Abstract
Metered dose inhalers (MDIs) are complex drug-device combination products widely used to treat pulmonary disorders. The efficacy, driven by aerosol performance of the products, depends on a multitude of factors including, but not limited to, the physicochemical properties of drug and nature and amount of excipient(s). Under the quality by design (QbD) paradigm, systematic investigations are necessary to understand how changes in critical quality attributes (CQAs) of formulation, device, and manufacturing process influence key product performance parameters, such as delivered dose (DD) and fine particle dose (FPD). The purpose of this work is to provide a better understanding of the effects of different levels of excipients and drug particle size distribution on the aerosol performance of MDI products, while using two fundamentally different MDI products as relevant model systems, Proventil® HFA (albuterol sulfate suspension) and Qvar® (beclomethasone dipropionate solution). These MDI products, as model systems, provided mid-points around which a design of experiments (DOE), consisting of 22 suspension and 9 solution MDI formulations, were defined and manufactured. The DOE included formulations factors with varying ethanol (2 to 20% w/w and 7 to 9% w/w for the suspension and solution, respectively) and oleic acid concentrations (0.005 to 0.25% w/w and 0 to 2% w/w for the suspension and solution, respectively) and drug volumetric median particle size distribution (PSD D50, 1.4 to 2.5 μm for the suspension). The MDI formulations were analyzed using compendial methods to elucidate the effect of these formulation variables (ethanol, oleic acid, and PSD D50) on DD and FPD. The outcomes of this study allowed defining design spaces for the formulation factors, such that DD and FPD would remain within specific pre-defined requirements. The systematic approach utilized in this work can contribute as a QbD tool to evaluate the extent to which the formulation factors govern the aerosol performance of MDI drug products, helping to design MDI formulations with desired product performance parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Metered dose inhalers (MDIs) have been employed to deliver drugs for the treatment of asthma for over 60 years. In that period, the requirement to transition from chlorofluorocarbon (CFC) propellants, implicated in atmospheric ozone depletion, to hydrofluorocarbon (HFA) propellants resulted in a significant increase in research and development activity to reformulate previously marketed drugs and prepare new products. This significant effort has occurred in parallel with regulatory emphasis on quality by design (QbD) as a means of ensuring the quality and performance of the drug product through improved understanding in how to control material properties and manufacturing processes. Few studies have been published where these properties and processes have been systematically varied to evaluate their influence on product quality and performance (1).
Especially in the last decade, increasing emphasis has been placed on control of product quality and performance through well-understood manufacturing processes. This approach is intended to eventually supersede traditional product quality control by iterative methods based on batch testing and disposal of product that falls outside of specifications. The critical quality attributes (CQAs) or associated interactions between CQAs of MDI product formulation and manufacture that may impact performance include (i) particle size and distribution of micronized particles to be used in suspension formulations, (ii) composition of the formulation with respect to propellant, cosolvent, and surfactant selection and concentrations, and (iii) device (i.e., canister, valve, and actuator). These factors often have significant impact on quality and performance variables, such as delivered dose uniformity (DDU), aerodynamic particle size distribution (APSD), plume geometry, spray pattern, and dissolution, specifically for poorly soluble or slowly dissolving drugs (1–3).
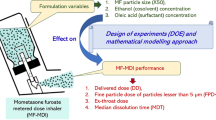
The intent by regulatory agencies when requiring developers to build quality into products based on sound scientific and engineering principles requires that the manufacturing variables and the testing protocols and procedures are suitable to achieve the desired monitoring and control of the product. The present study provides such data for two MDI systems: a suspension formulation modeled based on commercially available albuterol sulfate MDI (i.e., Proventil® HFA) and a solution formulation modeled based on commercially available beclomethasone dipropionate MDI (i.e., Qvar®). This study utilizes the approach detailed in Fig. 1 as a means of systematically identifying the impact of ethanol and oleic acid concentration on product performance of suspension and solution MDI formulations and micronized drug volumetric median particle size distribution (PSD D50) on product performance of suspension MDI formulations, which use drugs with different physicochemical properties. DDU and fine particle dose less than 5 μm (FPD) with the United States Pharmacopeia (USP) induction port and a more anatomically realistic mouth/throat model, the Alberta Idealized Throat (AIT), were selected as primary product performance endpoints for this analysis due to the relevance of these in vitro properties to pharmacokinetic outcomes (4). The data generated from the experiments described below were subsequently used to develop mathematical models relating formulation factors to the primary endpoints. These models were used to examine the design spaces for these formulations, providing an indication of the magnitude of impact each CQA has on the selected product performance measures. The systematic approach utilized in this work has the potential to contribute as a QbD tool to evaluate the extent to which the formulation factors govern the aerosol performance of MDI products, helping to design MDI formulations with desired product performance.
MATERIALS AND METHODS
Materials
Proventil® HFA Inhalation Aerosol (3M Drug Delivery Systems, Northridge, NJ, USA, batch no. 130150, no. 130369, no. 130374, and no. 130256, expiry May 2015, October 2015, October 2015, and July 2015, respectively) and Qvar® 40 (Teva Respiratory LLC, Horsham, PA, USA, batch no. 130101, no. 130102, and no. 130442, expiry February 2015, March 2015, and December 2015, respectively) were the suspension and solution commercial MDI batches used for the study, respectively. These commercial MDI batches were purchased from Community Care Pharmacy (Congers, NY, USA) and were within the expiry at the time of testing. Unmicronized albuterol sulfate was purchased from two different vendors, which included batch 2CB0171 (Spectrum Chemical Manufacturing Corp., New Brunswick, NJ, USA; retest date May 31, 2018) and batch 03561300413 (Teva Pharmaceutical Fine Chemicals S.R.L., Milano, Italy; retest date February 28, 2018) with volume median particle size distribution (PSD D50) ranging from 11 to 17 μm. Micronized beclomethasone dipropionate (BDP) was purchased from Teva API, Inc. (Woodcliff Lake, NJ, USA) with PSD D50 of 1.3 μm. Ethanol (200 proof, ≥99.5% dehydrated alcohol) and oleic acid (NF grade) were purchased from Fisher Scientific (Hampton, NH, USA). 1,1,1,2-tetrafluoroethane (HFA-134a, pharmaceutical grade) was purchased from Mexichem UK Limited (Cheshire, UK). Canisters (17 mL uncoated aluminum) were purchased from Presspart (Lancashire, UK) and metering valves (EPDM gasket, 28 and 50 μL) were purchased from Aptar Pharma (Congers, NY, USA). Plastic actuators supplied with the commercially available Proventil® HFA and Qvar® were used in the study. The actuators were washed with water and methanol at the frequency noted in the package insert of the commercial MDIs and only used for the number of actuations noted on the label of the commercial MDIs. All solvents were of high-performance liquid chromatography (HPLC) grade (Fisher Scientific, Hampton, NH, USA and Sigma-Aldrich, St. Louis, MO, USA). Ultrapure water was produced by MilliQ A-10 Water Purification System (Millipore, Billerica, MA, USA).
Analytical Methods
Drug Assay
HPLC mass spectrometry detection (HPLC-MSD) was used to assay albuterol base and BDP (see Supplementary Material, Table S1 for more details). For the albuterol assay, the deuterated internal standard (d3-albuterol) was purchased from CDN Isotopes Inc. (Pointe-Claire, Quebec, Canada), and the method had a linear range from 20 to 2700 ng/mL, 100 ± 2% accuracy, and 1% precision. For the BDP assay, the deuterated internal standard (d5-BDP) was purchased from BDG Synthesis (Wellington, New Zealand), and the method had a linear range from 20 to 1000 ng/mL, 100 ± 2% accuracy, and 1% precision.
Total Content Per Canister
The total albuterol sulfate and BDP content per canister samples were prepared by chilling the canister, then cutting it open to collect all drug in the canister to sample the entire content. The prepared samples were then analyzed using the HPLC-MSD methods described above. A similar approach was used to determine the oleic acid content per canister. In contrast, the ethanol content per canister was determined by sampling up to 15 actuations from the beginning of canister life to prepare each sample. The total ethanol and oleic acid content per canister was determined using gas chromatography with flame ionization detector (GC-FID)–the method for ethanol content had a linear range from 160 to 270 μg/mL, 100 ± 2% accuracy, and 0.4% precision; the method for oleic acid content had a linear range from 30 to 120 μg/mL, 100 ± 2% accuracy, and 0.7% precision.
Drug Particle Size Distribution
The PSD D50 of micronized albuterol sulfate was determined using static laser-light diffraction (Sympatec HELOS)–a dry dispersion method was used to analyze unmicronized and micronized bulk albuterol sulfate, and a wet dispersion method was used to analyze albuterol sulfate aerosolized from the sprays (the MDIs were actuated into a hexane/sorbitane trioleate (Span® 85) mixture of 97.5:2.5 v/v). The PSD D50 of micronized BDP was 1.3 μm, as per the certificate of analysis provided by the vendor, and the PSD D50 of BDP from the sprays was not determined because Qvar® is a solution formulation.
Delivered Dose Uniformity
The DDU through life stages–at beginning (B), middle (M), and end (E)–was conducted according to USP <601> (5). B, M, and E life stages are defined elsewhere (6). The sampling apparatus consisted of a 500 mL glass separatory funnel with cotton plug. The airflow rate through the experimental setup was 28.3 L/min (± 5%) and the volume of air sampled was 2 L. The MDIs were prepared (shaking, priming, and firing) and tested as per labeled instructions for use of the corresponding commercial products. The target ex-actuator delivered dose was the one specified by the label claim; this equates to 90 μg albuterol base and 40 μg BDP per actuation. The drug content was quantified using the HPLC-MSD drug assay methods described above, and the ex-actuator delivered dose for the B, M, and E life stages was determined, in this work, using two actuations in order to improve analytical sensitivity.
Aerodynamic Particle Size Distribution
The APSD was characterized at B and E life stages using a Next Generation Impactor (NGI) connected to a vacuum pump (0.25 hp, Series 0523, Gast Manufacturing Inc., Benton Harbor, MI, USA). The NGI method used Apparatus 6 of USP <601> (5) with uncoated cups and a terminal glass fiber filter. The NGI tests were conducted using the industry standard sampling induction port for cascade impactor testing, the USP induction port, as well as an anatomically more relevant mouth/throat model (Alberta Idealized Throat (AIT) from Copley Scientific Limited, Nottingham, UK). The USP induction port was uncoated, whereas the AIT was coated (7) with a thin layer of glycerol. The MDIs were prepared (shaking, priming, and firing) and tested as per labeled instructions for use of the corresponding commercial product. For each NGI test with the suspension formulations, two actuations per each B and E life stages were discharged into the NGI using an airflow rate of 30 L/min for the duration of time corresponding to 4 L inhaled volume. For each NGI test with the solution formulations, APSD was evaluated only at B life stage based on the experience obtained from evaluation of the suspension formulations. Following dose actuation, the NGI was dismantled, and each part was washed down using known volumes of mobile phase. The mass of drug deposited on each NGI stage (including the filter), valve stem, actuator, and induction port was quantified using the HPLC-MSD drug assay methods described above. Fine particle fraction less than 5 μm (FPF), fine particle dose less than 5 μm (FPD), mass median aerodynamic diameter (MMAD), and geometric standard deviation (GSD) were determined from the obtained NGI data. The FPF was calculated by linear interpolation between the ex-device cumulative percent mass on stage 4 to filter (≤3.99 μm) and the ex-device cumulative percent mass on stage 3 to filter (≤6.4 μm). The FPD was calculated by multiplication of the FPF and the total ex-device mass collected from the induction port to the filter (e.g., delivered dose as determined by NGI). MMAD and GSD were calculated based upon the inverse normal of the cumulative percent distribution of the aerodynamic diameter versus the log of the effective cut-off diameter (8). Linear regression of the two data points closest to 50% of the cumulative particle mass was used to compute MMAD and GSD (8). The cut-off diameters were those specified in the USP <601> for Apparatus 6 at 30 L/min (5). In all NGI tests, the mass balance (i.e., total recovery in percent of ex-valve label claim, as determined by NGI) was within 100 ± 15% of the ex-valve label claim of the commercial MDI products (i.e., 100 μg albuterol base or 50 μg BDP).
Establishment of Model System MDIs
Reverse Engineering of Commercial MDIs
The commercial MDIs were characterized according to drug and excipients content per canister, and DDU and APSD through life stages, using the methods described above and standard methodologies outlined in the FDA Draft Guidance for Industry (9) and USP (5). Seating time, shaking time and frequency, delay between waste and collected actuations, and delay between actuations (actuation hold time) were optimized for the DDU test using the commercially available product. The product handling parameters determined from the DDU testing of the commercial MDIs were then utilized for the DDU and APSD tests for all subsequent formulations (i.e., model systems and DOE MDIs) with the given drug. The drug product was handled as follows: at minimum 15 s elapsed between actuations and if more than ten actuations were performed consecutively, a 5 min break was introduced to allow the MDI to warm up to ambient temperature. During the time between actuations, the suspension MDIs were shaken vertically for approximately 10 s and actuated immediately after shaking ceased. Each actuation was held down for approximately 1 s. The PSD D50 of albuterol sulfate in the commercial MDIs was determined via static laser-light diffraction using the wet dispersion method described above.
Manufacture and Characterization of Model System MDIs
The formulations of suspension and solution model system MDIs were developed to be qualitatively (Q1) and quantitatively (Q2) the same as the corresponding commercial MDIs based on the data from reverse engineering conducted at Recipharm. Q1 and Q2 are defined elsewhere (6). Unmicronized albuterol sulfate was micronized using two jet mills (Glen Mills LHM001, Clifton, NJ, USA and Fluid Energy Processing and Equipment Company Jet-O-Mizer Model 00, Telford, PA, USA) operating at pressure range of 50–120 psi, feed rate of 0.5–3 g/min, and up to three passes through the jet mills. Drug PSD D50 was determined via static laser-light diffraction using the dry dispersion method described above.
The suspension model system MDI was manufactured via a one-step pressure filling process at a 3-L scale using a diaphragm filler (Pamasol, Pfäffikon, Switzerland), parallel in-line homogenization 13,500–17,500 rpm for 1 h; suspensions were filled into 17 mL canisters with 28 μL metering valves. The solution model system MDI was manufactured using a two-step pressure filling process (Pamasol, Pfäffikon, Switzerland); BDP dissolved in ethanol was added to the 17 mL canisters, 50 μL metering valves were crimped, and HFA-134a propellant was added through the metering valve. Different metering valve volumes were selected for the suspension and solution formulations based on shot weight and delivered dose data gathered from reverse engineering of suspension and solution commercial MDIs. At least 20 canisters were filled per each batch, with representative filling overages as that found in the commercial MDIs. DDU and APSD tests were conducted on the suspension and solution model system MDIs using the plastic actuators of suspension and solution commercial MDIs, respectively, and using the methods described above.
MDI Batches Based on Design of Experiments
Statistical DOE
The levels of excipients (ethanol and oleic acid concentrations in suspension and solution formulations) and drug PSD D50 (in suspension formulations) were varied according to a factorial statistical DOE approach which was used to develop a MDI batch manufacturing plan. The DOE approach considered the targets of the factors determined from the model system MDIs as central points, and the addition of low and high levels separated from the central points to ensure detection of effects. Table I summarizes the DOE plan for suspension (three-factor, three-level reduced factorial design) and solution (two-factor, three-level full factorial design) MDIs. Note that, for the suspension formulations, additional MDI batches were added to the DOE after the first 18 batches were tested, to further study the effect of ethanol in a lower and narrower range and the effect of oleic acid in a wider range than that studied using the first 18 batches. The DOE for the solution MDIs was partially guided by the findings from the DOE for the suspension MDIs; for instance, to evaluate the impact of oleic acid, the oleic acid concentration range was significantly increased in the DOE of the solution MDIs compared to that in the DOE of the suspension MDIs. Additionally, since the strong dependence of endpoints was noted for ethanol concentrations, the ethanol concentration range was also reduced.
Manufacture and Characterization of DOE MDIs
The batch manufacturing of suspension and solution MDIs was executed according to the DOE plan. A total of 22 (suspension, as 18 + 4 batches) and 9 (solution) batches were manufactured as described above for the model system MDIs, with representative filling overages as that found in the commercial albuterol sulfate or BDP MDI products. The DOE MDI batches using the most extreme combination of the factor levels (four for the suspension and two for the solution) were prepared first to confirm manufacturability and suitability of the DOE plan. The remaining batches were then manufactured following the full randomized order as per the DOE plan. At least 20 canisters were filled for each batch, and the batches were equilibrated at ambient conditions for 14 ± 2 days (suspension MDIs) and 7 ± 2 days (solution MDIs) prior to characterization. The suspension and solution DOE MDIs were characterized according to total content per canister (drug and excipients), DDU and APSD performance, using the plastic actuators of suspension and solution commercial MDIs, respectively, and using the methods described above.
Statistical Data Analysis
The results from DDU and APSD testing were subject to statistical two-way analysis of variance (ANOVA) with an a priori α level of 0.05 using SAS v9.2 and JMP v13.0 to explore the possible effects by the different formulation factors on the aerosol performance of suspension and solution MDIs. The primary endpoints were (i) overall mean DD from DDU tests and (ii) mean FPD at B and E life stages using USP induction port and AIT from APSD tests. For suspension MDIs, a between-canister standard deviation (SD) of 1.5 μg in overall mean DD was assumed, and based on this, the sample size (one canister per batch and two determinations per each B, M, and E life stages) was estimated to be sufficient to detect a 4 μg difference in overall mean DD between any two factors (96% probability). Similarly, a between-canister SD of 2.8 μg in mean FPD was assumed, and based on this, the sample size (one canister per batch and one determination per each B and E life stages) was estimated to be sufficient to detect a 6 μg difference in mean FPD between any two factors (82% probability). For solution MDIs, the sample size (two canisters per batch for DDU and APSD tests) was based on a desire to match the total sample size as that of the suspension DOE plan; as half the number of batches were studied for the solutions in comparison with the suspensions (9 vs. 18), twice the number of canisters per batch were tested. The secondary endpoints from DDU tests were mean DD at B, M, and E life stages, mean DD through life trend [(DD at B − DD at E) / DD at B) × 100], SD, and coefficient of variation (CV; SD as a percent of the overall mean DD). A formulation factor (ethanol and oleic acid concentrations or drug PSD D50) was considered to have a statistically significant effect on the endpoint if the associated p-value was <0.05.
Establishment of Design Spaces of MDI Aerosol Performance
Various multivariate mathematical models were tested to identify the best model capable of predicting differences in DD and APSD performance from the levels of formulation factors established for this study, as appropriate. Models were selected based on the explanatory power, i.e., how much (in percent) of the total variation can be explained by the selected model. For all cases, a multivariate linear regression model was initially evaluated, using all independent variables that appeared to have an influence on the endpoints of interest, regardless of their statistical significance levels. Based on the residuals analysis of the multivariate linear regression model, various empirical linear, log-linear, and power-linear models were selected for further evaluation with the inclusion of cross terms, as supported by the residuals analysis. The best models identified were used to assess the effects of 5% changes in formulation factors on the endpoints and to determine design spaces such that the performance characteristics under study remains within specified limits based on the experimental data and for exploratory purposes only.
RESULTS
Part I–Model System MDIs
Reverse Engineering of Commercial MDIs
The commercial suspension and solution MDIs were reverse engineered and characterized according to the total content per canister (drug and excipients), DDU through life stages, and APSD performance (at minimum n = 3 per each of the three batches) in order to establish model system MDIs as central targets for the statistical DOE plan. The results are summarized in the Supplementary Material (Table S2).
Formulation and Aerosol Performance of Model System MDIs
Table II shows the formulation of suspension and solution model system MDIs established based on the reverse engineered commercial MDIs. The aerosol performance of suspension and solution model system MDIs was characterized based on DDU through life stages and APSD profiles and compared to the corresponding commercial MDIs. The results are summarized in the Supplementary Material (Figs. S1, S2, and S3 and Tables S3 and S4). The mean delivered dose of albuterol base and BDP at each stage of canister life was within ±15% of label claim (Fig. S1). The overall mean DD for albuterol sulfate suspension model system MDI was 102% (ranging from 100 to 103% for mean DD at B, M, or E life stages) of that for the average of the suspension commercial MDI batches evaluated. The overall mean DD for BDP solution model system MDI was 101% (ranging from 96 to 107% for mean DD at B, M, or E life stages) of that for the average of the solution commercial MDI batches evaluated. While fairly large differences are seen for drug deposition on selected impaction stages (i.e., stages 1 and 4 to 6) or MDI components (i.e., actuator and valve stem), all the key APSD parameters (FPD, FPF, MMAD, GSD, and mass balance) differed <10% between the model system and commercially available MDIs (Tables S3 and S4).
Part II–MDIs Based on the DOE Plan
Drug PSD of Suspension DOE MDIs
Prior to the DOE MDI manufacture, albuterol sulfate was micronized to attain targeted PSD D50. The actual PSD D50 of albuterol sulfate used in the suspension DOE MDIs were 1.35, 1.67, and 2.41 μm, which approximately matched the targets shown in Table I (on average, the percent difference from targets was −3.5%).
Total Content Per Canister of DOE MDIs
The suspension and solution MDIs manufactured based on the DOE plan were characterized for the total drug, ethanol, and oleic acid content per canister (n = 2 per batch) to confirm that the actual levels matched the manufacturing targets of excipients (Table I) and drug (Table II). The percent difference from the targets was, on average, −2.2% (albuterol sulfate), −1.7% (ethanol), and +14.8% (oleic acid) for the suspension DOE MDIs and −6.2% (BDP), −4.2% (ethanol), and −6.3% (oleic acid) for the solution DOE MDIs.
Aerosol Performance of DOE MDIs
The DDU through life stages of suspension and solution DOE MDIs are shown in subpanels a and b of Fig. 2, respectively. The mean DD values for suspensions DOE batches ranged from 43.1 to 99.6 μg of albuterol base (Fig. 2a). The overall mean DD was 75.8 μg (SD = 10.9 μg), 16% below the label claim of 90 μg albuterol base, and there was a clear DD through life trend, i.e., the DD at B were typically higher than those at M, which in turn were higher than those at E life stage (Fig. 2a). The mean DD values for the solutions DOE batches were less variable–they ranged from 38.1 to 45.7 μg of BDP (Fig. 2b). The overall mean DD was 41.0 μg (SD = 2.2 μg), only 2.5% above label claim of 40 μg BDP, and no DD through life trend was observed (Fig. 2b).
Mean DDU through life stages of suspension (a) and solution (b) DOE MDIs. The actuation number is indicated below each B, M, and E life stage. Tests were performed using three canisters (batches 1 to 4) and one canister (batches 5 to 22) per batch of suspensions and two canisters per batch of solutions. Dashed lines represent ±15% of label claim. Mean values calculated based on all individual actuations (two per each life stage per canister) are shown
Individual FPD for suspension and solution DOE MDIs were collected at the B life stage using NGI with USP induction port and AIT, and the mean values are shown in subpanels a and b of Fig. 3, respectively. In addition, Fig. 3a presents the mean FPD for the suspension DOE MDIs at the E life stage using the NGI with the AIT. The mean FPD values of suspension DOE MDIs ranged from 15.9 to 67.4 μg (a factor of 4.2) and the mean FPD was 33.7 μg (SD = 11.6 μg) of albuterol base when using USP induction port and ranged from 15.9 to 67.4 μg (a factor of 4.2) and the mean FPD was 37.2 μg (SD = 13.7 μg) of albuterol base when using AIT. The mean FPD values for the solution DOE MDIs were slightly less variable, probably due to the more narrow range of ethanol studied. Mean FPD ranged from 11.5 to 32.4 μg (a factor of 2.8) for BDP. The mean FPD was 21.6 μg (SD = 5.7 μg) for BDP when using USP induction port, and it ranged from 13.5 to 31.7 μg (a factor of 2.3). The mean FPD was 21.6 μg (SD = 5.9 μg) for BDP when using the AIT.
Mean FPD of suspension DOE MDIs collected by NGI at B life stage (USP induction port and AIT) and E life stage (AIT) (a) and solution DOE MDIs collected by NGI at B life stage (USP induction port and AIT) (b). Tests were performed using three canisters (batches 1 to 4) and one canister (batches 5 to 22) per batch of suspensions and two canisters per batch of solutions
Statistical Data Analysis
The DD of suspension DOE MDIs exhibited a noticeable variability. The variability of individual DD was fairly high (SD = 8.9 μg of albuterol base and CV = 12.0%). The overall mean DD varied by 36.5% (61.4–88.9 μg), and the mean DD at B, M, and E life stages varied by 41.7% (64.5–99.6 μg), 34.3% (64.6–90.5 μg), and 60.2% (43.1–83.1 μg), respectively (Fig. 2a). The mean DD from B to E life stage decreased by 20.7% on average, which is greater than that seen for the commercial product (see Fig. S1) and resulted in a clear DD through life trend (Fig. 2a). In contrast, the individual DD of solution DOE MDIs were less variable (SD = 1.2 μg of BDP and CV = 2.9%). The overall mean DD varied by 13.7% (38.2–43.7 μg) and the mean DD at B, M, and E life stages varied by 17.4% (38.5–45.7 μg), 12.5% (38.1–43.2 μg), and 12.2% (38.1–43.1 μg), respectively (Fig. 2b). The mean DD from B to E life stages decreased by 1.1% on average, which resulted in no apparent DD through life trend (Fig. 2b).
The DD endpoints were subject to ANOVA, and the results are shown in Table III. At 5% level, the overall mean DDs for suspension DOE MDIs were not statistically significantly affected by any of the three factors except for the effect of ethanol at the B life stage. Therefore, the mean DD through life trend, SD, and CV for the individual DD were also affected by ethanol content. In the case of solution DOE MDIs, the effect of oleic acid on the overall mean DD and all secondary DD endpoints, except mean DD through life trend, was statistically significant. Furthermore, the effect of ethanol on the SD and CV of the overall mean DD of BDP was also statistically significant.
The size of the effects was evaluated to determine their relevance, since statistically significant effects may or may not be practically relevant with respect to specifications as established by pharmaceutical developers and agreed upon by regulators. In the case of suspension DOE MDIs, on average, the mean DD at B life stage decreased by 13.1% (88.1 to 76.6 μg) when the ethanol concentration increased from 2 to 20% w/w. The mean DD through life trend decreased from 45.0 to 10.1% (Fig. 4a), and the CV of the overall mean DD decreased from 26.2 to 5.9% (Fig. 4b) when the ethanol concentration increased from 2 to 20% w/w. In the case of solution DOE MDIs, the size of the effects was modest—on average, the overall mean DD and the mean DD at B, M, and E life stages decreased by 9.6% (43.0–38.9 μg), 10.9% (43.7–38.9 μg), 9.1% (42.7–38.8 μg), and 8.7% (42.6–38.9 μg), respectively, when the oleic acid concentration increased from 0 to 2% w/w (Fig. 4c). The CV for the overall mean DD decreased by 1.1 and 0.8% when the oleic acid and ethanol concentrations increased from 0 to 2% w/w and 7 to 9% w/w, respectively (Fig. 4d).
The mean FPD for suspension DOE MDIs exhibited a noticeable difference between formulations at B life stage (15.5–60.2 μg, USP induction port, a 3.9-fold difference and 15.9–67.4 μg, AIT, a 4.2-fold difference) and at E life stage (15.0–53.6 μg, AIT, a 3.6-fold difference), as shown in Fig. 3a. In contrast, the mean FPD for solution DOE MDIs was less affected–17.0 to 31.3 μg for the USP induction port (a factor 1.8) and 15.6 to 30.5 μg for the AIT (a factor 2.0)–both at the B life stage (Fig. 3b). The mean FPDs for USP induction port and AIT were not considerably different. The mean FPD across all suspension formulations at B stage life was 33.7 μg (SD = 11.6 μg) for the USP induction port and 37.2 (SD = 13.7 μg) for the AIT. Similarly, the mean FPD across all solution formulations at B stage life was 21.6 μg (SD = 5.0 μg) for the USP induction port and 21.6 μg (SD = 5.4 μg) for the AIT.
The FPD data were subjected to ANOVA. At 5% level, the mean FPD at B (USP induction port and AIT) and E life stages (AIT) of suspension DOE MDIs were statistically significantly affected by drug PSD D50 and ethanol, and the mean FPD at B life stage (USP induction port and AIT) of solution DOE MDIs was statistically significantly affected by oleic acid (Table IV).
The size of the effects was evaluated to determine their impact on FPD. In the case of suspension DOE MDIs, when the concentration of ethanol increased from 7 to 20% w/w, the FPD at B life stage with USP induction port decreased, on average, 58% (60.2–25.3 μg), 57.6% (43.4–18.4 μg), and 58.2% (37.1–15.5 μg) depending on the drug PSD D50 of 1.4, 1.65, and 2.5 μm, respectively (Fig. 5a). The FPD at B and E life stages with AIT decreased in a similar manner: 55.3% (67.4–30.1 μg), 59.9% (51.9–20.8 μg), and 64% (44.2–15.9 μg) as shown in Fig. 5b and 56.3% (53.6–23.4 μg), 57.2% (38.8–16.6 μg), and 50.5% (30.3–15.0 μg) as shown in Fig. 5c. The FPD decreased as the drug PSD D50 increased from 1.4 to 2.5 μm, irrespective of the canister life stage and type of induction port. For instance, FPD of albuterol base delivered from albuterol sulfate suspension MDIs using the USP induction port at B stage life ranged from 25.3 to 60.2, 18.4 to 43.4, and 15.5 to 37.1 μg for batches prepared with PSD D50 of 1.4, 1.65, and 2.5 μm, respectively. Interestingly, the decrease in FPD as a function of drug PSD D50 appears to have a greater impact at lower ethanol concentrations compared to higher concentrations. This may be due to a decrease in evaporation rate that results in an increase in MMAD or deposition of the drug in the induction port as a function of ethanol concentration and drug PSD (1). In the case of solution DOE MDIs, the FPD at B life stage decreased, on average, by 64.5% (32.4–11.5 μg) and 57.4% (31.7–13.5 μg) with USP induction port and AIT, respectively, when the oleic acid concentration increased from 0 to 2% w/w (Fig. 5d). The aforementioned decrease appeared to be non-linear, with 0% oleic acid resulting in greater FPD than that seen with 0.5 or 2% w/w oleic acid.
Changes in FPD of suspension (a, b, c) DOE MDIs according to different concentrations of ethanol in the canister, drug PSD D50, B and E canister life stage, and induction port (USP induction port and AIT). Changes in FPD at B life stage of solution (d) DOE MDIs according to different concentrations of oleic acid in the canister and induction port (USP induction port and AIT). Dots indicate the mean values of each batch, and lines represent the regression model noted in the determination of design spaces for MDI aerosol performance section
Determination of Design Spaces of MDI Aerosol Performance
All factors [ethanol (EtOH) and oleic acid (OA) concentrations and drug PSD D50] were considered for inclusion in the multivariate mathematical models, despite some factors having no statistically significant effect on the endpoints. This approach was taken because there could be some modest effects that, while not reaching statistical significance (Tables III and IV) likely due to the limited sample sizes, could still improve the model fit. Furthermore, the actual levels of the three factors (rather than the target levels) and data of all 22 suspension and 9 solution DOE MDIs were utilized in the pooled analysis.
The model Overall Mean DD = a1 + a2·(OA) a3 (a1 = 43.02, a2 = −2.923, a3 = 0.6006) explained approximately 83% of the total variation in DD for solution DOE MDIs. According to the model, for instance, if OA = 0.5% w/w changes by ±5%, the overall mean DD changes from 41.04 to 41.15 μg (a 0.11 μg difference, which is negligible considering the 32.8–46.0 μg specification for mean DD). Moreover, the model indicates that if OA ≤6.4% w/w, the overall mean DD would be within 85–115% of label claim (Fig. 6a, black area). As another example, if OA ≤4% w/w, the overall mean DD is predicted to remain within 91–109% of label claim. However, since these results are outside the studied range (OA = 0–2% w/w), they may not be reliable.
Design spaces for overall mean DD (a) and CV (b, c, d) and for FPD (e, f) of suspension and solution DOE MDIs. Overall mean DD [specified to be from (100 − L) to (100 + L)% of label claim (L was varied from 0 to 15%)] according to different concentrations of oleic acid (OA) in the solution MDI (a) CV of DD [specified to be ≤6%] according to different concentrations of ethanol (EtOH) and oleic acid (OA) in the solution MDI (b). CV of DD [specified to be ≤10%] according to different concentrations of ethanol (EtOH) and oleic acid (OA) and different drug PSD D50 in the suspension MDI (c, d). FPD [specified to be 21–43 μg of albuterol base] according to different concentrations of ethanol (EtOH) and drug PSD D50 in the suspension MDI (e). FPD [specified to be from (20 − f) to (20 + f) μg of beclomethasone dipropionate; K = (20 + f) / (20 − f)] according to different concentrations of oleic acid (OA) in the solution MDI (f)
The model CV = b1 + b2·(OA) + b3·(EtOH) + b4·(OA)·(EtOH) (b1 = 11.62, b2 = −5.31, b3 = −1.081, b4 = 0.62) explained approximately 44% of the total variation in CV of DD for solution DOE MDIs. Despite the low predictive power, the model was utilized to explore some cases. For instance, if OA = 0% w/w and EtOH = 8% w/w (the target levels) change by 5%, the CV of DD varies in the range 2.54–3.40%, which agrees with the experimental data (Fig. 4d). Moreover, the model shows what combinations of OA and EtOH concentrations are required to maintain the CV of DD ≤6% (Fig. 6b, black area)–if the OA concentration in the solution MDI is low, the EtOH concentration would need to be high.
The model CV = c1 + c2·ln(PSD D 50 ) + c3·ln(EtOH) + c4·ln(OA) (c1 = 29.80, c2 = 2.183, c3 = −7.45, c4 = 0.716) explained approximately 85% of the total variation in CV of DD for suspension DOE MDIs. According to the model, if drug PSD D50 = 1.58 μm, EtOH = 14.4% w/w, and OA = 0.03% w/w (the target levels) change by 5%, one factor at a time, the CV of DD varies in the ranges of 8.32–8.54% (PSD D50 factor), 8.07–8.81% (EtOH factor), and 8.39–8.47% (OA factor), the largest effect (0.74% difference) caused by a 5% change in EtOH. If all three factors change by 5% at the same time, the CV of DD varies 7.92–8.95% (1.03% difference). Moreover, the model shows the combinations of EtOH concentration and drug PSD D50 required to maintain the CV of DD ≤10% (black area in Fig. 6c and 6d, if the OA = 0.3 and 0.03% w/w, respectively)–if the EtOH concentration in the suspension MDI is high, the drug PSD D50 does not need to be tightly controlled to achieve the targeted CV of DD. Also, if the OA concentration in the suspension MDI decreases, the design space for CVs <10% increases.
The model FPD = d1 + d2·ln(PSD D 50 ) + d3·ln(EtOH) + d4·ln(PSD D 50 )·ln(EtOH) (d1 = 121.6, d2 = −68.44, d3 = −29.46, d4 = 16.99) explained approximately 86% of the total variation in FPD for suspension DOE MDIs. The model was developed based on the experimental results shown in Fig. 5a–c, i.e., the FPD was dependent on the drug PSD D50 and EtOH concentration (fairly linear from 7 to 20% w/w), but not on the OA concentration, irrespective of the canister life stage and type of induction port. According to the model, if drug PSD D50 = 1.58 μm and EtOH = 14.4% w/w (the target levels of PSD D50 and ethanol concentration for illustration purposes) change by 5%, one factor at a time, the FPD varies 31.34–33.64 μg (PSD D50 factor) and 31.41–33.58 μg (EtOH factor), i.e., a difference of 7% in both cases. However, if both factors change by 5% at the same time, the FPD varies in the range 30.32–34.81 μg, with a maximal difference of 15%. Moreover, the model shows what combinations of EtOH concentration and drug PSD D50 that would maintain the FPD within a given range. For instance, assume that the desirable range for FPD was 21–43 μg (Fig. 6e, black area)–if EtOH is 10% w/w, then PSD D50 needs to be within the range 1.5–3.0 μm and, if EtOH is 15% w/w, then PSD D50 needs to be within the range 1.0–2.5 μm.
The model FPD at B = e1 + e2·ln(OA) (e1 = 18.31, e2 = −1.39) explained approximately 65% of the total variation in FPD of solution DOE MDIs. The model was developed based on the experimental results shown in Fig. 5d, i.e., the FPD at B life stage was dependent on the OA concentration, irrespective of the type of induction port. The model provides the OA concentration that would be needed to maintain the FPD at B life stage within a specified range from (20 − f) to (20 + f), where f is function of K according to the ratio K = (20 + f) / (20 − f) (Fig. 6f, black area). For instance, in order to maintain the FPD at B life stage within 16.7–23.3 μg (K = 1.4) and 13.3–26.7 μg (K = 2), the OA concentration is <2 and <10% w/w, respectively. That is, for a narrower requirement on FPD at B life stage, the OA concentration needs to be lower. Note again that this assumes that extensive extrapolation is reasonable, which could not be verified with present data.
DISCUSSION
The product performance of MDIs may depend, among other things, on the nature and amount of excipient(s) used in the formulation (10). Under the QbD paradigm, systematic investigations are necessary to understand if and how changes in CQAs of the formulation influence the product performance of MDIs (11). This work utilized a systematic approach, outlined in Fig. 1, to provide a better understanding of the possible effects of different levels of excipient(s) and drug PSD on the aerosol performance of MDIs.
Two commercial MDI products (albuterol sulfate suspension and BDP solution) were reverse engineered and characterized (Table S2 in the Supplementary Material) in order to establish model system MDIs as an anchor point for developing a statistical DOE plan. The purpose of the model system MDIs was not to achieve in vitro equivalence to the commercial MDIs but to establish similarity with respect to formulation and key aerosolization parameters, in order to have a starting point which formulation factors could be varied. The formulations of model system MDIs (Table II) were Q1 and Q2 the same as the commercial MDIs. The DDU through life stages and APSD profiles of model system MDIs were comparable to the commercial MDIs, and the percent difference in key APSD parameters was consistently ≤10% (Figs. S1, S2, and S3 and Tables S3 and S4 in the Supplementary Material). These results demonstrated that suspension and solution model system MDIs were successfully established.
Batch manufacturing of suspension and solution MDIs was executed according to a DOE plan in which the levels of the formulation factors (ethanol and oleic acid concentrations and drug PSD D50) were varied using a factorial statistical design approach with the model system MDIs as the central targets (Table II). For suspension MDIs, a three-factor, three-level full factorial design resulted in 27 batches, which were reduced to 18 batches while allowing all main effects to be estimated appropriately (additional 4 batches were included to study the effects of lower ethanol and higher oleic acid concentrations based on the findings from the first 18 batches). For solution MDIs, a two-factor, three-level full factorial design resulted in nine batches. While the commercial solution MDI chosen for this work does not contain oleic acid, this excipient was included in the DOE plan to study its effects in solution MDIs in comparison with suspension MDIs. The reduced and full factorial statistical DOE plans for suspension (22 batches) and solution (9 batches) MDIs (Table I), respectively, were considered adequate to estimate all main effects of the factors and generate data for development of multivariate mathematical models to predict key aerosolization parameters of MDIs. The sample sizes utilized in this work were not designed for comparing individual batches but only for modeling and assessing the effects of the formulation factors.
The DOE batches of suspension MDIs were manufactured with micronized albuterol sulfate with PSD D50, on average, −3.5% from the target (Table I). The ethanol and oleic acid contents per canister were, on average, −1.7 and +14.8% (suspensions) and −4.2 and −6.3% (solutions) different from the targets (Table I), respectively. The drug content per canister was, on average, −2.2% (suspensions) and −6.2% (solutions) different from the targets (Table II). The higher percent difference for oleic acid in the suspension DOE MDIs (>10%) can be justified by the difficulty in weighing the lower amounts of this excipient (Table I) during the batch manufacturing process. Nevertheless, these results demonstrated that the manufacturability of suspension and solution DOE MDIs was successfully achieved.
The aerosol performance of DOE MDIs was affected by the different levels of factors. The mean DD of suspensions ranged from 68 to 99% of label claim and a clear DD through life trend was observed (Fig. 2a), whereas the mean DD of solutions ranged from 96 to 109% of label claim with no DD through life trend (Fig. 2b). A decrease in DD through life may be seen in MDIs due to the dynamic changes in liquid phase concentration (decrease in liquid volume and increase in vapor space) through the canister life (12), which may be further accentuated by the product handling. At the B life stage, the FPD of suspensions varied by a factor of 3.9 (USP induction port) and 4.2 (AIT) between formulations (Fig. 3a), whereas the FPD of different solution formulations varied by a factor of 2.8 (USP induction port) and 2.3 (AIT) (Fig. 3b). While this difference between factors may be due to the inherent differences between solution and suspension MDI formulations, it may also be due to the different ranges of ethanol and oleic acid concentrations evaluated for the two DOE plans.
The aerosol performance of DOE MDIs was statistically significantly affected by different factors (Tables III and IV). The mean DD was statistically significantly affected by ethanol (suspensions) and oleic acid (solutions), whereas the FPD was statistically significantly affected by drug PSD D50 and ethanol (suspensions) and oleic acid (solutions). However, several cases without effects were also found, despite some relatively large changes in concentrations of excipients studied. The possible effects of varying excipient levels must therefore be studied on a case-by-case basis, as only a few general conclusions can be drawn.
To increase drug or excipient solubility in MDIs, or to enhance valve function, the pharmaceutical industry has resorted to a large extent on use of cosolvents, typically ethanol (1,13), but at the potential cost of drug chemical instability (14) and decrease in product performance (15). For solution MDIs, Zhu et al. have shown that the FPF of budesonide and BDP decreased by 49% (16) and 38% (17), respectively, as the ethanol concentration increased from 5 to 30% w/w. Likewise, the FPF of fluticasone propionate decreased by 11% as the ethanol concentration increased from 15 to 30% w/w (17). Similar findings have been reported by Saleem and Smyth (18), Stein and Myrdal (15,19), and Gupta et al. (20). For suspension MDIs, Williams III and Liu (21) have shown that the FPF of bovine serum albumin (BSA, model protein) decreased by 38% (Brij 98), 11% (Aerosol OT), and 41% (Tween 80) as the ethanol concentration increased from 8 to 12%; additionally, the fraction of total emitted dose of BSA deposited on the throat and upper stages increased by 19, 12, and 37% according to the type of surfactant used (Brij 98, Aerosol OT, and Tween 80, respectively). Pu et al. (22) have shown that the FPF of a model suspension MDI decreased by 7.5% as the ethanol concentration increased from 1.5 to 2.5%. In our work, for the solution DOE MDIs (Table I), the mean DD and FPD were not statistically significantly affected by ethanol (Tables III and IV) likely due to the narrow concentration range studied (7 to 9% w/w) as compared to the previous literature cited above and possibly due to interactions of the drug with the formulation components. For the suspension DOE MDIs (Table I), as the ethanol concentration increased from 7 to 20% w/w, the mean DD at B life stage decreased by 13%, and the FPD decreased by 50–65% irrespective of the canister life stage, type of induction port used, and the drug PSD D50 (Fig. 5a–c). Furthermore, as the ethanol concentration increased from 2 to 20% w/w, the mean DD through life trend decreased by 35% (Fig. 4a). These findings are consistent with the previous literature cited above. The changes in dosing efficiency of MDIs as a function of ethanol concentration are attributed to the multiple effects of this semi-volatile cosolvent. The ethanol content has an effect on (i) the formulation density (i.e., the total mass of formulation atomized during actuation), (ii) the atomization process of the formulation (i.e., the size of the atomized droplets), and (iii) the evaporation rate of the droplets towards their residual particle sizes (1). Upon actuation of a MDI, the propellant rapidly evaporates (higher volatility component) leading to intermediate droplets that consist of cosolvent, non-volatile excipient(s) (if any) and the drug(s). The cosolvent also evaporates over the course of droplet formation (but at a slower rate than the propellant) leading to residual particles (substantially smaller than the corresponding initial droplets) containing drug and non-volatile components only (if complete evaporation occurs) to be deposited in the lungs (23). As the ethanol concentration increases, the vapor pressure of the formulation decreases (24,25) and this affects the atomization process. When the atomization force decreases, the initial emitted droplet size increases, resulting in larger residual particles after evaporation of the propellant in the aerosol spray. Furthermore, larger droplets increase the drug deposition on the mouth and throat. Thus, as the ethanol concentration increases, the MDI aerosol performance generally decreases (1).
Surfactants are typically utilized in MDIs for countless reasons. In solution MDIs, surfactants may increase drug solubility, moderate temperature-dependent drug solubility, and defeat valve sticking issues (1). In suspension MDIs, surfactants prevent irreversible particle agglomeration, hinder drug particle adhesion to the canister walls and valve components, reduce the risk of clogging of the spray orifice, reduce the separation rate between the drug and propellant, and prevent valve sticking problems (1). However, high surfactant concentration may decrease the MDI delivery efficiency, i.e., decrease the FPF and increase the drug deposition on the mouth, throat, and upper airways (18,21). For instance, for fluorescein Na solution MDIs, with increase in surfactant (Pluronic L81) concentration from 0 to 1.22% w/w, Saleem and Smyth (18) have shown that the FPF effectively did not change (49.6–50.7%), MMAD increased (1.56–3.70 μm), and the drug in the size range 1.36–3.99 μm increased. However, the FPF decreased (49.6–33.2%), the MMAD increased (1.56–5.93 μm), and the drug deposited on the NGI stages with cut-off diameter ≥6.4 μm increased, as the surfactant concentration increased from 0 to 5.45% w/w. Furthermore, the drug deposition in the USP induction port increased (80, 95, and 120 μg) as the surfactant concentration increased (0, 1.22, and 5.45% w/w, respectively). For suspension MDIs, Williams III and Liu (21) have shown that, as the surfactant/protein (Brij 98/BSA) molar ratio increased from 100:1 to 800:1, the deposition of BSA in the throat and upper stage of the impactor increased (40–55%). Furthermore, as surfactant/protein molar ratio increased from 500:1–800:1, the FPF decreased (47–36%). In our work, for the suspension DOE MDIs (Table I), the mean DD and FPD were not statistically significantly affected by oleic acid (Tables III and IV), likely due to the low concentrations studied (0.005, 0.006, 0.02, 0.1, and 0.25% w/w), despite some of these being significantly above the levels used for the commercial model products. For the solution DOE MDIs (Table I), as the oleic acid concentration increased from 0 to 2% w/w, the overall mean DD and mean DD at B, M, and E life stages decreased by 9–11% (Fig. 4c). In addition, the FPD at B life stage decreased by 61% according to a non-linear relationship with respect to oleic acid concentration, irrespective of the type of induction port used (Fig. 5d). This non-linear relationship between FPD and oleic acid suggests that the main effect on FPD is due to whether oleic acid is present or not; if present, the effect of the amount oleic acid is small and decreasing with increasing oleic acid concentration. These findings are consistent with the previous literature cited above. High vapor pressure MDIs with low concentration of non-volatile components (e.g., drug and surfactant) produce smaller and more respirable aerosol droplets (24). Thus, high surfactant concentrations generally decrease the MDI aerosol performance due to a decrease in evaporation rate of atomized droplets and consequent increase in MMAD (2,10).
Generally, apart from the orifice exit diameter, the final APSD of suspension MDIs is primarily governed by the properties of suspended drug particles, for instance, drug PSD, suspension density, and ability to resuspend (26). Myrdal et al. (1) have used experimental DOE results and simulation models to predict the APSD properties of residual particles from suspension MDIs and have shown that the MMAD increased (1.0–2.5 μm) as the PSD D50 of the micronized drug increased (0.43–2.29 μm). Experimentally, Pu et al. (22) have shown that the APSD profile of suspension MDIs shifted to larger aerodynamic particle sizes and broader APSD as the drug PSD D50 increased (1.1–2.2 μm). In our work, for the suspension DOE MDIs (Table I), the FPD was statistically significantly affected by drug PSD D50 (Table IV). The FPD decreased as the drug PSD D50 increased from 1.4 to 2.5 μm, irrespective of the ethanol concentration, canister life stage, and type of induction port (Fig. 5a–c). These findings are as expected and consistent with the previous literature cited above. The constraint of the minimum output droplet size is predominantly the primary particle size of suspended particles that is contained within the atomized droplets (16); droplets can obviously not be smaller than the largest particle contained therein. Therefore, if the size of the micronized drug suspended in the MDI is large, the MMAD generally increases whereas the FPF and FPD decrease.
The understanding of the extent to which the formulation factors (ethanol and oleic acid concentrations and drug PSD D50) govern DD and APSD performance allowed establishing the range of variation within design spaces for each of these factors that can be tolerated while still achieving acceptable aerosol performance (Fig. 6). It is important to note that the design spaces should not be extrapolated beyond the studied ranges of the factors without careful consideration.
In our work, the APSD properties of suspension and solution DOE MDIs were determined using the NGI with both the standard USP induction port and the AIT. The AIT is not a fully anatomically correct upper airway model but comprises the idealized geometry of its simplified pathway (27). Surprisingly, no substantial differences in FPD were observed either using the USP induction port or AIT (Fig. 3) for the studied model systems. Only some marginal differences in FPD were observed–for instance, in the case of suspension DOE MDIs, the overall mean FPD increased from 34 μg (USP induction port) to 37 μg (AIT), i.e., a slight increase of 8% which is due to a small, but discernible shift towards finer particle size with the AIT. The drug deposition on stages 4 to filter (≤3.99) of the NGI was higher with the AIT, whereas the drug deposition on stages 1 to 3 (≥3.99 μm) of the NGI was higher with the USP induction port (data not shown). While there has been conflicting results presented in literature regarding drug deposition in the USP induction port compared to that in the AIT, these findings are consistent with some of the literature cited. For instance, by using a full resolution Andersen eight-stage cascade impactor, Mitchel et al. (27) obtained equivalent values of FPM <4.7 μm for albuterol sulfate suspension MDIs regardless the type of induction port (36 μg with USP induction port and 39 μg with AIT, i.e., the same increase by 8% as presented herein). Additionally, Copley (7) reported similar FPM <5.0 μm results for the USP induction port and AIT (38 μg with USP induction port and 33 μg with AIT). It is known that the shape and volume of the induction port may affect the delivery characteristics of inhalation products (28). In the case of suspension DOE MDIs, our findings indicated that replacing the USP induction port by the AIT resulted in a slight shift to finer particle sizes which contributed to a slightly higher FPD of albuterol base. Whether these conclusions hold true for other flow rates, “anatomical” mouth/throat models, active pharmaceutical ingredients, or drug delivery systems (such as dry powder inhalers or soft mist inhalers) remains to be clarified.
CONCLUSIONS
Two commercially available MDI products (albuterol sulfate suspension and beclomethasone dipropionate solution) were selected as model system MDIs to provide a relevant anchor for product performance comparisons made by varying selected CQAs, such as ethanol and oleic acid concentrations, and micronized drug particle size, through factorial DOE. Statistical analysis showed that, in some cases, the formulation factors had profound effects on the aerosol performance, but in other cases, the effects were irrelevant with respect to pre-determined specifications established for this work or did not exist. Therefore, the effects of formulation compositions must always be assessed on a case-by-case basis. The impact of these CQAs on key product aerosol performance was subsequently used to define statistical models that delineated design spaces with pre-determined target ranges for DD and FPD. The utility of these models was demonstrated by presenting plausible formulations of albuterol sulfate and beclomethasone dipropionate to achieve desirable aerosol performance characteristics. These models also show that formulations with similar key aerosol properties can be designed using different formulation compositions.
References
Myrdal PB, Sheth P, Stein SW. Advances in metered dose inhaler technology: formulation development. AAPS PharmSciTech. 2014;15(2):434–55.
Ivey JW, Vehring R, Finlay WH. Understanding pressurized metered dose inhaler performance. Expert Opinion Drug Deliv. 2015;12(6):901–16.
Stein SW, Sheth P, Hodson PD, Myrdal PB. Advances in metered dose inhaler technology: hardware development. AAPS PharmSciTech. 2014;15(2):326–38.
Borgström L. The pharmacokinetics of inhaled hydrofluoroalkane formulations. J Allergy Clin Immunol. 1999;104(6):s246–s9.
US Pharmacopeia. General chapters: <601> inhalation and nasal drug products: aerosols, sprays, and powders—performance quality tests. Official from May 1, 2015; USP 38-NF 35:388–414.
Food and Drug Administration - Office of Generic Drugs. Albuterol sulfate inhalation aerosol metered. Draft Guidance. 2016.
Copley M. Improving the realism and relevance of mouth-throat models for inhaled product testing. www.ondrugdelivery.com. 2015; 32–7.
Shur J, Saluja B, Lee S, Tibbatts J, Price R. Effect of device design and formulation on the in vitro comparability for multi-unit dose dry powder inhalers. AAPS J. 2015;17(5):1105–16.
US Department of Health and Human Services - Food and Drug Administration. Metered dose inhaler (MDI) and dry powder inhaler (DPI) drug products—chemistry, manufacturing, and controls documentation. Guidance for Industry—Draft. 1998; 65.
Smyth HDC. The influence of formulation variables on the performance of alternative propellant-driven metered dose inhalers. Adv Drug Deliv Rev. 2003;55(7):807–28.
Lionberger RA, Lee SL, Lee L, Raw A, Lawrence XY. Quality by design: concepts for ANDAs. AAPS J. 2008;10(2):268–76.
Smith IJ, Bell J, Bowman N, Everard M, Stein S, Weers JG. Inhaler devices: what remains to be done? J Aerosol Med Pulm Drug Deliv. 2010;23(S2):S-25–37.
Conti DS, Grashik J, Yang L, Wu L, da Rocha SRP. Solvation in hydrofluoroalkanes: how can ethanol help? J Pharm Pharmacol. 2011;64(9):1236–44.
Zhu B, Traini D, Young P. Aerosol particle generation from solution-based pressurized metered dose inhalers: a technical overview of parameters that influence respiratory deposition. Pharm Dev Technol 2014(0):1–14.
Stein S, Myrdal P. The relative influence of atomization and evaporation on metered dose inhaler drug delivery efficiency. Aerosol Sci Technol. 2006;40(5):335–47.
Zhu B, Traini D, Chan H-K, Young PM. The effect of ethanol on the formation and physico-chemical properties of particles generated from budesonide solution-based pressurized metered-dose inhalers. Drug Dev Ind Pharm. 2013;39(11):1625–37.
Zhu B, Traini D, Lewis DA, Young P. The solid-state and morphological characteristics of particles generated from solution-based metered dose inhalers: influence of ethanol concentration and intrinsic drug properties. Colloids Surf A Physicochem Eng Asp. 2014;443:345–55.
Saleem IY, Smyth HD. Tuning aerosol particle size distribution of metered dose inhalers using cosolvents and surfactants. BioMed Res Int. 2013;2013:1–7.
Stein SW, Myrdal PB. A theoretical and experimental analysis of formulation and device parameters affecting solution MDI size distributions. J Pharm Sci. 2004;93(8):2158–75.
Gupta A, Stein SW, Myrdal PB. Balancing ethanol cosolvent concentration with product performance in 134a-based pressurized metered dose inhalers. J Aerosol Med. 2003;16(2):167–74.
Williams RO III, Liu J. Formulation of a protein with propellant HFA 134a for aerosol delivery. Eur J Pharm Sci. 1999;7(2):137–44.
Pu Y, Kline LC, Khawaja N, Van Liew M, Berry J. Comparison of optical particle sizing and cascade impaction for measuring the particle size of a suspension metered dose inhaler. Drug Dev Ind Pharm. 2015;41(5):737–43.
Sheth P, Stein SW, Myrdal PB. Factors influencing aerodynamic particle size distribution of suspension pressurized metered dose inhalers. AAPS PharmSciTech. 2014; 1–10.
Vervaet C, Byron PR. Drug-surfactant-propellant interactions in HFA-formulations. Int J Pharm. 1999;186(1):13–30.
Williams RO III, Liu J. Influence of formulation additives on the vapor pressure of hydrofluoroalkane propellants. Int J Pharm. 1998;166(1):99–103.
Zhu B, Xu N, Traini D, Lewis D, Young PM. The formation of aerosol particles from solution-based pressurized metered dose inhalers and implications of incomplete droplet drying: theoretical and experimental comparison. Aerosol Sci Technol. 2015; doi:10.1080/02786826.2015.1096897.
Mitchell J, Copley M, Sizer Y, Russell T, Solomon D. Adapting the Abbreviated Impactor Measurement (AIM) concept to make appropriate inhaler aerosol measurements to compare with clinical data: a scoping study with the “Alberta” Idealized Throat (AIT) inlet. J Aerosol Med Pulm Drug Deliv. 2012;25(4):188–97.
Guo C, Gillespie SR, Kauffman J, Doub WH. Comparison of delivery characteristics from a combination metered-dose inhaler using the Andersen cascade impactor and the next generation pharmaceutical impactor. J Pharm Sci. 2008;97(8):3321–34.
ACKNOWLEDGMENTS
Funding for this work was made possible, in part, by the Food and Drug Administration through grant 1U01FD004943-01. Views expressed in this publication do not necessarily reflect the official policies of the Department of Health and Human Services, nor does any mention of trade names, commercial practices, or organization imply endorsement by the US Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
The opinions expressed in this paper by the authors do not necessarily reflect the views or policies of the Food and Drug Administration (FDA).
Rights and permissions
About this article
Cite this article
Sheth, P., Sandell, D., Conti, D.S. et al. Influence of Formulation Factors on the Aerosol Performance of Suspension and Solution Metered Dose Inhalers: A Systematic Approach. AAPS J 19, 1396–1410 (2017). https://doi.org/10.1208/s12248-017-0095-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-017-0095-3