Abstract
The current work prepared chitosan/hydroxypropyl methylcellulose (HPMC) blends and studied the possibility of chitosan/HPMC blended patches for Zingiber cassumunar Roxb. The blended patches without/with crude Z. cassumunar oil were prepared by homogeneously mixing the 3.5% w/v of chitosan solution and 20% w/v of HPMC solution, and glycerine was used as plasticizer. Then, they were poured into Petri dish and produced the blended patches in hot air oven at 70 ± 2°C. The blended patches were tested and evaluated by the physicochemical properties: moisture uptake, swelling ratio, erosion, porosity, Fourier transform infrared spectroscopy, differential scanning calorimetry, and X-ray diffraction, and photographed the surface and cross-section morphology under SEM technique. Herbal blended patches were studied by the in vitro release and skin permeation of active compound D. The blended patches could absorb the moisture and became hydrated patches that occurred during the swelling of blended patches. They were eroded and increased by the number of porous channels to pass through out for active compound D. In addition, the blended patches indicated the compatibility of the blended ingredients and homogeneous smooth and compact. The blended patches made from chitosan/HPMC blends provide a controlled release and skin permeation behavior of compound D. Thus, the blended patches could be suitably used for herbal medicine application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Recently, the biodegradable polymers classified into three groups: namely natural, semisynthetic, and synthetic, based on their sources, received highly increasing attention for use in transdermal drug delivery system development in pharmaceutical applications such as pectin and gelatin for testosterone patches (1); deproteinized natural rubber latex, hydroxypropyl methylcellulose (HPMC), sodium carboxymethyl cellulose, methyl cellulose, ethyl cellulose, and polyvinyl alcohol for nicotine patches (2–4) and ketoprofen patches (5,6); sodium alginate and methyl cellulose for nifedipine patches (7); polylactic acid and poly (ε-caprolactone) for repaglinide patches (8); and poly (ε-caprolactone) and poly (DL-lactide-co-glycolide) for paclitaxel patches (9) and implantable biomaterials (10–12). This can be due to degradability in vivo, either enzymatically or nonenzymatically such as (I) abiotic reactions, i.e., oxidation, photodegradation, or hydrolysis, or (II) biotic reactions, i.e., degradations by microorganisms, to produce biocompatibility or nontoxic of by-products (13–15).
Among the suitable degradable polymers is chitosan, which is a potential useful as pharmaceutical material for transdermal patch development owing to its good biocompatibility and low toxicity (16,17). Chitosan is the most abundant basic biopolymer based on a linear amino polysaccharide of d-glucosamine and N-acetyl-d-glucosamine being obtained by the partial deacetylation of natural polymer chitin, the major compound of exoskeletons in crustaceans such as crabs, prawns, lobsters, the cuticles of insects, and the cell walls of fungi (16,18). The degree of deacetylation of chitosan can be determined by the proportion of d-glucosamine and N-acetyl-d-glucosamine. The amount of protonated amino groups in the polymeric chain on the proportion of acetylated and nonacetylated d-glucosamine units can specify the chitosan properties in terms of solubility, biodegradability, reactivity, and adsorption. When the solution is acids having pKa less than 6.2, the amino groups are completely protonated, resulting in positively charged, making chitosan soluble. Thus, the chitosan is soluble after stirring in acids such as acetic, nitric, hydrochloric, perchloric, and phosphoric, but it is insoluble in water, organic solvents, and aqueous bases (19,20). The degradation products of chitosan are nontoxic, nonimmunogenic, and noncarcinogenic making it safe for drug delivery application (17). Recently, chitosan is used to control release and also prepare as hydrogels in transdermal drug delivery systems of many drugs such as repaglinide (8), ciprofloxacin hydrochloride (21), warfarin (22), salicylic acid (23), prednisolone (24), and rhodamine B (25).
Herbal patches, also known as medicated adhesive patches, are designed to control release of active drug at a constant rate over a period of several hours or days after applying to the skin. Herbal patches provide a stronger, youthful, healthy body using a proven formula of natural herbal ingredients. The skin is a special membrane to control the rate at which the drug contained within the patches can (I) into the skin (dermal patches or topical patches) or (II) through the skin and into the bloodstream (transdermal patches) (26–28).
Zingiber cassumunar Roxb., Thai herb, is used for the relief of pain and inflammation in a great number of conditions involving the joints and muscles. It has a wonderful, uplifting peppery green eucalyptus aroma, and is highly regarded for its therapeutic properties in massage. Also, similar to ginger are the anti-inflammatory and analgesic actions, though it has an overall cooling, rather than warming effect. Z. cassumunar may be blended with other essential oils: helichrysum, ginger, marjoram, nutmeg, black pepper, or soothing oils such as lavender and neroli, or bergamot for a synergistic effect. Z. cassumunar essential oil is considered nontoxic, nonsensitizing, and nonirritating (29,30). (E)-4-(3′,4′-dimethoxyphenyl)-but-3-en-1-ol (compound D), the volatile oil, is extracted from the rhizomes of Z. cassumunar that is also reported as analgesic, and antipyretic properties exhibit inhibitory and anti-inflammation activity by using various experimental models of inflammation (31,32). It is also used as topical treatment for sprains, contusions, joint inflammations, muscular pain, abscesses, and similar inflammation-related disorders.
In recent studies, we prepared the herbal blended patches were the crude Z. cassumunar oil incorporating in polymer blends - consisted of chitosan, HPMC and using glycerine as plasticizer. These patches including blank and herbal blended patches were determined, identified, and evaluated the physicochemical properties such as moisture uptake, swelling ratio, erosion, porosity, Fourier transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), X-ray diffraction (XRD), scanning electron microscope (SEM), and in vitro release and skin permeation studies.
MATERIALS AND METHODS
Materials
The Z. cassumunar rhizome powder was provided from Charoensuk Osod, Thailand. Chitosan (degree of deacetylation = 85%) was obtained from Seafresh Industry Public Co., Ltd., Thailand. HPMC was obtained from Onimax, Thailand. Glycerine was obtained from Sigma-Aldrich, USA. All organic solvents were obtained from Merck KGaA, Germany.
Crude Z. cassumunar Oil Preparation and Separation of Compound D from Crude Z. cassumunar Oil
The Z. cassumunar powder was extracted in 95% ethanol, filtered through a 0.45 μm of polyamide membrane, and evaporated to obtain crude Z. cassumunar oil. The crude Z. cassumunar oil was dissolved in ethyl acetate and separated by column chromatography on silica gel technique to collect the compound D.
Herbal Blended Patch Preparation
The chitosan was dissolved in 1% acetic acid in distilled water in concentration of 3.5% w/v. The HPMC was dissolved in distilled water in concentration of 20% w/v. The blank blended patches were prepared by 2 g of 3.5% w/v chitosan that was mixed together with 5 g of 20% w/v of HPMC and homogeneously mixed with 2 g of glycerine as plasticizer. The herbal blended patches were prepared by dissolving 3 g of crude Z. cassumunar oil in absolute ethanol and continuously mixed in polymer blend solution. They were transferred into Petri dish and subsequently dried in hot air oven at 70 ± 2°C for 5 h.
Evaluation of Blank and Herbal Blended Patches
Moisture Uptake, Swelling Ratio, and Erosion Studies
The moisture uptake, swelling ratio, and erosion were cut 1 cm × 1 cm patch specimens. The moisture uptake and the patch specimens were weighed for their initial value (W 0). Then, the patches were moved to stability chamber (model Climate Chamber ICH/ICH L, Memmert GmbH + Co. KG, Germamany) which controlled the temperature at 25 ± 2°C and 75% relative humidity environment. The specimens were removed and weighed until constant (W u). The percentage of moisture uptake was calculated by Eq. (1) (33).
The swelling ratio and erosion study were also determined by drying patch specimens in hot air oven at 60 ± 2°C overnight. Then, the patches were weighed (W 0) and immersed in 5 mL of distilled water and moved to stability chamber (model Climate Chamber ICH/ICH L, Memmert GmbH + Co. KG, Germamany) which controlled the temperature at 25 ± 2°C and 75% relative humidity environment for 48 h. After removal of excess water, the hydrated patches were weighed (W s). They were then dried again at 60 ± 2°C overnight and weighed again (W d). The percentage of swelling ratio and the percentage of erosion were calculated by Eqs. (2) and (3), respectively.
Porosity Determination
After the patch specimens were equilibrated in water, the volume occupied by the water and the volume of the membrane in the wet state were determined. The porosity of patch specimens was obtained by Eq. (4) (34,35).
FTIR Study
The chitosan film, HPMC film, crude Z. cassumunar oil, blank blended patches, and herbal blended patches were scanned at a resolution of 4 cm−1 with 16 scans over a wavenumber region of 400–4,000 cm−1 using the FTIR spectrometer (model Nicolet 6700, DLaTGS detector, Thermo Scientific, USA.). The characteristic peaks of IR transmission spectra were recorded.
DSC Study
A sample was transferred into the DSC pan that was then hermetically sealed and run in the DSC instrument (model DSC7, Perkin Elmer, USA) from 20 to 350°C at the heating rate of 10°C/min under a liquid nitrogen atmosphere. The DSC thermogram was reported, and the endothermic transition was investigated.
XRD Study
The XRD (model X’Pert MPD, PHILIPS, Netherlands) was also employed to study the compatibility of the chitosan, hydroxypropylmethyl cellulose, blank blended patches, and herbal blended patches. The generator operating voltage and current of X-ray source were 40 kV and 45 mA, respectively, with an angular of 5–40° (2θ) and a stepped angle of 0.02° (2θ)/s.
SEM Photography
The surface and cross section of blank blended patches were photographed in the surface of herbal blended patches under SEM instrument (model Quanta 400, FEI, Czech Republic) with high vacuum and high voltage of 20-kV condition and using Everhart-Thornley detector.
In Vitro Release Study and Skin Permeation of Compound D
The in vitro release of compound D from the herbal blended patches was investigated using a modified Franz-type diffusion cell with an effective diffusion area of 1.77 cm2. The receptor medium was 12 mL of isotonic phosphate buffer solution pH 7.4/ethanol = 80:20, thermoregulated with a water jacket at 37 ± 0.5°C and stirred constantly at 600 rpm with a magnetic stirrer. The crude Z. cassumunar oil was applied on the cellulose membrane (MWCO 3,500 Da, CelluSep® T4, Membrane Filtration Product, Inc., USA) as a barrier between the donor compartment and the receptor compartment. The herbal blended patch preparations were cut and directly placed as a membrane between the donor and receptor cells. In the in vitro skin permeation, the newborn pigs of 1.4 to 1.8 kg weight that had died by natural causes shortly after birth were freshly purchased from a local pig farm in Chachoengsao Province, Thailand. They were trimmed with a scalpel and cleaned. The crude Z. cassumunar oil and herbal patch were applied onto the pig skin. The receptor compartment was 12 mL of isotonic phosphate buffer solution pH 7.4/ethanol = 80:20 and stirred constantly at 600 rpm by a magnetic stirrer, at a constant temperature of 37 ± 0.5°C. A 1-mL receptor solution was withdrawn at 0-, 0.5-, 1-, 2-, 3-, 4-, 6-, and 24-h time intervals, and an equal volume of fresh isotonic phosphate buffer solution pH 7.4/ethanol = 80:20 was immediately replaced. The compound D content in these samples was determined by the HPLC method. The experiments for each sample were performed in triplicate.
HPLC Condition
Compound D was analyzed by the RP-HPLC system using an Agilent 1260 Infinity system (Agilent Technologies, USA) with detection at 260 nm. A 4.6-mm × 250-mm diameter, 5-μm particle size C18 column (ACE 5, DV12-7219, USA.), a flow rate of 1 mL/min, and injection volume of 10 μL were used for this experiment. The mobile phase was a gradient elution of 2% acetic acid in ultrapure water (A) and methanol (B) of 60 to 50% of A, 50 to 30% of A, 30 to 20% of A, 20 to 50% of A, 50 to 60% of A, and 60% of A for 0–5, 5–15, 15–25, 25–30, 30–32, and 32–40 min, respectively (36). The compound D content was calculated comparing with the validated calibration curve. The HPLC method provided limit of detection of 0.20 μg/mL, limit of quantification of 0.80 μg/mL, good accuracy (95.38–104.76%), precision (less than 2% CV), and linearity with good correlation coefficient (r 2) >0.9999 in the required concentration range of 2–40 μg/mL of pure compound D. However, the method validation was described in previous publication (36,37).
RESULTS AND DISCUSSION
Evaluation of Blank and Herbal Blended Patches
The appearances of blank blended patches after drying were photographed by digital camera (Fig. 1). The blank blended patches were yellowish transparent membrane (Fig. 1a). When crude Z. cassumunar oil was mixed in blank blended patches, called herbal blended patches, it expressed the dark yellow patches due to the individual appearance of crude Z. cassumunar oil (Fig. 1b).
Moisture Uptake, Swelling Ratio, Erosion, and Porosity Studies
The moisture uptake, swelling ratio, erosion, and porosity of blank blended patches are shown in Table I. The chitosan and HPMC were swelling and hydrophilic polymers that were reported in previous work and increasingly used of these polymer in pharmaceutical industry for production of controlled drug delivery systems (2,16). Swelling in polymer compacted of chitosan and HPMC occurs upon immediate hydration of the polymer. As the dry patches became hydrated patches and adsorbed the moisture from environmental during studied experimentation, the mobility of the polymer chains of chitosan and HPMC increased, therefore increasing the hydrodynamic volume of the polymer compact that allowed swelling of these patches. And then, some polymer or ingredients were dissolved and eroded from their patches which presented the pores in these patches.
When crude Z. cassumunar oil was added in blank blended patches, the moisture uptake, swelling ratio, erosion, and porosity were found not significantly changed. Furthermore, the moisture uptake and swelling ratio values were observed, and the erosion and porosity values of the blended patches might significantly effect on release behavior of active compound D. These indicated that some hydrophilic parts of polymers or ingredients could be dissolved and eroded from the patches, to increase the number of porous channels to pass through out for active compound D. Thus, the moisture uptake, swelling, and erosion behaviors of the polymeric patches play important roles during the early stages of patch degradation (38).
FTIR Study
Figure 2a shows the FTIR spectra of crude Z. cassumunar oil and compound D. The crude Z. cassumunar oil is composed of several essential oils (39–41); thus, we cannot identify the functional groups for their compound. However, the compound D exhibited a topical anti-inflammatory effect that inhibited edema formation when tested using various experimental models (32,42–44). Therefore, we separated the compound D from crude Z. cassumunar oil by column chromatography on silica gel technique and then identified their structure with FTIR, 1H-NMR, 12C-NMR, and mass spectrometry. The identification of compound D was IR νmax 3,478–3,417 (broad, OH), 1,514 (aromatic), 2,931 and 2,361 cm−1 (weak, short chain aliphatic); 1H-NMR δ 2.45 (q, 2H), 3.73 (t, 2H), 3.86 (s, 3H), 3.88 (s, 3H), 6.05 (m, 1H), 6.42 (d, 1H), 6.79 (d, 1H), 6.87 (dd, 1H), and 6.91 (d, 1H); 13C-NMR δ 36.48 (C-2), 55.79 (OMe), 55.90 (OMe), 62.07(C-1), 108.44 (C-2′), 111.04 (C-5′), 119.12 (C-6′), 124.27 (C-3), 130.32 (C-1′), 132.53 (C-4), 148.53 (C-4′), and 148.96 (C-3′); and mass spectroscopy m/z 208 [M]+. These separation method and results were reported in our previous publication (36). Thus, we successfully separated the main active compound D from crude Z. cassumunar oil which could be used as standard compound D for this research.
In Fig. 2b, the principle FTIR absorption peaks of the pure chitosan were observed and corresponded to the stretching vibrations of –OH groups which overlapped to the stretching vibration of N–H in the range from 3,750 to 3,000 cm−1. The bending vibrations of methylene and methyl groups were also visible at 1,375 and 1,426 cm−1, respectively. The spectrum bands in the range of 1,680–1,480 cm−1 were identified to the vibrations of carbonyl bonds of the amide group and to the vibrations of protonated amine group. The vibrations of CO group were found in the range from 1,160 to 1,000 cm−1. In addition, the spectrum band located at around 1,150 cm−1 related to asymmetric vibrations of CO in the oxygen bridge resulting from deacetylation of chitosan. Finally, the small spectrum peak at ∼890 cm−1 corresponded to wagging of the saccharide structure of chitosan (45). The HPMC showed the broad spectra of the stretching vibration of O–H at 3,446 cm−1 and the stretching of the C–O–C anhydroglucose ring at 1,054 cm−1. The acquired spectra were similar to those reported for HPMC (46,47). Blank blended patches and herbal blended patches had not found new absorption band; thus, they had found all ingredients and compound D in their patches.
DSC Study
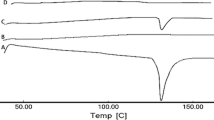
DSC technique was used as tool for monitoring the effects of additives on the thermal behavior of composition in blank blended patches, and herbal blended patches; this technique was used to deliver qualitative information about the physicochemical status of ingredients in the preparations (48). DSC thermograms of chitosan, blank blended patches, and herbal blended patches are shown in Fig. 3. Thermogram of chitosan showed an initial broad peak at 76°C, and enthalpy of peak (∆H) was 242.49 J/g over a large temperature range that was attributed to water loss due to evaporation of absorbed water, and this represents the energy required to vaporize water present in the samples. Under the experimental conditions, the degradation DSC peak was observed for chitosan polymer that occurs at 326.67°C, and ∆H was 55.60 J/g (Fig. 3a). The transition of both blank blended patches and herbal blended patches occurred at an initial very broad peak at 97.83°C and ∆H was 188.31 J/g, and 121.17°C and ∆H was 138.88 J/g, respectively, which had resulted from loss of moisture on heating. These events were followed by a broad endothermic peak at 200 to 292°C in both blank blended patches and herbal blended patches, which might be attributed to glycerine component. Further, decomposition of both blank blended patches and herbal blended patches was observed as a broad endotherm at 263.84°C and ∆H was 402.058 J/g, and 271.83°C and ∆H were 514.559 J/g, respectively, under identical experimental DSC conditions (Fig. 3b, c). However, blank blended patches and herbal blended patches were not seen as an asymmetric peak representing thermal event of a new form.
XRD Study
The XRD technique was based on the elastic scattering of X-rays from structures which had long-range order. It was an efficient analytical technique to identify and characterize crystalline and amorphous form of sample. Thus, this work used the XRD technique to identify and characterize crystalline and amorphous form of chitosan, HPMC, blank blended patches, and herbal blended patches that had been studied in range of 5–40° (2θ) shown in Fig. 4. The XRD profile of chitosan showed peaks at ∼10° and ∼23° (2θ) (49). The intensity results of pure HPMC at 7.95° and 20.13° (2θ) represented their semicrystalline characters because of the strong intermolecular interaction between HPMC chains through intermolecular hydrogen bonding (50). In addition, these peaks were not found in the blank blended patches and herbal blended patches which exhibited an amorphous phase, due to the broad diffraction halo, so that the crystalline peaks of chitosan and HPMC disappeared.
SEM Photography
The SEM technique was used to photograph the high-resolution morphology in surface and cross section (Fig. 5) of blank blended patches. The surface of blank blended patches was homogeneously smooth and dense with no visual pores (Fig. 5a). Although the cross section of blank blended patches was dense and smooth morphology without poring and cavities, it was seen minimal cracking due to the water being lost rapidly through surface evaporation in blank blended patch preparation (Fig. 5c) (51). When crude Z. cassumunar oil was added in blank blended patches, the herbal blended patches became rough and uneven surface patches (Fig. 5b). This was due to the crude Z. cassumunar oil leading to conglomeration and aggregation in the matrix of herbal blended patches. This conglomeration and aggregation might result in high dense, condense, and compact patches without poring, cracking, or cavities in the cross-section morphology of herbal blended patches more that had the great diffusion of the crude Z. cassumunar oil in the patches (Fig. 5d).
In Vitro Release and Skin Permeation Study of Compound D
The determination of compound D content in herbal blended patches was extracted by sonication method in absolute ethanol. The different five sites of the herbal blended patches were cut into 1 cm × 1 cm. They were soaked with 10 mL ethanol in volumetric flask and sonicated at 25°C for 30 min. Then, they were diluted with ethanol in appropiate concentration and filtered through a 0.45 μm. The content of compound D was analyzed with HPLC method. The content of compound D in herbal blended patches was found 1.34 ± 0.26 mg/cm2.
In in vitro experimental, the compound D in crude Z. cassumunar oil was added in concentration of 0.58 mg/cm2 to study the in vitro release and skin permation behavior. The release behavior of compound D in crude Z. cassumunar oil across cellulose dialysis membrane is shown in Fig. 6a. It could be seen that all compound D release profiles presented a fast initial burst release during the first 6 h, which might be due to rapid diffusion of compound D in the receptor medium. The final concentration of compound D release from crude Z. cassumunar oil after 24 h was 0.53 ± 0.11 mg/cm2, and then, it was converted to percentage cumulative release of 90.43 ± 19.28% when compared to initial concentration (Fig. 6c). As well as, the herbal blended patches could release the compound D in final concentration of 1.14 ± 0.20 mg/cm2 (Fig. 6b) or in the percentage cumulative release of 85.62 ± 14.68% (Fig. 6c). It presented a fast initial burst release during the first 6 h, exhibited a similar effect on the compound D release behavior from crude Z. cassumunar oil, which might due to rapid diffusion of compound D on the surface of patches. In addition, from the results of moisture uptake, swelling ratio, erosion, and porosity studies might create a space and a large free volume within the blended patches, leading to increase molecular mobility and segmental relaxation, and therefore enhancing compound D diffusion (38). The previous research suggested that the amorphous region of the matrix patches could accordingly enhance drug diffusion (2,3,52). However, the percentage cumulative release of compound D from herbal blended patches was found to be significantly lower than that from crude Z. cassumunar oil. Thus, the formulation of blended patches was observed to had a significant impact on compound D release. The herbal blended patches could highly entrap compound D in their formation and slightly released the compound D.
The in vitro skin permeation study was carried out in a modified Franz diffusion cell using newborn pig skin as partition membrane. The mean cumulative amount of compound D permeated from crude Z. cassumunar oil and herbal blended patches after 24 h was 0.23 ± 0.11 (Fig. 7a) and 0.49 ± 0.22 mg/cm2 (Fig. 7b), respectively. Then, they were calculated as percentage cumulative permeation of 38.55 ± 18.48 and 36.55 ± 16.73%, respectively (Fig. 7c). From the skin permeation results, it was found that the compound D slightly detected in receptor medium because the compound D might be accumulated in the stratum corneum layer of newborn pig skin. Thus, the newborn pig skin was removed from modified Franz diffusion cell apparatus and extracted in absolute ethanol, and then analyzed the remaining compound D content by HPLC method. It found 60.54 ± 39.55 and 49.53 ± 20.56%, respectively. Even though another publication reported the glycerine that could enhance drug permeability by acts as penetration enhancer (2,3), the herbal blended patches comprised the glycerine as plasticizer; it not affected on this study due to a little amount in herbal blended patches. In addition, structure of compound D was reported in previous publication (32,41,44) act as hydrophilicity less than hydrophobicity. Thus, it might highly accumulate in stratum corneum layer of newborn pig skin and could slightly across this layer into receptor medium. However, this mechanism effect will be further studied in our future work.
CONCLUSIONS
Our study demonstrated that the formulation, physicochemical characterization, and in vitro study of herbal blended patches prepared from the blending of crude Z. cassumunar oil, chitosan, and HPMC with glycerine as plasticizer. The moisture uptake, swelling ratio, erosion, and porosity confirmed the hydrophilic patches that could be used for herbal blended patches. They suggested the controlled release of active compound D from their patches. FTIR, DSC, and XRD studies showed compatible blended patches that were homogeneous blended patches with amorphous region. In addition, the SEM morphology confirmed the homogeneous smooth and compact in both surface and cross-section preparation. The in vitro release presented the suitable blended patches which could entrap the compound D in their blended patch formation and control the compound D release into receptor medium. Moreover, in vitro skin permeation of compound D slightly found in receptor medium, but it was accumulated in newborn pig skin. Thus, we successfully prepared the new blended patches made from chitosan and HPMC for herbal medicine application that might be developed for industrials in the future.
REFERENCES
Mazzitelli S, Pagano C, Giusepponi D, Nastruzzi C, Perioli L. Hydrogel blends with adjustable properties as patches for transdermal delivery. Int J Pharm. 2013;454(1):47–57. doi:10.1016/j.ijpharm.2013.06.081.
Pichayakorn W, Suksaeree J, Boonme P, Amnuaikit T, Taweepreda W, Ritthidej GC. Deproteinized natural rubber latex/hydroxypropylmethyl cellulose blending polymers for nicotine matrix films. Ind Eng Chem Res. 2012;51(25):8442–52. doi:10.1021/ie300608j.
Pichayakorn W, Suksaeree J, Boonme P, Amnuaikit T, Taweepreda W, Ritthidej GC. Nicotine transdermal patches using polymeric natural rubber as the matrix controlling system: effect of polymer and plasticizer blends. J Membr Sci. 2012;411–412:81–90. doi:10.1016/j.memsci.2012.04.017.
Pichayakorn W, Suksaeree J, Boonme P, Amnuaikit T, Taweepreda W, Ritthidej GC. Deproteinized natural rubber as membrane controlling layer in reservoir type nicotine transdermal patches. Chem Eng Res Des. 2012;91(3):520–9. doi:10.1016/j.cherd.2012.09.011.
Suksaeree J, Charoenchai L, Monton C, Chusut T, Sakunpak A, Pichayakorn W, et al. Preparation of a pseudolatex-membrane for ketoprofen transdermal drug delivery systems. Ind Eng Chem Res. 2013;52(45):15847–54. doi:10.1021/ie402345a.
Suksaeree J, Monton C, Sakunpak A, Charoonratana T. Formulation and in vitro study of ketoprofen pseudolatex gel for transdermal drug delivery systems. Int J Pharm Pharm Sci. 2014;6(2):248–53.
Babu VR, Sairam M, Hosamani KM, Aminabhavi TM. Preparation of sodium alginate-methylcellulose blend microspheres for controlled release of nifedipine. Carbohydr Polym. 2007;69(2):241–50. doi:10.1016/j.carbpol.2006.09.027.
Vijayan V, Reddy KR, Sakthivel S, Swetha C. Optimization and charaterization of repaglinide biodegradable polymeric nanoparticle loaded transdermal patchs: in vitro and in vivo studies. Colloids Surf B: Biointerfaces. 2013;111:150–5. doi:10.1016/j.colsurfb.2013.05.020.
Lao LL, Venkatraman SS, Peppas NA. Modeling of drug release from biodegradable polymer blends. Eur J Pharm Biopharm. 2008;70(3):796–803. doi:10.1016/j.ejpb.2008.05.024.
Abedalwafa M, Wang F, Wang L, Li C. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: a review. Rev Adv Mater Sci. 2013;34(2):123–40.
Llorens E, Armelin E, del Mar Pérez-Madrigal M, del Valle LJ, Alemán C, Puiggalí J. Nanomembranes and nanofibers from biodegradable conducting polymers. Polymers. 2013;5(3):1115–57. doi:10.3390/polym5031115.
Roether JA, Rai R, Wolf R, Tallawi M, Boccaccini AR. Biodegradable poly(glycerol sebacate)/poly(3-hydroxybutyrate)-TiO2 nanocomposites: fabrication and characterisation. Mater Sci Technol. 2014;30(5):574–81. doi:10.1179/1743284713Y.0000000499.
Engineer C, Parikh J, Raval A. Review on hydrolytic degradation behavior of biodegradable polymers from controlled drug delivery dystem. Trends Biomater Artif Organs. 2011;25(2):79–85.
Leja K, Lewandowicz G. Polymer biodegradation and biodegradable polymers—a review. Pol J Environ Stud. 2010;19(2):255–66.
Mohanty AK, Misra M, Hinrichsen G. Biofibres, biodegradable polymers and biocomposites: an overview. Macromol Mater Eng. 2000;276–277(1):1–24. doi:10.1002/(sici)1439-2054(20000301)276:1<1::aid-mame1>3.0.co;2-w.
Agnihotri SA, Aminabhavi TM. Controlled release of clozapine through chitosan microparticles prepared by a novel method. J Control Release. 2004;96(2):245–59. doi:10.1016/j.jconrel.2004.01.025.
Ge Y-b, Chen D-w, Xie L-p, Zhang R-q. Optimized preparation of daidzein-loaded chitosan microspheres and in vivo evaluation after intramuscular injection in rats. Int J Pharm. 2007;338(1–2):142–51. doi:10.1016/j.ijpharm.2007.01.046.
Papadimitriou S, Bikiaris D, Avgoustakis K, Karavas E, Georgarakis M. Chitosan nanoparticles loaded with dorzolamide and pramipexole. Carbohydr Polym. 2008;73(1):44–54. doi:10.1016/j.carbpol.2007.11.007.
Aranaz I, Mengibar M, Harris R, Panos I, Miralles B, Acosta N, et al. Functional characterization of chitin and chitosan. Curr Chem Biol. 2009;3(2):203–30. doi:10.2174/187231309788166415.
Santiago de Alvarenga E. Characterization and properties of chitosan. In: Elnashar M, editor. Biotechnology of biopolymers. Croatia: InTech; 2011. p. 91–108.
Chen Y, Zhang Y, Feng X. An improved approach for determining permeability and diffusivity relevant to controlled release. Chem Eng Sci. 2010;65(22):5921–8. doi:10.1016/j.ces.2010.08.028.
Khalil SKH, El-Feky GS, El-Banna ST, Khalil WA. Preparation and evaluation of warfarin-β-cyclodextrin loaded chitosan nanoparticles for transdermal delivery. Carbohydr Polym. 2012;90(3):1244–53. doi:10.1016/j.carbpol.2012.06.056.
Michalak I, Mucha M. The release of active substances from selected carbohydrate biopolymer membranes. Carbohydr Polym. 2012;87(4):2432–8. doi:10.1016/j.carbpol.2011.11.013.
Kofuji K, Ito T, Murata Y, Kawashima S. The controlled release of a drug from biodegradable chitosan gel beads. Chem Pharm Bull (Tokyo). 2000;48(4):579–81.
Hye Kim J, Il Kim S, Kwon I-B, Hyun Kim M, Ik LJ. Simple fabrication of silver hybridized porous chitosan-based patch for transdermal drug-delivery system. Mater Lett. 2013;95:48–51. doi:10.1016/j.matlet.2012.12.078.
Rathva SR, Patel NN, Shah V, Upadhyay UM. Herbal transdermal patches: a review. Int J Drug Dis Herb Res. 2012;2(2):397–402.
Tangyuenyongwatana P, Kowapradit J, Opanasopit P, Gritsanapan W. Cellular transport of anti-inflammatory pro-drugs originated from a herbal formulation of Zingiber cassumunar and Nigella sativa. Chin Med. 2009;4(1):19.
Walters KA. Topical and transdermal therapeutic systems. In: Walters KA, editor. Dermatological and transdermal formulations (drugs and the pharmaceutical sciences). New York: Informa Healthcare; 2002. p. 1–112.
Pongprayoon U, Soontornsaratune P, Jarikasem S, Sematong T, Wasuwat S, Claeson P. Topical antiinflammatory activity of the major lipophilic constituents of the rhizome of Zingiber cassumunar. Part I: the essential oil. Phytomedicine. 1997;3(4):319–22. doi:10.1016/S0944-7113(97)80003-7.
Pongprayoon U, Tuchinda P, Claeson P, Sematong T, Reutrakul V, Soontornsaratune P. Topical antiinflammatory activity of the major lipophilic constituents of the rhizome of Zingiber cassumunar. Part II: hexane extractives. Phytomedicine. 1997;3(4):323–6. doi:10.1016/S0944-7113(97)80004-9.
Kanjanapothi D, Soparat P, Panthong A, Tuntiwachwuttikul P, Reutrakul V. A uterine relaxant compound from Zingiber cassumunar. Planta Med. 1987;53:329–32.
Panthong A, Kanjanapothi D, Niwatananant W, Tuntiwachwuttikul P, Reutrakul V. Anti-inflammatory activity of compound D {(E)-4-(3′,4′-dimethoxyphenyl)but-3-en-2-ol} isolated from Zingiber cassumunar Roxb. Phytomedicine. 1997;4(3):207–12. doi:10.1016/S0944-7113(97)80069-4.
Rajesh N, Siddaramaiah H, Gowda DV, Somashekar CN. Formulation and evaluation of biopolymer based transdermal drug delivery. Int J Pharm Pharm Sci. 2010;2 Suppl 2:142–7.
Chen Z, Deng M, Chen Y, He G, Wu M, Wang J. Preparation and performance of cellulose acetate/polyethyleneimine blend microfiltration membranes and their applications. J Membr Sci. 2004;235(1–2):73–86. doi:10.1016/j.memsci.2004.01.024.
Suksaeree J, Boonme P, Taweepreda W, Ritthidej GC, Pichayakorn W. Relationships between hydraulic permeability and porosity of natural rubber blended films. Isan J Pharm Sci. 2012;8(1):89–95.
Suksaeree J, Madaka F, Monton C, Sakunpak A, Chusut T, Charoonratana T. Method validation of (E)-4-(3′,4′-dimethoxyphenyl)-but-3-en-1-ol in Zingiber cassumunar Roxb. with different extraction techniques. Int J Pharm Pharm Sci. 2014;6(3):295–8.
Suksaeree J, Charoenchai L, Pichayakorn W, Boonme P. HPLC method development and validation of (E)-4-(3,4-dimethoxyphenyl)-but-3-en-1-ol in Zingiber cassumunar Roxb. from Thai Herbal Compress ball. Int J Pharm Pharm Sci Res. 2013;3(3):115–7.
Limpongsa E, Umprayn K. Preparation and evaluation of diltiazem hydrochloride diffusion-controlled transdermal delivery system. AAPS PharmSciTech. 2008;9(2):464–70. doi:10.1208/s12249-008-9062-8.
Han A-R, Kim M-S, Jeong YH, Lee SK, Seo E-K. Cyclooxygenase-2 inhibitory phenylbutenoids from the rhizomes of Zingiber cassumunar. Chem Pharm Bull. 2005;53(11):1466–8.
Kaewchoothong A, Tewtrakul S, Panichayupakaranant P. Inhibitory effect of phenylbutanoid-richZingiber cassumunar extracts on nitric oxide production by murine macrophage-like RAW264.7 cells. Phytother Res. 2012;26(12):1789–92.
Masuda T, Jitoe A. Phenylbutenoid monomers from the rhizomes of Zingiber cassumunar. Phytochemistry. 1995;39(2):459–61. doi:10.1016/0031-9422(94)00883-U.
Jeenapongsa R, Yoovathaworn K, Sriwatanakul KM, Pongprayoon U, Sriwatanakul K. Anti-inflammatory activity of (E)-1-(3,4-dimethoxyphenyl) butadiene from Zingiber cassumunar Roxb. J Ethnopharmacol. 2003;87(2–3):143–8. doi:10.1016/S0378-8741(03)00098-9.
Ozaki Y, Kawahara N, Harada M. Anti-inflammatory effect of Zingiber cassumunar Roxb. and its active principles. Chem. Pharm Bull (Tokyo). 1991;39(9):2353–9.
Panthong A, Kanjanapothi D, Niwatananun V, Tuntiwachwuttikul P, Reutrakul V. Anti-inflammatory activity of compounds isolated from Zingiber cassumunar. Planta Med. 1990;56(6):655.
Silva SMLB, Carla RC, Fook MVL, Raposo CMO, Carvalho LH, Canedo EL. Application of infrared spectroscopy to analysis of chitosan/clay nanocomposites. In: Theophanides T, editor. Infrared spectroscopy - materials science, engineering and technology. Croatia: InTech; 2012. p. 43–62.
Anuar NK, Wui WT, Ghodgaonkar DK, Taib MN. Characterization of hydroxypropylmethylcellulose films using microwave non-destructive testing technique. J Pharm Biomed Anal. 2007;43(2):549–57. doi:10.1016/j.jpba.2006.08.014.
Larsson M, Viridén A, Stading M, Larsson A. The influence of HPMC substitution pattern on solid-state properties. Carbohydr Polym. 2010;82(4):1074–81. doi:10.1016/j.carbpol.2010.06.030.
Dhanikula A, Panchagnula R. Development and characterization of biodegradable chitosan films for local delivery of paclitaxel. AAPS J. 2004;6(3):88–99. doi:10.1208/aapsj060327.
Clark GL, Smith AF. X-ray diffraction studies of chitin, chitosan, and derivatives. J Phys Chem. 1935;40(7):863–79. doi:10.1021/j150376a001.
Abdelaziz M, Ghannam MM. Influence of titanium chloride addition on the optical and dielectric properties of PVA films. Phys B. 2010;405(3):958–64. doi:10.1016/j.physb.2009.10.030.
Moon TY, Cooper RH. Method of preventing surface cracking of portland cement mortar and concrete containing a film forming polymer modifier. US. 1979.
Guo R, Du X, Zhang R, Deng L, Dong A, Zhang J. Bioadhesive film formed from a novel organic–inorganic hybrid gel for transdermal drug delivery system. Eur J Pharm Biopharm. 2011;79(3):574–83. doi:10.1016/j.ejpb.2011.06.006.
Acknowledgments
The authors would like to acknowledge the Faculty of Pharmacy and the Research Institute of Rangsit University for financial supports (Grant No. 74/2555).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suksaeree, J., Monton, C., Madaka, F. et al. Formulation, Physicochemical Characterization, and In Vitro Study of Chitosan/HPMC Blends-Based Herbal Blended Patches. AAPS PharmSciTech 16, 171–181 (2015). https://doi.org/10.1208/s12249-014-0216-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0216-6