaroxaban concentration in plasma was linear over the range of 10–2000 ng/ml which presented in Fig. 10. Area of peak for 4.3 ng/ml was the lower limit of quantification. Extraction recovery at 10.24, 512, and 1536 ng/ml and its range from 88.48 to 93.50. Relative standard deviation (RSD) of inter- and intra-day precision were all below 10% (37,38). All above results accorded with the analytical requirement of blood plasma sample of beagle dog.
The plasma concentration versus time of rivaoxaban was determined after oral administration of SNEDDS formulation (20 mg rivaroxaban) and commercial formulation (20 mg rivaroxaban) to fasted and fed adult beagle dogs were presented in Fig. 11. The corresponding pharmacokinetic parameters were listed in Table III.
As the data showed, the AUC0-∞ and Cmax of rivaroxaban were obtained from commercial formulations under fasted condition (2676 ± 152 ng h/ml and 575.3 ± 114.1 ng/ml) were obvious lower than that obtained from equal dosage of same formulation under fed condition (4545 ± 167 ng h/ml and 813 ± 121.4 ng/ml). This result confirmed a fact that food intake had a significant influence on the absorption of rivaroxaban in commercial formulation. However, the AUC0-∞ and Cmax of rivaroxaban obtained from SNEDDS formulations under fasted condition (4311 ± 147 ng h/ml and 820.9 ± 131.8 ng/ml) were similar to that taken orally same dosage of SNEDDS formulations under fed condition (4730 ± 175 ng h/ml and 832.9 ± 122.9 ng/ml). This observation revealed that SNEDDS technology used in rivaroxaban successfully enhanced drug bioavailability in fasted condition and no food effect was achieved as well in rivaroxaban-SNEDDS.
Based on the statistical analysis, the Cmax, AUC0-t and AUC0-∞ of commercial formulation showed a significant difference under the fasted and fed condition (P < 0.05); on the contrary, there had no difference presented in SNEDDS formulation (P > 0.05). In fed state, the test/reference ratio for AUC0-t, AUC0-∞ and Cmax (90% confidence interval) was 96.85~108.71%, 93.72~108.12%, and 91.34~106.81%, respectively. All of them were within 80~125%, which suggested that two formulations were bioequivalence.
CONCLUSIONS
In this study, rivaroxaban-SNEDDS was successfully prepared by an optimized composition of IPM, Tween80, and 1,2-propanediol, which finally gained a fast self-emulsifying time, a suitable droplet size and a proper zeta potential. The in vitro drug dissolution study exhibited a rapid release characteristic with over 95% cumulative drug release in 35 min. The pharmacokinetics parameters of rivaroxaban-SNEDDS in beagle dogs were the same in both the fasted and fed state, indicating an enhanced oral bioavailability in fasted state and no food effect characteristics were obtained from SNEDDS formulation.
References
Yu LX, Straughn AB, Faustino PJ, Yang Y, Parekh A, Ciavarella AB, et al. The effect of food on the relative bioavailability of rapidly dissolving immediate-release solid oral products containing highly soluble drugs. Mol Pharm. 2004;1(5):357–62.
Ribas A, Zhang W, Chang I, Shirai K, Ernstoff MS, Daud A, et al. The effects of a high-fat meal on single-dose vemurafenib pharmacokinetics. J Clin Pharmacol. 2014;54(4):368–74.
Musson DG, Kramer WG, Foehr ED, Bieberdorf FA, Hornfeldt CS, Kim SS, et al. Relative bioavailability of sapropterin from intact and dissolved sapropterin dihydrochloride tablets and the effects of food: a randomized, open-label, crossover study in healthy adults. Clin Ther. 2010;32(2):338–46.
Thilakarathna SH, Rupasinghe HPV. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients. 2013;5(9):3367–87.
Okawara M, Hashimoto F, Todo H, Sugibayashi K, Tokudome Y. Effect of liquid crystals with cyclodextrin on the bioavailability of a poorly water-soluble compound, diosgenin, after its oral administration to rats. Int J Pharm. 2014;472(1–2):257–61.
Kubitza D, Becka M, Wensing G, et al. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939—an oral, direct factor Xa inhibitor—after multiple dosing in healthy male subjects. Eur J Clin Pharmacol. 2005;61(12):873–80.
Stampfuss J, Kubitza D, Becka M, Mueck W. The effect of food on the absorption and pharmacokinetics of rivaroxaban. Int J Clin Pharmacol Ther. 2013;51(7):549–61.
Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–60.
Gao Y, Li ZG, Sun M, et al. Preparation, characterization, pharmacokinetics, and tissue distribution of curcumin nanosuspension with TPGS as stabilizer. Drug Dev Ind Pharm. 2010;36(10):1225–34.
Wan S, Sun Y, Qi X, Tan F. Improved bioavailability of poorly water-soluble drug curcumin in cellulose acetate solid dispersion. AAPS PharmSciTech. 2012;13(1):159–66.
Wang D, Chen G, Ren L. Preparation and characterization of the sulfobutylether-β-cyclodextrin inclusion complex of amiodarone hydrochloride with enhanced oral bioavailability in fasted state. AAPS PharmSciTech. 2017;18(5):1526–35.
Singh B, Beg S, Khurana RK, Sandhu PS, Kaur R, Katare OP. Recent advances in self-emulsifying drug delivery systems (SEDDS). Crit Rev Ther Drug Carrier Syst. 2014;31(2):121–85.
Li F, Song S, Guo Y, et al. Preparation and pharmacokinetics evaluation of oral self-emulsifying system for poorly water-soluble drug Lornoxicam. Drug Deliv. 2014;22(4):1–11.
Xi J, Chang Q, Chan CK, Meng ZY, Wang GN, Sun JB, et al. Formulation development and bioavailability evaluation of a self-nanoemulsified drug delivery system of oleanolic acid. AAPS PharmSciTech. 2009;10(1):172–82.
Cho HJ, Lee DW, Marasini N, Poudel BK, Kim JH, Ramasamy T, et al. Optimization of self-microemulsifying drug delivery system for telmisartan using Box–Behnken design and desirability function. J Pharm Pharmacol. 2013;65(10):1440–50.
Miao Y, Sun J, Chen G, et al. Enhanced oral bioavailability of lurasidone by self-nanoemulsifying drug delivery system in fasted state. Drug Dev Ind Pharm. 2015;42(8):1.
Zhu JX, Tang D, Feng L, Zheng ZG, Wang RS, Wu AG, et al. Development of self-microemulsifying drug delivery system for oral bioavailability enhancement of berberine hydrochloride. Drug Dev Ind Pharm. 2013;39(3):499–506.
Gershanik T, Benita S. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur J Pharm Biopharm. 2000;50(1):179–88.
Pandav G, Ganesan V. Efficacy of different block copolymers in facilitating microemulsion phases in polymer blend systems. Macromolecules. 2013;46(20):8334–44.
Patel N.D, Patel K.V, PanChal L.A, et al. An emerging technique for poorly soluble drugs: self emulsifying drug delivery system. International Journal of Pharmaceutical & Biological Archives. 2011;2(2):621–29.
Kyatanwar AU, Jadhav KR, Kadam VJ. Self micro-emulsifying drug delivery system (SMEDDS): review. J Pharm Res. 2015;1:75–83.
Kassem AM, Ibrahim HM, Samy AM. Development and optimisation of atorvastatin calcium loaded self-nanoemulsifying drug delivery system (SNEDDS) for enhancing oral bioavailability: in vitro and in vivo evaluation. J Microencapsul. 2017;34(3):319–33.
Pan G, Jia X, Wei H, et al. Comparison among several preparation methods for pseudo-ternary phase diagrams of pharmaceutical microemulsions. China Pharmacy. 2006;17(1):21–23.
Basalious EB, Abdallah AM. Phospholipid based self-nanoemulsifying self-nanosuspension (p-SNESNS) as a dual solubilization approach for development of formulation with diminished food effect: fast/fed in vivo pharmacokinetics study in human. Eur J Pharm Sci. 2017;109:244–52.
Khan AW, Kotta S, Ansari SH, Sharma RK, Ali J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2015;22(4):552–61.
Miao Y, Chen G, Ren L, et al. Controlled release of glaucocalyxin—a self-nanoemulsifying system from osmotic pump tablets with enhanced bioavailability. Pharm Dev Technol. 2015;22(2):148–55.
Xu F, Wang LL, Shi ZQ, Chen F, Sun DM. Formulation optimization of Zuojin floating-bioadhesive pellets by central composite design-response surface methodology. J Chin Med Mater. 2015;38(9):1969–73.
Michaelsen MH, Wasan KM, Sivak O, et al. The effect of digestion and drug load on halofantrine absorption from self-nanoemulsifying drug delivery system (SNEDDS). AAPS J. 2015;18(1):180–6.
Thomas N, Holm R, Garmer M, et al. Supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS) enhance the bioavailability of the poorly water-soluble drug simvastatin in dogs. AAPS J. 2013;15(1):219–27.
Kumar R, Kumar S, Sinha VR. Evaluation and optimization of water-in-oil microemulsion using ternary phase diagram and central composite design. J Dispers Sci Technol. 2016;37(2):166–72.
Kang JH, Oh DH, Oh YK, Yong CS, Choi HG. Effects of solid carriers on the crystalline properties, dissolution and bioavailability of flurbiprofen in solid self-nanoemulsifying drug delivery system (solid SNEDDS). Eur J Pharm Biopharm. 2012;80(2):289–97.
Kassem AA, Mohsen AM, Ahmed RS, Essam TM. Self-nanoemulsifying drug delivery system (SNEDDS) with enhanced solubilization of nystatin for treatment of oral candidiasis: design, optimization, in vitro, and in vivo, evaluation. J Mol Liq. 2016;218:219–32.
Miao Y, Chen G, Ren L, Ouyang P. Preparation and evaluation of ziprasidone-phospholipid complex from sustained-release pellet formulation with enhanced bioavailability and no food effect. J Pharm Pharmacol. 2016;68(2):185–94.
Basalious EB, Shawky N, Badr-Eldin SM. SNEDDS containing bioenhancers for improvement of dissolution and oral absorption of lacidipine. I: development and optimization. Int J Pharm. 2010;391(1–2):203–11.
Zhang J, Li J, Ju Y, et al. Mechanism of enhanced oral absorption of morin by phospholipid complex based self-nanoemulsifying drug delivery system. Mol Pharm. 2015;12(2):504–13.
Yadav P, Yadav E, Verma A, Amin S. In vitro characterization and pharmacodynamic evaluation of furosemide loaded self nano emulsifying drug delivery systems (SNEDDS). J Pharm Investig. 2014;44(6):443–53.
Rv T, Meijer J, Takusagawa S, et al. Development and validation of LC-MS/MS methods for the determination of mirabegron and its metabolites in human plasma and their application to a clinical pharmacokinetic study. J Chromatogr B Anal Technol Biomed Life Sci. 2012;887-888(7):102–11.
Verma S, Singh SK. LC-ESI-MS/MS estimation of loratadine-loaded self-nanoemulsifying drug delivery systems in rat plasma: pharmacokinetic evaluation and computer simulations by GastroPlus™. J Pharm Biomed Anal. 2016;124:10.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xue, X., Cao, M., Ren, L. et al. Preparation and Optimization of Rivaroxaban by Self-Nanoemulsifying Drug Delivery System (SNEDDS) for Enhanced Oral Bioavailability and No Food Effect. AAPS PharmSciTech 19, 1847–1859 (2018). https://doi.org/10.1208/s12249-018-0991-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-018-0991-6