Abstract
Direct measurement of red blood cell (RBC) survival in humans has improved from the original accurate but limited differential agglutination technique to the current reliable, safe, and accurate biotin method. Despite this, all of these methods are time consuming and require blood sampling over several months to determine the RBC lifespan. For situations in which RBC survival information must be obtained quickly, these methods are not suitable. With the exception of adults and infants, RBC survival has not been extensively investigated in other age groups. To address this need, we developed a novel, physiology-based mathematical model that quickly estimates RBC lifespan in healthy individuals at any age. The model is based on the assumption that the total number of RBC recirculations during the lifespan of each RBC (denoted by N max) is relatively constant for all age groups. The model was initially validated using the data from our prior infant and adult biotin-labeled red blood cell studies and then extended to the other age groups. The model generated the following estimated RBC lifespans in 2-year-old, 5-year-old, 8-year-old, and 10-year-old children: 62, 74, 82, and 86 days, respectively. We speculate that this model has useful clinical applications. For example, HbA1c testing is not reliable in identifying children with diabetes because HbA1c is directly affected by RBC lifespan. Because our model can estimate RBC lifespan in children at any age, corrections to HbA1c values based on the model-generated RBC lifespan could improve diabetes diagnosis as well as therapy in children.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Understanding of RBC survival is useful in both clinical care and research. For example, information about RBC survival is useful in the evaluation of new RBC storage media, in optimizing RBC transfusion therapy, in elucidating mechanisms of RBC senescence, and in understanding the pathophysiology of hemoglobinopathies such as sickle cell disease (1–3). RBC survival information is also important in diabetes mellitus because HbA1c, an important marker of long-term glycemic control, is directly affected by RBC lifespan (4,5).
Although the direct measurement of RBC survival has progressed from the original accurate but limited differential agglutination technique (6) to the current reliable, safe, and accurate biotin method (7), all the methods for direct measurement of RBC survival are time consuming and require several months to determine the RBC lifespan. This is not practical for situations in which RBC survival information is needed for immediate clinical decision making (such as anemic neonates). In addition, RBC survival has not been investigated for age groups other than infants and adults. To address this need, we developed a novel, physiology-based mathematical model that can quickly estimate RBC lifespan in any human age group. This model is based on the assumption that the total number of recirculations of a RBC during a life time (i.e., N max) is a relatively constant value in different age groups. We first validated this model using RBC lifespan data from our biotin-labeled red blood cell (BioRBC) studies conducted in infants (8) and adults (9) and then extended the model to estimate RBC lifespan at any age.
MATERIALS AND METHODS
Prior Clinical Studies
In the development of the model, the previous studies that we conducted in adults and infants were evaluated separately. The clinical study methods employed in these prior studies, including the study design and details of biotin method, have been previously published (8,9) and are summarized below. All studies were approved by the University of Iowa Committee on Research on Human Subjects (study performance site) and the institutional review board of the University of Arkansas for Medical Sciences (study analysis site).
For adults, aliquots of autologous RBC were labeled with biotin, and BioRBC was infused as previously described (9). Survival of BioRBC was determined at 20-min, 24-h, and 1- to 2-week intervals until the BioRBC enumerated by flow cytometry had decreased to the limit of detection, i.e., <0.06% of total RBC. For infants, aliquots of allogeneic RBC were transfused as 15 ml/kg of packed RBC; the first 14 ml/kg of allogeneic donor RBC was transfused over 3–4 h. The remaining 1 ml/kg of donor RBC and an additional 0.5 to 3 ml/kg of autologous blood from the study infants were biotinylated at either of two biotin densities between 6 and 36 μg/ml and transfused sequentially within 10 min after completion of the 14-mg/kg clinical transfusion (8). Beginning at 24-h post-transfusion, leftover anticoagulated whole blood from clinically ordered lab testing was salvaged up to four times per week for quantitation of the proportions of BioRBC by flow cytometric enumeration as previously described (8).
Physiologically Based Mathematical Modeling
Calculation of N max
The total number of times an erythrocyte is oxygenated and deoxygenated (i.e., the total number of recirculations during its life time), N max, before being taken out of the circulation is calculated assuming a constant blood volume (BV) and cardiac output (CO) as follows:
where LS is the average erythrocyte lifespan.
Central Hypothesis
Our overall hypothesis is as follows: inter- and intra-species variability of N max is small enough to enable accurate RBC lifespan estimations based on cardiac output and blood volume alone. This concept did not originate with our group. In 1960, Allison evaluated the total number of RBC recirculations in three different species—rat, dog, and man (10)—and found that the total number of recirculations of an erythrocyte during their life time is almost the same. This was observed in these three species despite more than 100-fold difference in cardiac output, more than 250-fold difference in blood volume, and more than 2-fold RBC survival difference (10). The average value of N max that Allison observed was ∼1.75 × 105 recirculations, with small variability (CV = 7.5%) (Fig. 1a). Based on this, we hypothesized that N max is a relatively constant number not only in different animal species, but even more so in different age groups within a single species, thus making the N max equation (Eq. 1) a suitable starting point for estimation of RBC lifespan . The schematic representation of our hypothesis is illustrated in Fig. 1b where erythrocytes reach their lifespan when their number of recirculations reaches N max. The slope, which is calculated as CO/BV, represents the “circulatory speed,” i.e., the number of erythrocyte recirculations per unit time. Accordingly, if the circulation speed in one age group is shorter than another, then the RBC will make more trips per unit time and will reach the same N max more quickly, thereby resulting in shorter lifespan.
Testing Our Hypothesis Using Published RBC Lifespan Data
To test our hypothesis, we estimated the Nmax value of a neonatal RBC based on our data obtained from a RBC survival study conducted in infants using biotin-labeled RBC (8). Based on Eq. 1, the information needed to estimate N max includes cardiac output, blood volume, and RBC lifespan. Based on our infant RBC study, the estimated average lifespan of neonatal RBC is 52.1 ± 10.8 days. For the cardiac output and blood volume, as it is difficult to obtain reliable values for each infant subject, literature-reported values from newborns were used (11,12). Unlike older children and adults, cardiac output and blood volume in rapidly growing infants undergo substantial change over short periods of time (12,13). It has been reported that both cardiac output and blood volume, even after body weight normalization, are not constant in infants (12,13). Because these body weight (BW)-normalized parameters change substantially with maturation, infant body weight also needs to be taken into account. In doing so, the equation for N max estimation in newborns becomes:
where CO in Eq. 2 now (in contrast to Eq. 1) specifically represents BW-normalized cardiac output and BV represents BW-normalized blood volume.
The literature information on BW-normalized cardiac output and blood volume of newborn is limited. Using published data for these two parameters in newborns within a few days of birth and in infants at around 1 year old (11,12), we assume that over the first year of life, the change in BW-normalized CO is linear with BW and change in BW-normalized blood volume is linear with respect to age.
The BW-normalized cardiac output was found to decrease with increasing BW (13). Accordingly, the following equation captures the BW-related change of cardiac output in infants:
where a is the BW-normalized cardiac output value of newborns and b represents the decrease of CO per unit of BW during the first year of life. BW0 is BW at birth. For newborns, BW = BW0
Similarly, the BW-normalized blood volume of infants decreases with increasing postnatal age (12). Thus, the following linear equation estimates the change in infant blood volume:
where c is the BW-normalized blood volume value of newborn and d represents the decrease of blood volume per unit of time after birth.
For estimating the BW of infants, Leffler’s formula (14) was used to capture the change in BW over time:
where m is the BW of newborn and h represents the increase of BW per unit of time during the first year of life.
Incorporating Eqs. 3, 4, and 5 into Eq. 2 gives:
After integration, the final equation for calculation of N max of a RBC in infants becomes:
where the value of LS is 74,880 min (i.e., 52 days) based on the data from our infant BioRBC study (15) and parameters a, b, c, d, m, and h are the parameters defining the relationships in Eqs. 3–5.
Physiologically Based Mathematical Model for Different Age Groups
After validating our hypothesis in newborns (see the “Results” section), we extended the physiologically based mathematical model to older age groups. Because N max is relatively constant across different age groups, we fixed N max to the average value in the rat, dog, man, and human infant. Using this N max value, RBC lifespan can then be estimated at any age as long as cardiac output and blood volume information are known at that age. This is simply done numerically by solving Eq. 6 for LS, which can be done by many software tools including Excel. Fortunately, ample data on cardiac output and blood volume have been published in humans. According to literature information, we divided age into the following four groups: 0–1 year, 1–10 years, 10–18 years, and >18 years.
-
For BW-normalized cardiac output, based on the literature reports (11,13), the following equations are used to describe its change in different age groups:
$$ \mathrm{B}\mathrm{W}-\mathrm{normalized}\ \mathrm{C}\mathrm{O} = \left\{\begin{array}{ll}{a}_0\hfill & \mathrm{AGE}=0\ \left(i.e.,\ \mathrm{newborn}\right)\hfill \\ {}{a}_1\ \hbox{--}\ {b}_1 \times \mathrm{B}\mathrm{W}\hfill & 0<\mathrm{AGE}\le 12\ \mathrm{months}\hfill \\ {}{a}_2 \times {\mathrm{BW}}^{b_2}\hfill & 1<\mathrm{AGE}\le 10\ \mathrm{years}\hfill \\ {}{a}_3 \times {\mathrm{BW}}^{b_3}\hfill & 10<\mathrm{AGE}\le 18\ \mathrm{years}\hfill \\ {}{a}_4 \times {\mathrm{BW}}^{b_4}\hfill & \mathrm{AGE}>18\ \mathrm{years}\hfill \end{array}\right. $$(8)where a 1 represents BW-normalized CO at the start of the study and it is calculated as a 0 − b 1 × (BW − BW0), with a 0 and BW0 being BW-normalized CO and BW0, respectively, at birth.
-
For BW-normalized blood volume, the value is relatively constant after the first year of life. The following equations were used to describe BW-normalized blood volume in different age groups (12,16,17):
$$ \mathrm{B}\mathrm{W}-\mathrm{normalized}\ \mathrm{B}\mathrm{V}=\left\{\begin{array}{ll}{c}_0\hfill & \mathrm{AGE}=0\ \left(i.e.,\ \mathrm{newborn}\right)\hfill \\ {}{c}_1\ \hbox{--} \kern0.5em {d}_1 \times t\hfill & 0<\mathrm{AGE}\le 12\ \mathrm{months}\hfill \\ {}{c}_2\hfill & 1<\mathrm{AGE}\le 10\ \mathrm{years}\hfill \\ {}{c}_3\hfill & 10<\mathrm{AGE}\le 18\ \mathrm{years}\hfill \\ {}{c}_4\hfill & \mathrm{AGE}>18\ \mathrm{years}\hfill \end{array}\right. $$(9)In this equation, c 1 represents BW-normalized BV at the start of the study and is calculated as c 0 − d 1 × AGE0, with c 0 being BW-normalized BV at birth and AGE0 is the postnatal age at the start of the study. t is time since the subject enters the study.
-
BW increases linearly during the first year of life and during 1–10 years, albeit the growth rates are different in these two age groups (14). For child older than 10 years, we assumed that the increase in BW is negligible during the lifespan of an erythrocyte. The following equations were used to describe change in BW in different age groups.
$$ \mathrm{B}\mathrm{W}=\left\{\begin{array}{ll}{m}_0\hfill & \mathrm{AGE}=0\ \left(i.e.\ \mathrm{newborn}\right)\hfill \\ {}{m}_1+{h}_1 \times t\hfill & 0<\mathrm{AGE}\le 1\ \mathrm{year}\hfill \\ {}{m}_2 + {h}_2 \times t\hfill & 1<\mathrm{AGE}\le 10\ \mathrm{year}\mathrm{s}\hfill \\ {}{m}_3\hfill & 10<\mathrm{AGE}\le 18\ \mathrm{year}\mathrm{s}\hfill \\ {}{m}_4\hfill & \mathrm{AGE}>18\ \mathrm{year}\mathrm{s}\hfill \end{array}\right., $$(10)where m 1 represents BW at the start of the study and is calculated as m 0 + h 1 × Age0, m 0 is BW at birth, and Age0 is the postnatal age at the start of the study.
For a red cell that is produced (i.e., enters blood circulation) at time T (relative to T = 0 at birth), the N max during that red cell’s lifespan is given by:
$$ {N}_{\max }={\displaystyle {\int}_{\mathrm{T}}^{\mathrm{T}+\mathrm{L}\mathrm{S}}\frac{\mathrm{CO}}{\mathrm{BV}}dt}, $$(11)where CO represents BW-normalized cardiac output in the time period from T to T + LS and BV represents BW-normalized blood volume in the time period from T to T + LS.
Incorporating Eqs. 8, 9, and 10 into Eq. 11 gives the following final equations for calculation of RBC lifespan (LS) in different age groups:
-
For 0–1 year old:
$$ {N}_{\max }=\left(\frac{b_1 \times {h}_1 \times \left({c}_0-{d}_1\times T\right)}{\ {d_1}^2}-\frac{\left({a}_0-{b}_1\times {h}_1\times T\right)-{b}_1\times {m}_1}{d_1}\right)\times \ln \left(1-\frac{d_1}{\left(\ {c}_0-{d}_1\times T\right)} \times \mathrm{L}\mathrm{S}\right)+\frac{b_1\times {h}_1}{d_1}\times \mathrm{L}\mathrm{S} $$(12) -
For 1–10 year old:
$$ {N}_{\max }=\frac{a_2}{\ {c}_2\times \left({b}_2+1\right)\times {h}_2}\times \left[{\left({m}_2+{h}_2\times LS\right)}^{b_2+1}-{m_2}^{b_2+1}\right] $$(13)where BV is a constant value for the 1- to 10-year-old population.
-
For 10–18-year olds:
$$ {N}_{\max }=\frac{a_3\times {\mathrm{BW}}^{b_3}}{\ {c}_3}\times \mathrm{L}\mathrm{S} $$(14) -
For >18-year olds:
$$ {N}_{\max }=\frac{a_4\times {\mathrm{BW}}^{b_4}}{c_4}\times \mathrm{L}\mathrm{S} $$(15)T ranges for Eq. 12 is 0 to (1-RBC lifespan) year; T ranges for Eq. 13 is 1 year to (10-RBC lifespan) year; T ranges for Eq. 14 is 10 year to (18-RBC lifespan) year; and T ranges for Eq. 15 is >18 years. The unit of RBC lifespan is in years.
For the above equations in different age groups, N max was fixed to 1.75 × 105. BW-normalized cardiac output, BW-normalized blood volume, and BW, along with their dynamic change, in these four different age groups were obtained from the literature. T is the age of the individual relative to birth. The only unknown information is LS. The equations were solved for LS in MatLab (MathWorks, Natick, MA). However, any other software that is able to solve a nonlinear equation for one variable such as Excel could be employed instead.
RESULTS
Clinical Studies
A summary of subject demographics is presented in Table I. For infants, the mean postnatal age was 3.2 days, with a range of 0.2 to 14 days. The mean postnatal weight was 741 g, with a range of 513 to 1042 g.
Figure 2 depicts the survival curves for (1) autologous adult BioRBCs transfused into an individual adult, (2) autologous neonatal BioRBCs transfused into an individual infant, and (3) allogeneic adult BioRBCs transfused in the same individual infant. Long-term survival of the autologous neonatal BioRBCs in infants was considerably shorter than that of autologous adult RBCs in adults. The estimated lifespans of autologous adult BioRBC in adult and autologous neonatal BioRBC in neonate are 120 and 52 days, respectively, consistent with literature reports. Contrary to current understanding, the survival of adult donor BioRBCs in infants was also shortened and indeed was almost identical to that of autologous neonatal BioRBCs. We infer that RBC survival is primarily controlled by extrinsic environmental factors, e.g., circulatory environmental events.
The survival curves for the autologous adult BioRBC transfused in an individual adult (9), as well as autologous neonatal BioRBC and allogeneic adult donor BioRBC transfused in an individual infant
Physiologically Based Mathematical Modeling
As shown in Fig. 1a, the RBC lifespans in the rat, dog, and man range from 55 days in rat to 120 days in man. The slope of each curve is calculated as CO/BV and reflects the number of trips that the RBCs make per unit time. The slope is steepest in rats and shallowest in humans, indicating that the RBC makes more trips per unit time in rats than in humans. Although the slopes and RBC lifespans differ among the three species, the total number of trips that a RBC can make before removal from circulation (i.e., N max as shown on the y-axis) is very similar.
When we estimated the N max of neonatal RBC, the dynamic changes of BW-normalized cardiac output, BW-normalized blood volume, and BW were all incorporated in our model. The initial values of BW-normalized cardiac output and BW-normalized blood volume (the intercepts of Eqs. 3 and 4, i.e., parameters a and c) are 273 ml/min/kg and 109 ml/kg, respectively, and were obtained directly from the newborn literature (11,12). The dynamic changes of BW-normalized cardiac output, BW-normalized blood volume, and BW (the slopes of Eqs. 3, 4, and 5; i.e., parameters b, d, and h) during the first year of life are shown in Table II. Parameters m and LS are 0.81 kg and 52 days, respectively, and were obtained from our infant BioRBC studies (15). When all of these parameters were entered into Eq. 7, the estimated N max of neonatal RBCs was 1.84 × 105 cycles (Fig. 3), a value which is remarkably similar to those for the rat, dog, and man (10).
After this validation of the N max principle, a physiologically based mathematical model was built to estimate RBC lifespan in different age groups. As BW-normalized cardiac output, BW-normalized blood volume, and BW undergo specific changes at different ages, age-specific equations were applied in four age groups: 0–1 year, 1–10 years, 10–18 years, and >18 years. The initial values, as well as the dynamic changes of BW-normalized cardiac output, BW-normalized blood volume, and BW, were obtained from the literature (Table II), and estimates are provide in Table III and Fig. 4. The RBC lifespans are estimated to be 62, 74, 82, and 86 days at age 2, 5, 8, and 10 years, respectively. Our model estimates that RBC lifespan is longer in males than in females (even after body weight corrections). For example, the mean estimated RBC lifespan is 133 and 110 days, respectively, in men and women. This gender difference in RBC lifespan estimation is mainly caused by the gender difference in BW-normalized blood volume. As the BW-normalized blood volume in women is less than that in men (65 vs 75 ml/kg) (16,17), RBC in women will make more trips per unit time and reach N max more quickly resulting in shorter RBC lifespan. The observation that RBC senescence is accelerated in the female of the species by about 10 to 15% (21) potentially arises from the circulatory mechanism postulated here. Further investigations are warranted to evaluate gender effects on RBC lifespan.
Model-estimated RBC lifespan in different age groups in human. a RBC lifespans that were estimated using only Eqs. 12–15 without a correction function. b Estimated RBC lifespans with a correction function applied. For the 0–1-year-old group, the correction function is y = 1 − 0.1265*t (in years). For the 1–10-year-old group, the correction function is y = 1.1897 − 0.0204*t (in years). For the 10–18-year-old group, the correction functions are y = 1.0362 − 0.00214*t (in years) and y = 1.3502 − 0.01793*t (in years), for boys and girls, respectively
Figure 4 depicts RBC lifespan as a continuous function from infants to adulthood. Because different equations were used for the four different age groups, there are discontinuities between the RBC lifespan estimates at the age group transitions (Fig. 4a). Because the best equation for estimating the true RBC lifespan at the transition points is currently unclear, the mean value of the two estimates offered as the best estimate and linear correction functions were utilized such that the final predictions of RBC lifespan at 1 year, 10 years, and 18 years are equal to the mean value of the estimates obtained by the two equations. The linear correction function is expressed as Y = a − b*t (t is age in years). Children at a different age will have a specific Y value which is obtained by plugging age (in years) in the equation. The corrected RBC lifespan in each age group is then calculated as follows: estimated RBC lifespan × Y.
For the 0–1-year-old group, the correction function is y = 1 − 0.1265*t (in years). For the 1–10-year-old group, the correction function is y = 1.1897 − 0.0204*t (in years). For the 10–18-year-old group, the correction functions are y = 1.0362 − 0.00214*t (in years) and y = 1.3502 − 0.01793*t (in years), for boys and girls, respectively. The final predicted RBC lifespan with correction factors is also provided in Fig. 4b.
The final estimated RBC lifespan with correction factors was further evaluated against the square root of age (i.e., \( \sqrt{t} \)). The fitting was done in SigmaPlot using the built-in Weibull function. The Weibull function used to fit the data is listed as the following:
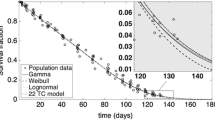
As shown in Fig. 5a, b, Weibull function can capture the whole data range sufficiently. The estimated parameters (a, b, c, d, and e) for male and female are listed in Table IV. The advantage of using this Weibull equation is that RBC lifespan can be quickly estimated as long as the age is known and the estimates across all age groups can be made using only one equation.
The estimated RBC lifespan with correction factors vs the square root of age (\( \sqrt{t} \)) in a male and b female. Weibull function was used to fit the data. The estimated parameters (a, b, c, d, and e) are listed in Table IV
DISCUSSION
In the current study, we successfully developed a novel physiology-based mathematical model capable of easily and quickly estimating human RBC lifespan at any age. Consistent with the N max assumption, the model estimates that the younger the age, the shorter the RBC lifespan (Fig. 4). For example, the model generated the following estimated RBC lifespans in 2-year-old, 5-year-old, 8-year-old, and 10-year-old children: 62, 74, 82, and 86 days, respectively. Our model may have important clinical implications in anemic neonates, where RBC survival information needs to be quickly applied, and diabetic pediatric patients, whose HbA1c level ties closely to RBC lifespan.
Several methods for measuring RBC lifespan have been developed (22,23). The first reliable method to determine RBC lifespan was developed by Winifred Ashby in 1919 using differential agglutination of RBC with antibodies to antigens on the RBC surface (6). This technique is accurate but cumbersome to perform and cannot be applied to transfusion of autologous RBC (e.g., to assess the effect of RBC storage media). As a result, the differential agglutination was largely replaced in the 1950s by methods based on labeling of RBC with a radioactive isotope. Among the radioactive isotopes, the one most commonly used is chromium 51 (51Cr) (23). Although the 51Cr method has been the reference method for determination of RBC survival for several decades, 51Cr method has disadvantages, including the following: (1) 51Cr binds reversibly to hemoglobin and elutes from RBCs at rates that vary unpredictably among individuals from 0.5 to 2.5% per day, leading to substantial error in measurement of RBC survival; (2) only one population of RBC can be studied simultaneously in the same subject; and (3) exposure of subjects to radiation (24,25). Because radiation exposure for research purposes has become ethically unacceptable in studies of vulnerable populations such as infants, children, and pregnant women (23), studies in these populations are no longer acceptable to regulatory bodies in Europe and the USA.
In the past two decades, multiple studies have provided strong evidence that labeling RBC with nonradioactive biotin is practical, safe, and accurate for measuring RBC survival, even in vulnerable populations (7,26,27). Recently, our group successfully measured autologous RBC in adults and the post-transfusion survival of both neonatal autologous and adult allogeneic RBC in infants using the biotin method (8,9); the mean lifespan of autologous neonatal RBC was 52 days (15), which is considerably shorter than the approximated 120-day RBC lifespan observed for autologous and allogeneic adult RBC by us (9,26) and others (28). The phenomenon of shorter lifespan of fetal RBC in the fetus than that of adult RBC in adults has been reported previously (29,30). Although the mechanism(s) of the RBC lifespan differences remain to be elucidated, both intrinsic and extrinsic factors have been proposed as contributors (31,32). Our observations using the multi-density biotin method allowed a novel head-to-head comparison and provide strong evidence that extrinsic factors (e.g., factors and events in the circulatory environment) are the primary determinants of RBC survival in healthy humans at different ages.
Human RBCs in healthy adults exhibit nonrandom removal (23), exhibiting senescence that is highly dependent on RBC age, i.e., as if there were an “alarm clock” for removal of senescent cells. Although RBC senescence has been extensively studied and several mechanisms governing senescence have been proposed, there is no widely accepted model of RBC senescence (22,23). Allison reported that the total number of RBC recirculations was remarkably similar (∼1.75 × 105 cycles) in three very different animal species (rat, dog, and man) and speculated that the oxygenation/deoxygenation that RBCs experience with each circulation contributes to membrane stress and senescence (10). Li and Li (32) proposed that the RBC goes through an osmotic shrinking and swelling cycle with each circulation of the body, leading to mechanical failure of the red cell membrane. These investigators performed an in vitro experiment to evaluate the effect of osmotic stress on cell lysis (32). They found that 50% of human RBCs lyse after 1.0 × 105 osmotic cycles (i.e., N max = 2.0 × 105 cycles), a number very close to that of Allison’s (10).
In the present study, we extrapolated the N max concept of limited work capacity (i.e., N max is relatively constant) to neonatal RBCs and found that it also holds true in human infants. Therefore, although the exact mechanism of RBC senescence is not completely understood, it appears that the “alarm clock” for cell senescence and removal is triggered when a normal RBC circulates approximately 1.75 × 105 times. This phenomenon allows us to estimate RBC lifespan simply based on the two physiological parameters—cardiac output and blood volume—that control the number of circulatory cycles that RBCs experience per unit time.
Importantly, because of this purely extrinsic design feature of the model, we speculate that the model offers a useful tool for comparative evaluation of the contribution of intrinsic factors expressed as differences in the LS parameter. Our model for RBC LS estimation may have important clinical applications in diabetes research. For example, it is known that the level of HbA1c is dependent on not only blood glucoses but also the mean age of RBC in the circulation. It has been reported that HbA1c increases linearly with the increase in mean RBC age, with the values ranging from very low in reticulocytes to several folds higher in the oldest RBC (4). Although RBC lifespan is a known determinant of HbA1c level, its variation has been considered insignificant to impact clinical decisions. This may not be the case based on our model estimation, which clearly indicates that RBC lifespans are much shorter in the pediatric population compared to adults. For example, based on our model estimation, the RBC lifespan in an 8-year-old child is around 82 days, which is only two thirds of the adult RBC lifespan (120 days). As the mean age of RBC in the circulation of children is considerably younger, even the blood glucose level is same as that in adults, the percentage of HbA1c will be lower in children. Therefore, we estimate that, without taking RBC lifespan into account, with current cutoff values of HbA1c for diagnosis, children are prone to have false negatives for diabetes diagnosis compared to adults, and the younger the child, the higher the risk of missing cases of diabetes. Indeed, in line with our estimation, it has been reported that HbA1c is not as reliable as a test for identifying children with diabetes and pre-diabetes compared with adults, and using of the current cutoff value of HbA1c in children may lead to missed cases (33–35). In addition, Hosking et al. reported that HbA1c level rises with age through childhood (36). Except for infants, RBC lifespan in other children groups has not been evaluated before. As our physiologically based mathematical model can estimate RBC lifespan in different ages of children, our model could play an important role in improving diabetes diagnosis as well as improving clinical care decisions to the treatment of diabetes in children.
Cohen et al. evaluated RBC survival in six healthy adults and six adults with diabetes mellitus (4). They found that the RBC mean age (i.e., T 50) in these two populations is very close: T 50 = 50.7 ± 6.9 days in healthy adults and 47.9 ± 6.0 days in subjects with diabetes mellitus (the difference is only 5.5%, p = 0.47). Based on Cohen’s data, we infer that diabetes has minimal impact on cardiac output and blood volume. In addition to diabetes, it seems that cancer also has little impact on cardiac output and blood—Luten et al. (2) evaluated the RBC survival in 10 cancer patients, and they found that the mean RBC lifespan is 116 days (with CV% of 13.9%), which is in line with the RBC lifespan of 120 days observed in healthy subjects. Additionally, Korell et al. evaluated RBC survival in both patients with chronic kidney disease and healthy subjects using 51Cr method and, their data showed that there is little difference in disappearance of 51Cr label from the circulation between the CKD patients and healthy subjects (37), indicating minimal impact of chronic kidney disease on cardiac output and blood volume. Based on the information listed above, it is reasonable to speculate that our model can be used to estimate RBC lifespan in not only healthy subjects but also patients with diabetes, cancer patients, and patients with chronic kidney disease. Overall, there is very limited information on effect of disease state on cardiac output and blood volume. For other diseases and conditions that have not as yet been tested, cautions are needed when the model is applied to those patients.
The current version of our mathematical model for RBC lifespan estimation has important limitations. The model estimates that the age-dependent RBC lifespan uses population mean value of cardiac output and blood volume in different ages of healthy subjects. If a patient has certain diseases where cardiac output and/or blood volume are influenced, the patient-specific cardiac output and blood volume need to be obtained before using this mathematical model to estimate the RBC lifespan in that particular patient. In addition, our model is not suitable for estimating RBC lifespan in patients who have abnormal RBC lifespan due to abnormal RBCs (i.e., intrinsic factors). An example of this is sickle cell disease. For patients with sickle cell disease, their red cells are sickle-shaped, which are not flexible and cannot change shape easily. As a result, those cells tend to burst apart or hemolyze and have a much shorter lifespan (10–20 days) than the normal RBCs estimated by our model (38).
Another limitation of our current model is that it only estimates the mean age-dependent RBC lifespan. Overall, the variability of RBC survival is reported to be low. Using the biotin method, we found that the average RBC lifespan is 52.1 days with CV% of 20.7% in infants (15) and 121.8 days with CV% of 6.9% in adults (9). Cohen et al. also found that the variability of RBC mean age is relatively low, with CV% of 13.6% in healthy adults and 12.5% in DM subjects (4). We also evaluated RBC survival using 51Cr method, and the RBC lifespan is 116.0 days with CV% of 13.8% (26).
In summary, we constructed a novel physiologically based mathematical model which can be used to quickly estimate RBC lifespan in different age groups in humans. Our model may also have important clinical applications that are especially relevant to neonatal anemia and diabetes in the pediatric population. Future work will require the following: (1) validating our model by conducting clinical trials to evaluate RBC lifespan in children and adolescents, and incorporating the variability of RBC lifespan in the model after the clinical data in children and adolescents is available; (2) strengthening our model by incorporating extrinsic factors (inflammation, oxygen injury, disease states, biomarkers, and other as yet unknown factors), along with intrinsic factors (e.g., RBC deformability and mean corpuscular volume) as covariates in our model, potentially allowing estimating RBC lifespan in not only healthy subjects but also patients with specific diseases. Further extension of this research will be important in better defining HbA1c as a biomarker for diabetes by correcting for the influence of RBC lifespan estimable by this and future work.
Abbreviations
- RBC:
-
red blood cell
- CO:
-
cardiac output
- BV:
-
blood volume
- BW:
-
body weight
- BioRBCs:
-
biotin-labeled red blood cells
References
Franco RS, Yasin Z, Lohmann JM, et al. The survival characteristics of dense sickle cells. Blood. 2000;96(10):3610–7.
Luten M, Roerdinkholder-Stoelwinder B, Schaap NP, de Grip WJ, Bos HJ, Bosman GJ. Survival of red blood cells after transfusion: a comparison between red cells concentrates of different storage periods. Transfusion. 2008;48(7):1478–85. doi:10.1111/j.1537-2995.2008.01734.x.
Strauss RG, Mock DM, Widness JA, Johnson K, Cress G, Schmidt RL. Posttransfusion 24-hour recovery and subsequent survival of allogeneic red blood cells in the bloodstream of newborn infants. Transfusion. 2004;44(6):871–6. doi:10.1111/j.1537-2995.2004.03393.x.
Cohen RM, Franco RS, Khera PK, et al. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood. 2008;112(10):4284–91. doi:10.1182/blood-2008-04-154112.
Khera PK, Smith EP, Lindsell CJ, et al. Use of an oral stable isotope label to confirm variation in red blood cell mean age that influences HbA1c interpretation. Am J Hematol. 2015;90(1):50–5. doi:10.1002/ajh.23866.
Ashby W. The determination of the length of life of transfused blood corpuscles in man. J Exp Med. 1919;29(3):267–81.
Mock DM, Widness JA, Veng-Pedersen P, et al. Measurement of posttransfusion red cell survival with the biotin label. Transfus Med Rev. 2014;28(3):114–25. doi:10.1016/j.tmrv.2014.03.003.
Widness JA, Nalbant D, Matthews NI, et al. Tracking donor RBC survival in premature infants: agreement of multiple populations of biotin-labeled RBCs with Kidd antigen-mismatched RBCs. Pediatr Res. 2013;74(6):689–97. doi:10.1038/pr.2013.163.
Mock DM, Matthews NI, Zhu S, et al. Red blood cell (RBC) survival determined in humans using RBCs labeled at multiple biotin densities. Transfusion. 2011;51(5):1047–57. doi:10.1111/j.1537-2995.2010.02926.x.
Allison AC. Turnovers of erythrocytes and plasma proteins in mammals. Nature. 1960;188:37–40.
Hirsimaki H, Kero P, Wanne O, Erkkola R, Makoi Z. Doppler-derived cardiac output in healthy newborn infants in relation to physiological patency of the ductus arteriosus. Pediatr Cardiol. 1988;9(2):79–83. doi:10.1007/BF02083704.
Sisson TR, Lund CJ, Whalen LE, Telek A. The blood volume of infants. I. The full-term infant in the first year of life. J Pediatr. 1959;55(2):163–79.
de Simone G, Devereux RB, Daniels SR, et al. Stroke volume and cardiac output in normotensive children and adults. Assessment of relations with body size and impact of overweight. Circulation. 1997;95(7):1837–43.
Leffler S, Hayes M. Analysis of parental estimates of children’s weights in the ED. Ann Emerg Med. 1997;30(2):167–70.
Kuruvilla DJ, Widness JA, Nalbant D, Schmidt RL, Mock DM, Veng-Pedersen P. A method to evaluate fetal erythropoiesis from postnatal survival of fetal RBCs. AAPS J. 2015;17(5):1246–54. doi:10.1208/s12248-015-9784-y.
Williams L. Reference values for total blood volume and cardiac output in humans. 1994.
http://reference.medscape.com/calculator/estimated-blood-volume
http://www.disabled-world.com/artman/publish/height-weight-teens.shtml
Widness JA, Kuruvilla DJ, Mock DM, Matthews NI, Nalbant D, Gress GA, et al. Autologous Infant and Allogeneic Adult Red Cells Demonstrate Similar Concurrent Post-Transfusion Survival in Very Low Birth Weight Neonates. J Pediatr. 2015;167(5):1001–6.
Sisson TR, Whalen LE, Telek A. The blood volume of infants. II. The premature infant during the first year of life. J Pediatr. 1959;55:430–46.
Landaw SA. Factors that accelerate or retard red blood cell senescence. Blood Cells. 1988;14(1):47–67.
Franco RS. Measurement of red cell lifespan and aging. Transfus Med Hemother: Off Organ Dtsch Ges Transfusionsmed Immunhamatol. 2012;39(5):302–7. doi:10.1159/000342232.
Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84(2):109–14. doi:10.1002/ajh.21298.
Recommended methods for radioisotope red-cell survival studies. A report by the ICSH panel on diagnostic applications of radioisotopes in haematology. Br J Haematol. 1971;21(2):241–50.
Mollison P, Engelfriet C, Contreras M. The transfusion of red cells. Blackwell Scientific Publications; 1987. p 99–115.
Mock DM, Lankford GL, Widness JA, Burmeister LF, Kahn D, Strauss RG. Measurement of red cell survival using biotin-labeled red cells: validation against 51Cr-labeled red cells. Transfusion. 1999;39(2):156–62.
Hoffmann-Fezer G, Maschke H, Zeitler HJ, et al. Direct in vivo biotinylation of erythrocytes as an assay for red cell survival studies. Ann Hematol. 1991;63(4):214–7.
Mollisson P. Blood transfusion in clinical medicine. 7th ed. Blackwell Scientific Publications; 1983. p. 93–110.
Pearson HA. Life-span of the fetal red blood cell. J Pediatr. 1967;70(2):166–71.
Hollingsworth JW. Lifespan of fetal erythrocytes. J Lab Clin Med. 1955;45(3):469–73.
Egberts J, Hardeman MR, Luykx LM. Decreased deformability of donor red blood cells after intrauterine transfusion in the human fetus: possible reason for their reduced life span? Transfusion. 2004;44(8):1231–7. doi:10.1111/j.1537-2995.2004.04014.x.
Li CK, Li EK. Mechanical fatigue as a possible determinant of in vivo longevity of red blood cells. IEEE Trans Biomed Eng. 1983;30(4):226–8.
Lee JM, Wu EL, Tarini B, Herman WH, Yoon E. Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr. 2011;158(6):947–52 e1-3. doi:10.1016/j.jpeds.2010.11.026.
Nowicka P, Santoro N, Liu H, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care. 2011;34(6):1306–11. doi:10.2337/dc10-1984.
Vehik K, Cuthbertson D, Boulware D, et al. Performance of HbA1c as an early diagnostic indicator of type 1 diabetes in children and youth. Diabetes Care. 2012;35(9):1821–5. doi:10.2337/dc12-0111.
Hosking J, Metcalf BS, Jeffery AN, Streeter AJ, Voss LD, Wilkin TJ. Divergence between HbA1c and fasting glucose through childhood: implications for diagnosis of impaired fasting glucose (Early Bird 52). Pediatr Diabetes. 2014;15(3):214–9.
Korell J, Vos FE, Coulter CV, Schollum JB, Walker RJ, Duffull SB. Modeling red blood cell survival data. J Pharmacokinet Pharmacodyn. 2011;38(6):787–801. doi:10.1007/s10928-011-9220-6.
McCurdy PR, Mahmood L, Sherman AS. Red cell life span in sickle cell-hemoglobin C disease with a note about sickle cell-hemoglobin O ARAB. Blood. 1975;45(2):273–9.
Acknowledgments
The authors acknowledge the helpful discussions with Robert M. Cohen, MD (University of Cincinnati), and Robert S. Franco, PhD (University of Cincinnati), regarding the clinical applications of the present study. This work was supported in part by National Institutes of Health (NIH) US Public Health Service Program Project Grant 2 P01 HL046925 and the National Center for Research Resources, a part of the NIH, Grant Number UL1TR000039. We acknowledge Sysmex for the loan of their hematology analyzer. We thank Iowa’s neonatal nurse research team. We also acknowledge the research laboratory teams in University of Iowa and University of Arkansas for Medical Sciences. We are grateful to the families of study subjects in allowing their infants to participate.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
An, G., Widness, J.A., Mock, D.M. et al. A Novel Physiology-Based Mathematical Model to Estimate Red Blood Cell Lifespan in Different Human Age Groups. AAPS J 18, 1182–1191 (2016). https://doi.org/10.1208/s12248-016-9923-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-9923-0