Abstract
The following research study focuses on improving the solubility of zaleplon (BCS class II drug) via micronization technique in order to enhance its oral delivery in orodispersible formulations. Zaleplon along with a surfactant solution was micronized by ultrasonication. The micronization process reduced the particle size of the crystalline drug about six-fold from its original size of 155.5 μm. The micronized zalepon dispersion was lyophilized to allow for a change in the state of matter (to a powder). The superior dissolution parameters (Q5, Q30, IDR, MDR, MDT, DE, and RDR) of zaleplon in microcrystalline form over the original crystalline form in in vitro dissolution studies had unraveled that micronization technique is an efficient tool in enhancing drug solubility. The micronized zaleplon solid dispersion (after lyophilization) was loaded into orodispersible tablet (ODT) and orodispersible film (ODF) formulations. The positive quality of ODT with adequate hardness and smooth texture was attributing to the presence of Pearlitol Flash® as a ready to use ODT platform. On the other hand, the ODF loaded with micronized zaleplon and prepared with Lycoat® RS 720 (as a film former) ensured adequate tensile strength. The disintegration time of ODT and ODF was 30 ± 5 and 35 ± 5 s, respectively. Thus, the orodispersible formulations containing micronized zaleplon have a strong potential for rapid disintegration following superior absorption in solution state through oral cavity into the blood stream, envisaging better oral delivery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Insomnia is a sleep disorder that could occur due to various physical, psychological, and socioeconomic stresses in populations of different ages and genders [1, 2]. Irrespective of the root cause, more than 67% of geriatric population suffer from sleep disturbances resulting in a deplorable quality of their life [3,4,5]. Commonly, short-acting benzodiazepine and non-benzodiazepine hypnotics are prescribed in order to improve sleep quality without residual effects; the effects are commonly noticed in the case of these long-acting hypnotics [6, 7]. Zaleplon is a short-acting non-benzodiazepine hypnotic agent prescribed to older adults with sleep affliction [6]. Additionally, this drug is recommended for elderly patients with electroshock-induced convulsions [8]. Zaleplon currently marketed in the US market as an immediate release capsule, under the brand name SONATA®. Being a BCS class II drug, its poor aqueous solubility limits the oral bioavailability [9]. Moreover, its extensive hepatic metabolism limits the systemic absorption to < 30% [10].
In order to improve the bioavailability of dissolution rate limited drugs, formulation scientists have reported numerous conventional and advanced formulation strategies such as solid dispersions, molecular inclusion complexes with cyclodextrins, salt formation, self-emulsifying drug delivery systems, micro-/nanoemulsions, liposomes, and niozomes [11,12,13,14]. Micronization, a technique of reducing the particle size to 10–30-μm range, has gained prominence as an industrial unswerving technique. It is cost-effective and highly reproducible due to its consistent particle size distribution [15]. The micronized crystals of drug have been shown to have a high dissolution rate and consequently, this strategy of solubility enhancement is an efficient practice to improve the rate and extent of absorption of BCS class II drugs across biological membranes [16].
Orodispersible formulations are the new dosage forms, which disintegrate or dissolve instantly when placed on the tongue [17,18,19]. For geriatric and pediatric patients with dysphagia, the replacement of swallowable dosage forms with orodispersible formulations is effective. The orodispersible formulation provides an increased patient compliance by an instantaneous disintegration or dissolution in the oral cavity [20, 21]. The drug, presented in these formulations, enters the systemic circulation via oral mucosa and enhances the oral bioavailability by bypassing first-pass hepatic metabolism. However, these formulations proved ineffective when loaded with pharmaceutical actives of poor aqueous solubility and/or limited permeability [22].
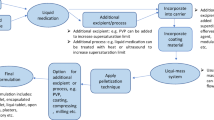
Therefore, this research is first addressing zaleplon’s solubility by micronizing it into an ultrafine crystalline form. Micronized particle size distribution was evaluated using a laser particle size analyzer. A solid dispersion of microcrystalline zaleplon was then generated by lyophilization. The lyophilized product was investigated for in vitro dissolution behavior, to determine the solubility enhancement by micronization. In order to further enhance the oral delivery of micronized zaleplon, a solid dispersion of the microcrystalline drug was formulated as, orodispersible tablets (ODTs) and orodispersible films (ODFs) using novel formulation excipients. The chemical inertness, between the drug and all other ODF excipients, was examined via Fourier transmission infrared spectroscopy. These dosage forms were evaluated for their characteristic properties to exemplify the best quality of the formulations. ODFs were also evaluated for the presence of micronized drug particles under optical microscope. The disintegration time was also recorded to evaluate the orodispersible nature of the formulations.
MATERIALS AND METHODS
Materials
Zaleplon was provided as a gift sample from Symed Labs, Hyderabad, India. Neosorb® P60W (sorbitol), Lycoat® RS 720 (a non-GMO pregelatinized hydroxypropyl pea starch), and Pearlitol Flash® (a compound of mannitol and starch) were generous samples provided by Roquette America, Inc. (Geneva, IL USA). Pluronic® P188, Tween 80, VitE-TPGS, and sucralose were procured from Sigma Chemicals (St. Louis, MO). The other chemicals and solvents utilized in this study were of analytical and HPLC grade, respectively. Deionized (DI) water was used during the experimentation.
METHODS
Preparation of Micronized Zaleplon Suspension
A Sonics Vibra Cell VCX-130 ultrasound probe sonicator (Newton, CT) was used to prepare the micronized zaleplon suspension [15]. Zaleplon (5.0 mg) was weighed and wetted with 15 ml of a surfactant solution consisting of a combination of Pluronic® P188, Tween 80, and VitE-TPGS. Sucralose was added in equal quantities of the drug in order to help in cake formation. After high-speed homogenization (IKA RW 20 digital, IKA Works Inc., Wilmington, NC), the resultant suspension was broken down by ultrasound (Vibra-Cell, Sonics, Newton, CT) probe sonication for 1 h in pulse mode with process time intervals of 15 min. The resulting suspension containing zaleplon microcrystals was utilized in the following experiments.
Preparation of Micronized Zaleplon Powder via Lyophilization
The micronized dispersion of zaleplon was frozen at − 80°C under liquid nitrogen and isopentane. Furthermore, the frozen dispersion was evaporated until dry at − 45°C in a Labconco Freezone® 4.5-L freeze dryer (Labconco, Kansas City, MO) under vacuum (185 × 10−3 Mbar) overnight to obtain micronized zaleplon lyophilized powder (MZLP).
Physicochemical Characterization of Micronized Zaleplon Lyophilized Powder (MZLP)
Particle Size Analysis
Particle size analysis was performed using a particle size analyzer with a dry dispersion unit (Malvern Light Scattering System, Malvern PA). Refractive index values for dispersant (air) and samples were 1.0 and 1.5, respectively. The absorption coefficient was 0.01 with an air pressure of 0.3 bar. Samples of final granulate from ten different batches were used in particle size analysis. Six different representative samples from the same batch were measured. The samples were collected in 75-mL glass bottles, using a metal spoon. To obtain homogenous dispersion, bottles with samples were stirred lengthwise, 20 s in one direction and 20 s in the other direction. Amount of 1-g granulate was placed in measuring cell of the laser sizer. After the particle dispersion was complete, the measurement was performed and the particle size distribution of each granulate was recorded.
Powder X-ray Diffractometry (PXRD)
To understand the crystalline nature of pure zaleplon and MZLP, a powder X-ray diffractometric study was executed. The diffractogram of pure zaleplon and MZLP was recorded by operating the X-ray diffractometer (Bruker D8-Focus XRD, Netherlands) with a Cu anode tube over the interval of 5–70°/2θ at 40-kV voltage and 30-mA current. A constant scanning speed of 10°/min was maintained during the process.
Estimation of Drug Content
The amount of drug present in MZLP was estimated by dissolving the appropriate quantity of sample in a mobile phase. The solution was then vortexed and filtered using a 0.45-μm filter membrane (Millipore, USA). The filtrate was measured for the drug content with the aid of high performance liquid chromatography (HPLC) as described below. This experiment was carried out in triplicate for variability estimation.
In vitro Dissolution
The effect of micronization on dissolution behavior of drug was ascertained by performing an in vitro dissolution study using a USP type II (paddle) dissolution apparatus (Hanson SR-8 plus, Hanson Research Corporation, Chatsworth, CA), equipped with a paddle operated at a speed of 75 rpm. Weighed quantities of pure zaleplon (5 mg) and MZLP equivalent to 5 mg of pure zaleplon were placed in a hemispherical beaker containing 500 mL of phosphate buffered saline (pH 6.8) as dissolution medium and maintained at 37 ± 0.5°C. At predetermined time points, 5 mL of the sample was withdrawn followed by replenishment with equal volume of fresh dissolution media to ensure a fixed volume of dissolution medium during the experiment. The collected samples were quantified for zaleplon by HPLC after filtering through 0.45-μm filter membrane (Millipore, USA). This experiment was performed in triplicate.
Dissolution Parameters
The percentage cumulative amount of drug released in 5 and 30 min (Q5 and Q30, respectively) was derived from the in vitro dissolution release profile. Dissolution efficiency (DE) was depicted from the area under dissolution curve illustrated by means of trapezoidal rule and represented as a percentage in the area of rectangle representing 100% dissolution in the same time. DE was quantified by the following Eq. (1): [23].
The rate at which the drug gets dissolved in the initial 15 min of the study was termed as initial dissolution rate (IDR) and calculated using the following Eq. (2):
To understand the plausible average time taken for a molecule to be dissolved in the medium, under in vitro dissolution conditions, the mean dissolution time (MDT) of the drug was calculated with the aid of the Eq. (3). [24]:
while j denotes the in vitro dissolution sample number, n is the number of sample time points, tj defines midpoint of the jth time period (easily calculated with [t + (t − 1)] / 2) and △Mj is the additional amount of drug dissolved between tj and t − 1.
The mean dissolution rate (MDR), which specifies the cumulative amount of drug added to the bulk of dissolution media per unit time under in vitro dissolution conditions, is determined via Eq. (4) [24]:
where the number of in vitro dissolution sample times is denoted by n, between tj and t − 1 time points, the additional amount of drug dissolved is given as △Mj, and △t claims the midpoint time between tj and t – 1 (it can also be simplified with (t + t − 1) / 2).
The ratio of dissolution efficiencies of micronized MZLP with that of pure zaleplon exhibits the relative dissolution rate (RDR) (Eq. (5)) [25].
where DE is the dissolution efficiency.
Preparation of Orodispersible Formulations of Micronized Zaleplon
Preparation of Orodispersible Tablets (ODTs)
Zaleplon ODTs were formulated by a direct compression technique on a Korsch XP1 (Korsch America Inc., South Easton, MA) tablet press. The accurate quantities of MZLP, containing 5 mg of pure zaleplon, were blended with ODT platform Pearlitol Flash® for 10 min in Turbula mixer (Table I) (Glenmills Inc., Clinton, NJ). The press was fitted with 8-mm convex punches and a compression force of 20.2 kN was applied. The punched ODTs were ejected out with an ejection force of 890 N.
Preparation of Orodispersible Films (ODFs)
The ODFs containing zaleplon were formulated by a solvent casting method [26]. To an aqueous plasticizer solution of Neosorb® P60W, sweetener, color, a mixture of lecithin, and Tween 80 were dissolved in a few drops of water. Ethanol was then added and solution was mixed for about 3 min. The film forming agent Lycoat® RS 720 was added to the resultant mixture and subjected to mixing for 15 min. The zaleplon microparticle suspension was then added to the mixture and homogenized (IKA RW 20 digital, IKA Works Inc., Wilmington, NC) at 1000 rpm for about 15 min. The resulted mixture was casted on to a plastic support (byco-charts from BYC Gardner GmbH, Germany) using an automatic RK Control Coater equipped with a 200-μm wire wound rod applicator. The coated suspension was dried overnight in a humidity chamber at 25°C and 60% relative humidity (RH) conditions and cut into 3 × 2-cm2 strips when dry. Similarly, a placebo film (no drug) was also prepared.
Evaluation of ODTs
Physical parameters such as hardness, thickness, and friability of zaleplon ODTs were evaluated. These ODT parameters were assessed with the help of Schleuniger 6D tablet hardness tester, vernier calipers, and VanKel friabilator, respectively. To assess the tablet weight and drug content variations in the ODTs, weight of ten tablets was recorded, tablets were ground using a mortar and pestle, and the drug content estimated as per USP HPLC method described below. Disintegration time was recorded by performing a USP27 method for investigating the disintegration behavior of ODTs.
Properties of ODFs
Optical Microscopic Study
The optical microscopic studies were performed to reveal the presence of micronized drug in ODF. The placebo as well as zaleplon-containing ODFs were placed on a glass slide and observed for the presence of any crystalline particulates in the ODFs using an optical microscope (Axiolab A1; Carl Zeiss). The photomicrographs of both placebo and drug-loaded ODFs were recorded using a Carl Zeiss camera attached to the microscope.
Fourier Transform Infrared (FTIR) Spectroscopy
Fourier transform infrared (FTIR) spectroscopy was performed to evaluate the chemical compatibility between the drug and base constituents of ODF. The infrared spectra of pure zaleplon, Lycoat® RS 720, physical mixture of zaleplon and Lycoat® RS 720, placebo ODF, and ODF of zaleplon were recorded with a FTIR spectrophotometer (Paragon 1000, Perkin Elmer, USA) by the conventional KBr pellet method in a scanning range of 4000–500 cm−1 with a 4-cm−1 resolution.
Texture Analysis of ODFs
Tensile testing was conducted using a TA1 Texture Analyzer equipped with a 5-kg load cell (Stable Micro Systems, Surrey, UK). The film was cut into 3 × 2-cm2 strips and equilibrated at 25°C for 1 week. Tensile tests were performed according to ASTM International Test Method for Thin Plastic Sheeting (D 882-02). Each test strip was placed in tensile grips on the texture analyzer. Initial grip separation was 20 mm and the cross head speed was 12.5 mm/min. The test was considered complete when the film broke. Tensile strength, elongation at break, and tensile energy to break were computed to evaluate tensile properties of the films.
-
a)
Tensile strength (TS) was calculated by dividing the maximum load by the original cross-sectional area of the specimen and was expressed in force per unit area (MPa).
-
b)
Percent elongation at break (E%) was calculated by dividing the extension at the moment of rupture of the specimen by the initial gage length of the specimen and multiplying by 100 according to Eq. (6):
where L0 is the initial gage length of the specimen and L the length at the moment of rupture.
-
c)
Tensile energy to break was defined by the area under the stress–strain curve. The value is in units of energy per unit volume of the specimen’s initial gage region. The result was expressed in energy per unit volume. An average of five measurements was taken for each type of specimen.
HPLC Analysis
The quantification of zaleplon, in all the samples of this study, was performed as per the reported method using a HPLC system (Waters, 1525) equipped with an autosampler (Waters, 717 plus) and a variable wavelength dual λ absorbance detector (Waters, 2487) [27]. The mobile phase, consisting of a mixture of de-ionized water and acetonitrile (45:55, v/v), was allowed to pass through the Phenomenex Luna C18 (2) 100 R analytical column (4.6 mm × 150 mm, 5.0 μm) with a flow rate of 1.0 mL/min at 32°C. After loading samples in to the HPLC vial, 20 μL of solution from each vial was automatically injected into the HPLC system and the column effluent was examined for UV absorption at 232 nm.
The limit of detection and quantification were 0.1 and 10 μg/mL, respectively. The inter-day and intra-day variation in the assay was 4.8 ± 1.4%. The concentration versus peak area ratio plot was linear (r2 > 0.9993) over the concentration range of interest and the zaleplon content in samples was quantified using this plot.
RESULTS AND DISCUSSION
Micronization of zaleplon was performed by ultrasonication, a fast processing, cost-, and labor-effective technique producing high stability microcrystals [15]. Other micronization methods like mechanical milling, spray drying, and super critical fluid pose typical issues such as poor stability of micronized product, costly processing conditions, manual labor requirement, loss of material, risk of dust explosion, and time consuming processing [28]. The formed micronized dispersion of zaleplon was lyophilized with the aid of sucralose, as a cake-forming agent. The free flowing MZLP attributes to the stearic stabilizing effect by the surfactant solution during the micronization process due to low agglomeration of formed ultrafine particles [29].
Physicochemical Characterization of MZLP
Particle size of pure zaleplon and MZLP was measured using a Malvern light scattering particle size analyzer. The particle size of the pure drug was reduced from 155.5 ± 5.2 to 23.79 ± 2.25 μm upon micronization (Fig. 1). The ultrasound energy involved between the two impingement jets of drug and surfactant solution leads to fine mixing and attributed to the formation of ultrafine crystal particles. The surfactant solution acts as a stabilizing agent and has a great affinity towards newly formed hydrophobic surfaces. It ceased the crystal growth formation of microcrystals by forming a protective layer around the particle ensuring the stable micronized dispersion of drug [29].
The diffractograms of pure zaleplon and MZLP illustrated in Fig. 2 depict the crystalline nature of both pure zaleplon and MZLP. The decrease in the particle size might be reflected in the reduction of the drug peak intensity displayed in the micronized zaleplon powder diffractogram.
The drug content analysis confirmed that every 30 mg of MZLP is equivalent to 5 mg of zaleplon (100%). Thus, 30 mg of MZLP and 5 mg of pure drug were examined for in vitro dissolution characteristics in a USP type II dissolution apparatus.
The increased dissolution behavior of MZLP over the pure crystalline drug is depicted in Fig. 3. The cumulative drug release, in the bulk of dissolution medium by the end of 30 min of the study (Q30), was found to be higher in micronized form (96.12 ± 2.46%) with respect to the native form of zaleplon (61.11 ± 5.1%), thus indicating the efficiency of micronization in improving the solubility of zaleplon. The greater IDR of micronized zaleplon in comparison to the pure drug suggests that the reduced particle size enhanced the surface area of the drug. Thus, making it better exposed to dissolution media and resulting in an improved dissolution with high dissolution velocity (Table II) [16]. The MDT and MDR of the drug in a micronized form were 7.68 ± 1.27 and 3.80 ± 0.51/min respectively, whereas MDT and MDR of pure crystalline drug were 17.83 ± 1.02 and 1.34 ± 0.30/min, respectively. The high MDR and low MDT of the drug in micronized lyophilized powder attribute to the increased wetting behavior of microcrystals due to the presence of surfactant surface protective layer around them (Table II). Hence, the MZLP had shown a faster dissolution with a higher dissolution rate [16, 29]. Furthermore, the rise in the DE from 52.47 ± 2.1 to 91.13 ± 1.6 min of zaleplon upon micronization confirmed the caliber of this technique in improving the solubility of this drug. An increase in the dissolution rate of drug by 1.73 ± 0.16-fold, upon micronization over pure zaleplon, exemplifies that the micronization had imparted good dissolution behavior. This was not only because of reduced particle size but also by increasing the wetting property in the presence of surfactant [16].
Characterization of ODT
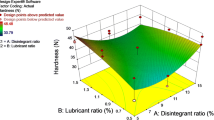
The ODTs containing micronized drug were formulated with Pearlitol Flash® ODT platform. The hardness and thickness of the tablets were 107.02 ± 9.51 N and 2.27 ± 0.25 mm, respectively. The percentage of friability of these tablets was 96 ± 2.14, indicating that the compact ODT had viable tablet strength and remained intact under mechanical stress (Table III). The presence of Pearlitol Flash® with its good flow properties and high compressibility has ensured the tablet’s hardness and a smooth surface without any visible tableting flaws (Table III) [30]. Moreover, the presence of Pearlitol Flash®, along with a lubricant, not only ensued a good flow property of ODT but also accounted to its low weight variation of less than 5% and a drug content (106 ± 2.3%), which fall within the limits specified in USP27 [30].
Disintegration time of ODTs was recorded by performing the disintegration study according to the USP27 method. All the ODTs rapidly dispersed within 30 ± 5 s in pH 6.8 phosphate buffered saline media. A high content of Pearlitol Flash®, a compound of spray dried mannitol and starch, along with a water absorptive trait, might have aided the fast disintegration of ODT without any disintegrant/super disintegrant. Moreover, Pearlitol Flash® as a component in ODT will leave the patient with a cool feel in their mouth due to the negative heat of the solution and leaves an acceptable taste because of the sweet taste of mannitol [13, 30].
Properties of ODF
ODFs were prepared by a casting method. The Lycoat® RS 720, a non-GMO pregelatinized hydroxypropyl pea starch, was used as a film forming polymer in ODF formulation based on its finest plasticization tendency [31]. The ODF formed by new film forming Lycoat® RS 720 was reported to exhibit rapid disintegration [31]. Neosorb® P60W, a non-calorific and non-acidogenic sweetener, was incorporated in the film to confer the acceptable sweet taste of ODF. The drug containing the ODF of a 3 × 2-cm2 dimension was cut and analyzed for the amount of drug present in the ODF. The relative amount of drug present in each ODF of mass 32.64 ± 1.44 mg was found to be 98 ± 3.6% falling in the range of 85% to 115% as per USP 27 guidelines (Table IV).
The MZLP-loaded ODF had showed a disintegration time of 35 ± 5 s. The presence of Lycoat® RS 720 and Neosorb® P60W had ensured the rapid disintegration of ODFs [31] (Table IV). Therefore, the formed ODF is expected to readily disperse in saliva following a rapid absorption via oral cavity mucosa into the blood stream, while avoiding the pre-systemic metabolism in liver [18, 19].
Microscopic Study of ODF
The optical microscopic studies were performed to investigate the presence of micronized zaleplon in ODFs. The microscopic studies had revealed that there was a clear transmission of light through the placebo film and indicates the absence of drug particle. But, the ODF loaded with micronized zaleplon had obstructed the transmission of bright background light due to the presence of microcrystalline drug particles (Fig. 4). The microscopic photographs delineate the presence of micronized drug in ODF, and furthermore, the uniform distribution of drug in ODF was made evident from drug content estimation studies on randomly collected ODF (Fig. 4) (Table IV).
Fourier Transmission Infrared Spectroscopic (FTIR) Studies
FTIR spectroscopic studies were executed to envisage the chemical inertness between the drug and components of ODF. The characteristic peaks corresponding to cyanide and amide carbonyl group of zaleplon at 2232 and 1651 cm−1, respectively, were found to be absent in the placebo ODF indicating the absence of drug (Fig. 5). The presence of IR stretching at 2229.32 and 1647 cm−1 in both the physical mixture and micronized drug-loaded ODF without any extra peaks suggests that all components of ODF were found to be chemically compatible with the drug (Fig. 5). However, the reduction in the intensity of absorption peaks might be due to a physical interaction between excipients and drug molecules.
Texture Analysis
The quality of obtained ODFs consisting of micronized zaleplon was confirmed by evaluating tensile strength and elongation of ODFs with the aid of texture analyzer. The tensile strength of the ODF with MZLP and placebo was 0.34 ± 0.17 and 0.32 ± 0.15 N, respectively. These strengths conferred that the ODFs not only have the optimum strength to withstand the stress during the film processing but also show that drug loading has not altered the tensile strength of the ODF (Table IV). This tensile strength of ODF attributes to the superior plasticizing quality of Lycoat® RS 720 as a result of greater chain mobility, which is because of the free volume space due to plasticization effect of the micronized drug [31,32,33]. Furthermore, the percentage elongation of drug-loaded film (14.24 ± 0.05) indicates sufficient intactness of the film. Moreover, the lower energy required to break the films represents the faster disintegration behavior of the OD films, which could be due to the hygroscopic behavior of Neosorb® P60W. However, the reduction in the tensile energy required to break the ODF of micronized zaleplon (0.004 ± 0.002 MPa) in comparison to the placebo film (0.007 ± 0.001 MPa) might be due to the increased surface area of solid drug particles after micronization.
CONCLUSION
The particle size analysis and in vitro dissolution study showed that micronized zaleplon displayed a greater dissolution behavior due to an increased surface area for dissolution. The micronized drug suspension was transformed to solid dispersions (powder state) via lyophilization and processed for preparation of orodispersible formulations containing zaleplon. The novel orodispersible excipients employed in ODT and ODF formulations of micronized zaleplon addressed improving strategies in zaleplon’s oral delivery.
Abbreviations
- BCS:
-
biopharmaceutics classification system
- Q5 :
-
cumulative drug release in 5 min
- Q30 :
-
cumulative drug release in 30 min
- DE:
-
dissolution efficiency
- MDT:
-
mean dissolution time (minutes)
- MDR:
-
mean dissolution rate
- IDR:
-
initial dissolution rate
- RDR:
-
relative dissolution rate to pure drug
- MZLP:
-
micronized zaleplon lyophilized powder
- FTIR:
-
Fourier transform infrared
- PXRD:
-
powder X-ray diffractometry
References
Qiu L, Sautter J, Liu Y, Age GD. Gender differences in linkages of sleep with subsequent mortality and health among very old Chinese. Sleep Med. 2011;12(10):1008–17. https://doi.org/10.1016/j.sleep.2011.04.014.
Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497–502. https://doi.org/10.1016/j.jpsychores.2004.02.010.
Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425–32. https://doi.org/10.1093/sleep/18.6.425.
Leblanc MF, Desjardins S, Desgagné A. The relationship between sleep habits, anxiety, and depression in the elderly. Nat. Sci. Sleep. 2015;7:33–42.
Bankar MA, Chaudhari SK, Chaudhari KD. Impact of long term yoga practice on sleep quality and quality of life in the elderly. J Ayurveda Integr Med. 2013;4:28–32.
Ancoli-Israel S, Walsh JK, Mangano RM, Fujimori M. Zaleplon, a novel nonbenzodiazepine hypnotic, effectively treats insomnia in elderly patients without causing rebound effects. Prim Care Companion J Clin Psychiatry. 199;1:114–120.
Béland SG, Préville M, Dubois MF, Lorrain D, Voyer P, Bossé C, et al. Scientific Committee of the ESA Study. The association between length of benzodiazepine use and sleep quality in older population. Int J Geriatr Psychiatry. 2011;26(9):908–15. https://doi.org/10.1002/gps.2623.
Dooley M, Plosker G. Zaleplon a review of its use in the treatment of insomnia. Drugs. 2000;60(2):413–45. https://doi.org/10.2165/00003495-200060020-00014.
Waghmare A, Pore Y, Kuchekar B. Development and characterization of zaleplon solid dispersion systems: a technical note. AAPS Pharm Sci Tech. 2008;9(2):536–43. https://doi.org/10.1208/s12249-008-9077-1.
Drover DR. Comparative pharmacokinetics and pharmacodynamics of short-acting hypnosedatives zaleplon, zolpidem and zopiclone. Clin Pharmacokinet. 2004;43(4):227–38. https://doi.org/10.2165/00003088-200443040-00002.
Valleri M, Mura P, Maeshrelli F, Cirri M, Ballerini R. Development and evaluation of glyburide fast dissolving tablets using solid dispersion technique. Drug Dev Ind Pharm. 2004;30:525–34.
Popescu C, Manda P, Juluri A, Janga KY, Cidda M, Murthy SN. Enhanced dissolution efficiency of zaleplon solid dispersions via modified ß-cyclodextrin molecular inclusion complexes. J Pharma Pharma Sci. 2015;1:1–10.
Janga KY, Jukanti R, Velpula A, Sunkavalli S, Bandari S, Kandadi P, et al. Bioavailability enhancement of zaleplon via proliposomes: role of surface charge. Eur J PharmBiopharm. 2012;80(2):347–57. https://doi.org/10.1016/j.ejpb.2011.10.010.
Gurrapu A, Jukanti R, Bobbala SR, Kanuganti S, Jeevana JB. Improved oral delivery of valsartan from maltodextrin based proniosome powders. Adv Powder Technol. 2012;23(5):583–90. https://doi.org/10.1016/j.apt.2011.06.005.
Jalay JT. A review on micronization techniques. J. Pharm. Sci Tech. 2011;3:651–81.
Rasenack N, Muller BW. Micron-size drug particles: common and novel micronization techniques. Pharm Dev Technol. 2004;9(1):1–13. https://doi.org/10.1081/PDT-120027417.
Köllmer M, Popescu C, Manda P, Zhou L, Gemeinhart RA. Stability of benzocaine formulated in commercial oral disintegrating tablet platforms. AAPSPharmSciTech. 2013 Dec;14(4):1333–40.
Hoffmann EM, Breitenbach A, Breitkreutz J. Advances in orodispersible films for drug delivery. Expert Opin Drug Delivery. 2011;8:299–316.
Hariharan M, Bogue A. Orally dissolving film strips (ODFS): the final evolution of orally dissolving dosage forms. Drug Delivery Technol. 2009;9:24–9.
Chaturvedi A, Srivastava P, Yadav S, Bansal M, Garg G, Sharma PK. Fast dissolving films: a review. Curr Drug Delivery. 2011;8(4):373–80. https://doi.org/10.2174/156720111795768022.
Arya A, Chandra A, Sharma V, Pathak K. Fast dissolving oral films: an innovative drug delivery system and dosage form. Int J Chem Tech Res. 2010;2:576–83.
Jyoti A, Gurpreet S, Seema S, Rana AC. Fast dissolving films: a novel approach to oral drug delivery. Int Res J Pharm. 2011;2:69–74.
Malladi M, Jukanti R, Nair N, Wagh S, Padakanti H, Mateti A. Design and evaluation of taste masked dextromethorphan hydrobromide oral disintegrating tablets. Acta Pharma. 2010;60:267–80.
Al-Hamidi H, Edwards AA, Mohammad MA, Nokhodchi A. To enhance dissolution rate of poorly water-soluble drugs: glucosamine hydrochloride as a potential carrier in solid dispersion formulations. Colloids Surf B. 2010;76(1):170–8. https://doi.org/10.1016/j.colsurfb.2009.10.030.
Valleri M, Mura P, Maeshrelli F, Cirri M, Ballerini R. Development and evaluation of glyburide fast dissolving tablets using solid dispersion technique. Drug Dev Ind Pharm. 2004;30(5):525–34. https://doi.org/10.1081/DDC-120037483.
Mishra R, Amin A. Formulation development of taste-masked rapidly dissolving films of cetirizine hydrochloride. Pharm Technol. 2009;33:48–56.
Janga KY, Jukanti R, Velpula A, Sunkavalli S, Bandari S, Kandadi P, et al. In situ absorption and bioavailability studies of zaleplon loaded self-nanoemulsifying powders (SNEPs). J Microencapsul. 2013;30:167–72.
Vandana KR, Raju YP, Chowdary VH, Sushma M, Kumar NV. An overview on in situ micronization technique—an emerging novel concept in advanced drug delivery. Saudi Pharm J. 2014;22:283–9.
Roya T, Varshosaz J, Mostafavi SA, Ali N. Dissolution enhancement of gliclazide using pH change approach in presence of twelve stabilizers with various physico-chemical properties. J Pharm Pharm Sci. 2009;12:250–65.
Hulse WL, Forbes RT, Bonner MC, Getrost M. The characterization and comparison of spray-dried mannitol samples. Drug Dev Ind Pharm. 2009;35(6):712–8. https://doi.org/10.1080/03639040802516491.
El-setouhy DA, El-malak NSA. Formulation of novel tianeptine sodium orodispersible film. AAPS Pharm Sci Tech. 2010;11(3):1018–25. https://doi.org/10.1208/s12249-010-9464-2.
Bruce C, Manning M. Melt extruded thin strips containing coated pharmaceutical. U.S. Patent application 2012030863, December 2012.
Jani R, Patel D. Hot melt extrusion: an industrially feasible approach for casting orodispersible film. Asain J Pharma Sci. 2015;10:292–305.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manda, P., Popescu, C., Juluri, A. et al. Micronized Zaleplon Delivery via Orodispersible Film and Orodispersible Tablets. AAPS PharmSciTech 19, 1358–1366 (2018). https://doi.org/10.1208/s12249-017-0924-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0924-9