ABSTRACT
The increasing use of nanotechnology, including nanoparticles, in the preparation of drug products requires both manufacturing and analytical considerations in order to establish the quality metrics suitable for performance and risk assessment. A range of different nanoparticle systems exists including (but not limited to) nano-drugs, nano-additives, and nano-carriers. These systems generally require more complex production and characterization strategies than conventional pharmaceutical dosage forms. The advantage of using nanoparticle systems in pharmaceutical science is that the effective and desired function of the material can be designed through modern manufacturing processes. This paper offers a systematic nomenclature which allows for greater understanding of the drug product under evaluation based on available data from other nanoparticle reports. Analytical considerations of nano-drugs, nano-additives, and nano-carriers and the way in which they are measured are directly connected to quality control. Ultimately, the objective is to consider the entire nano-drug, nano-additive, and nano-carrier product life cycle with respect to its manufacture, use, and eventual fate. The tools and approaches to address the needs of these products exist; it should be the task of the pharmaceutical scientists and those in related disciplines to increase their understanding of nanomedicine and its novel products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Therapeutic agents on the nanoscale are increasingly widespread in the biomedical and pharmaceutical industry (1–3). The first FDA-approved nanoscale drug, Doxil, was a liposome formulation produced in 1995 for passive targeting of solid tumors (e.g., AIDS-related KS, myeloma) (4–8). Since then, “old” drugs that fit today’s nano-drug criteria have emerged with new names and a myriad of new ones have also been developed (4). For example, currently approved drugs that meet today’s nanomedicine criteria have been re-introduced to the market with revised labels. In addition, currently approved drugs that meet today’s nanomedicine criteria have been reformulated to incorporate nanomedicine-based features. Nanoscale therapeutic agents are generally regarded as a part of the field of nanomedicine—the application of nanotechnology to prevent, diagnose, or treat disease in the human body.
The overlap of nanotechnology and biomedical research gave rise to the field of nanomedicine, of which nano-drugs, nano-additives, and nano-carriers are major components. This paper highlights the most recent perspectives in the area of manufacturing and analytical characterization of nanoparticle systems in the field of nanomedicine by assessing their performance, risk, and life cycle.
NANOMEDICINE NOMENCLATURE AND CATEGORIZATION
Nanomedicine is the medical application of nanoscience and is inclusive of drugs (or pharmaceuticals or therapeutic agents), devices, sensors, and machines. A nano-drug is an example of a therapeutic agent that is the drug alone on the nanoscale; can be a hard solid (e.g., tablets), soft solid (ointments), liquid (suspensions), or gas (aerosol); it can also be referred to as a nano-pharmaceutical. A nano-additive is an inactive ingredient or excipient on the nanoscale that are added to dosage forms. A nano-carrier is a non-drug component(s) prepared as nanoparticle(s) in which the drug is either dispersed (in a single particle) or to which drug is added (particles of drug and carrier). Both nano-additives and nano-carriers are often proposed as a transport module for a drug.
Therapeutic agents within nanomedicine encompass an extensive list of dosage forms, which can be classified under the following categories: nano-drugs (or pharmaceuticals) abbreviated as “ND,” nano-additives (or excipients) abbreviated as “NA,” and nano-carriers (or drug carrier systems) abbreviated as “NC” (9,10). NDs can include suspensions of nanoparticles and nanocrystals, among other entities. Suspensions of nanoparticles in a solvent (e.g., water) persist as aqueous dispersions due to the very low solubility of the drug in water. Nanocrystals are examples of NDs and can be described as very small particles of the drug in solid, crystal formation.
Drugs, additives, and carrier systems on the nanoscale are designed for very specific applications (11,12). To that end, the agents can include either active ingredients or a mixture of active and inactive ingredients. In nanomedicine, an inactive ingredient is often referred to as an NA. NAs are described as natural or synthetic substances formulated with active pharmaceutical ingredients (APIs) of a therapeutic agent. The purpose of the excipient depends on the formulation and application of the drug and can include characteristics such as stability, long-term storage, adding mass in the form of “fillers” or “diluents”), or increasing bioavailability (i.e., absorption or solubility).
NCs are drug delivery systems that carry the API by encapsulating it within the core of a platform or bonded to the surface (13,14). There are several subtypes of NCs: solid-lipid particles, self-assembled structures, polymer particles, and metal particles (15,16). Each subtype has unique qualities that render it more or less effective with particular APIs. Finally, there are several other nanotechnologies currently under research and development, whose support from private and public sector investments amount to more than an estimated US$12.4 billion (17,18).
MANUFACTURING PERSPECTIVES OF NANO-DRUGS, NANO-ADDITIVES, AND NANO-CARRIERS
Therapeutic agents on the nanoscale are individually complex in terms of component, function, and performance. The six components that dictate the function and performance of NDs, NAs, and NCs include the following:
-
1.
The presence and concentration of the active ingredient (small or large molecular weight)
-
2.
Surface properties of the drug, additive, or carrier
-
3.
Chemical composition of the drug, additive, or carrier
-
4.
Presumed targeting moieties (subcellular or cellular)
-
5.
Physical formulation (solid or liquid)
-
6.
Route of administration
NDs, NAs, and NCs can be created using a “bottom-up” (i.e., molecular assembly) or a “top-down” approach (i.e., attrition) (19–21). A bottom-up approach is often described as a small molecule placed in a solvent and undergoes seeded growth under high temperature and pressure. Once the seed grows to the optimal size, the reaction is quenched and the resultant particles are isolated and subsequently formulated for its applications. A top-down approach is described as milling, grinding, or crushing a micrometer-sized material or larger down to a particle on the nanoscale. In some cases, the most desirable endpoint is creating a homogeneous size population (22–24); however, this is not always the rationale case. Recent published data suggest that certain applications benefit from differing shape profiles (i.e., non-spherical particles) or distributions (25–27). A particle system is homogeneous in its size population when the particles’ primary diameter is uniform throughout the entire contents (i.e., in one, two, or all three dimensions).
There are notable advantages to creating a homogeneous nanoparticle system (28,29). The surface area of all individual particles is the same as monodisperse particles; therefore, surface functionalization or “capping agent” molarity will be the same per particle when formulated for a specific application. Also, a homogenous particle system ensures reproducibility and a high level of quality control.
The pharmaceutical preparation of an ND has a number of pharmaceutical drivers; for compounds whose water solubility or dissolution rate limits their oral bioavailability, size reduction to the nanoscale can increase in vivo dissolution rate and fraction absorbed (30). Poorly soluble drugs can be “milled” to a specified size and size range resulting in not only useful bioavailability but also sustained release features.
The development of NDs often involves a top-down approach in which the active ingredient is milled (or otherwise subjected to particle reduction strategies) in either an aqueous environment or in a dry formulation. Some people consider top-down strategies to be more controllable and more robust as a function of process and design space for this type of manipulation (21). However, there are bottom-up processes (e.g., micelle formation through molecular self-assembly) that are extremely controlled and top-down strategies (e.g., emulsion formation through shear dispersion) that may not be as easily controlled (31,32). If a solid active ingredient is milled, it is ground under high energy in the presence of a milling media such as highly reticulated polystyrene or zirconium milling beads. Milling is generally monitored by an appropriate particle-sizing technique, and the experimental design is defined by milling curves first at small scale and then at production scale; the production scale development of nanoscale formulations when completed using ball milling usually involves high-energy mills which can recirculate the material, re-milling it until a consistent product is generated. The milling media can also abrade under the conditions of milling; care should be taken that significant contamination of the suspension by the milling media does not occur. The process of high-energy milling can be carried across scales from 1 mL to more than 1000 L; scaling is a function of the product and the equipment (22–24). NAs may be prepared in a similar manner to NDs prior to the inclusion in a formulation. A variety of constructive methods are employed to prepare NCs and NAs including extrusion for liposomes (33), microfluidization for micelles (34), and precipitation for polymers (35,36).
NDs, NAs, and NCs are manufactured in either batch-to-batch loads or in a continuous process. Operations have historically been batch processes where each step introduces a source of error impacting the quality of the product. In nanoscale drug and carrier production, continuous processes could be adopted; the design space should dictate the process control; the process control should dictate the product quality.
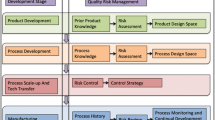
In general, design space development requires consideration of the critical quality attributes of the product usually related to composition, structure, and stability, in the context of the target product profile as illustrated in Fig. 1. The sequence shown illustrates in broad terms the critical quality attributes that would be considered to ensure that the target product profile is met and the performance assured. These considerations are familiar to most pharmaceutical scientists. The principle of experimental design employed for product optimization can be illustrated from the literature where a potential asthma therapy, consisting of nanoscale ipratropium bromide poly(lactide-co-glycolide) constructs as spray-dried particles, was developed through a particle engineering approach to achieve optimal particle morphology, physicochemical, dissolution, and aerodynamic characteristics to extend the duration of bronchodilation in an animal model (35).
Attributes at all scales of scrutiny: Drug, carrier, and dose require consideration of critical quality attributes to address the desired biological outcome and ensure the quality, efficacy, and safety of the product. This scheme illustrates the way in which a continuous process can be linked to a desired therapeutic outcome, thereby demonstrating the adoption of critical quality attributes that support the target product profile (38–40)
Poly(lactic acid) particles (5 and 30% polymer) incorporating ipratropium bromide (95 and 70% drug, respectively) were prepared by spray drying (Buchi B2900 Spray drier) and selected for evaluation in a guinea pig model of bronchodilation following the evaluation of the dissolution properties of a number of particle formulations. The spray-drying procedure required optimization of the manufacturing variables according to a multivariate statistical experimental design.
It was possible to map the process space for feed flow rates and feedstock concentrations to attain the desired particle size and surface area attributes of engineered inhalation particles based on this experimental design study. Zanen et al. have demonstrated that the optimum particle sizes for inhaled IPB would be 2.8 μm (MMAD), hence the range of 2.5 to 3 μm (37). Regions of interest for particle surface area would be as high as possible based on the likelihood that these would be the most porous particles, but based on limitations of this equipment and formulation/solvent system, a field between 2.5 and 3.6 m2/g was chosen. This process space was mapped, and any combination of process parameters can be selected to yield particles of the desired attributes.
In guinea pig studies of bronchodilation, an apparent difference was noted between the 5% (w/w) PLA and 30% (w/w) poly(lactic acid) particles for time of onset (1.67 vs. 9.78 min, respectively) and duration of effect (13 vs. 54 min, respectively). A statistical difference (Student’s t test, p < 0.05) was not noted for any of the measures for pulmonary function. However, a trend was noted for the duration of effect (Student’s t test, p < 0.1).
Spray drying and coating methods are used to generate hollow, spherical, porous ipratropium bromide/glycine particles within a respirable range (1–5 μm). Engineered, sustained release particles were crystalline and demonstrated excellent physicochemical stability and a broad range of release profiles (patent application submitted). Experimental design techniques were utilized to identify critical manufacturing variables and optimize process to yield desirable particle attributes. In vivo evaluation showed bronchoprotection trends for time of onset and duration of effect for poly(lactic acid)-coated particles with respect to ipratropium bromide alone. The nanoparticles helped carry the drug through the mucus to the epithelial surface following the deaggregation of the micro-aggregate. This case study illustrates the way in which a continuous process, through the application of multivariate statistical experimental design, can be linked to a desired therapeutic outcome, thereby demonstrating the adoption of critical quality attributes that support the target product profile (38–40). This example of incorporation of nanoparticles into a functional dosage form points out the importance of the final product characteristics in addition to the substituent nanoparticle component.
Incorporation of nanoparticles into drug delivery applications is facilitated by reference to available data on nanomaterial. Curated databases, such as the Nanomaterial Registry (https://www.nanomaterialregistry.com/) and CaNanoLab (https://cananolab.nci.nih.gov/), allow efficient access to a large amount of available data (41,42).
There are many different ways that researchers report physicochemical properties. For example, particle size may be reported as a composite (aggregate) diameter and primary particle size, in terms of mean hydrodynamic diameter (from dynamic light scattering measurements) or projected area diameter (from microscopic measurements) (43,44). The complexity of characterizing nanoparticles has been addressed through structured databases. The curated NIH Nanomaterial Registry considers a range of properties defining key characteristics of nanoparticles (NIH https://www.nanomaterialregistry.org/). Data curation involves not only the property (PS) but also the measurement technique and parameters. Figure 2 illustrates the way in which the Nanomaterial Registry approaches the data and the terms employed for describing nanoparticle systems (45,46). This data structure allows the examination of trends or identification of information that may not have been easily accessible before the data were integrated. Ideally, this approach will allow decision-making and risk management strategies to be developed as increasing amounts of data are curated. It is evident that nanoparticles exist in large populations and are found in a variety of environments or conditions. As a consequence, the way in which data are collected and expressed depends on the following considerations:
Nano-drugs, like other nanoparticles, exist in a population (p) within an environment (e) that is indistinguishable to the observer. Therefore, their measured characteristic is a function of the way in which they are observed, ƒ (p,e). The information about nanoparticles must consider the following important details: (1) the fundamental property being measured, (2) the unique way the property is expressed, (3) the measurement technique itself and the instrument employed, and (4) the way in which the sample is collected and prepared for examination
-
1.
Physicochemical property being measured
-
2.
The manner in which the property is reported
-
3.
Measurement technique and the instrument employed
-
4.
The record in which the sample is collected and prepared for examination
Not all data are published with the same attention to detail. Consequently, curated databases can ascribe measures of confidence to the data to assist those using the database with respect to adequacy of the descriptors of the experiment. This approach is the first step in assuring the quality of the measurement and data generated. Simply stating a size, without attention to the method, instrument, and procedures employed, would be least valuable as it would be difficult to repeat or compare to other studies. In contrast, conducting an experiment under a known protocol such as those promulgated by the ASTM International (https://www.astm.org/) or the Nanotechnology Characterization Lab within the National Cancer Institute (http://ncl.cancer.gov/) in which specification are supplied would be the most valuable and therefore give the greatest degree of confidence in the data acquired.
In summary, registries can be employed to explore aggregated publicly available data to inform decision-making for the application of interest to the researcher. In serving this purpose, they minimize unnecessary duplication of effort in areas where data are numerous and maximize the value of new experimental data obtained. Moreover, comprehensive databases allow gaps to be identified and guide exploration of less studied nanomaterial compositions, properties, and outcomes to serve the application of interest, e.g., efficacy, safety, environmental disposition, or risk assessment, thereby expanding the general knowledge of nanomaterials for the benefit of the scientific community and society.
ANALYTICAL PERSPECTIVES OF NANO-DRUGS, NANO-ADDITIVES, AND NANO-CARRIERS
Nanoscale therapeutic agents are typically characterized by their physicochemical and functional properties. More importantly, physicochemical attributes of ND directly influence its functionality (44,47). Physicochemical properties may furthermore have an impact on the following aspects of ND, NA, or NC: bulk properties (i.e., Born forces, viscosity), process, performance, stability, and appearance. The emphasis on physicochemical properties of ND products is necessary in that scientists and engineers must understand the degree to which changes in physicochemical properties can impact ND but also how these changes influence their function (44,47). Given that the functional and physicochemical properties of nanoparticles are inherently different from one another, understanding the physical properties of pharmaceuticals is key for successful drug and process development.
When characterizing NDs, NAs, and NCs, there are several characteristics that influence the behavior of the particle in a system. Pharmacokinetics is described as the movement of drugs in the body (i.e., the processes of absorption, distribution, metabolism, and excretion (ADME)). These kinetics can be influenced by the physicochemical properties of the NDs, NAs, and NCs including particle size, composition, morphology, charge, surface, and stability (47). The size of the therapeutic agent primarily influences pharmacokinetics in that it dictates the amount of molecular absorption of the drug to the surface of the nano-entity. Similarly, the chemical and physical chemical composition of the particle controls the activity of the chemical, which in turn influences the drug’s pharmacokinetics (43).
Morphology, as it relates to therapeutic agents on the nanoscale, can be described as the physical form, shape, and/or structure(s) of NDs, NAs, and NCs. Structure is dependent on the electron configuration (i.e., the distribution of electrons of an atom, molecule, or nanoparticle in molecular orbitals), which also affects the pharmacokinetic behavior of the drug.
The surface charge of NDs, NAs, and NCs also affect pharmacokinetics. For nano-carriers, charge may be of tremendous influence on the amount of drug absorbed to the platform’s surface. For NDs, charge can change bioavailability by increasing dissolution or improving localization of particles to certain tissues or cell types. The stability of any ND, NA, or NC can be altered synthetically and is dependent on the biological matrix.
The relative contribution of each physicochemical property to the pharmacokinetics of the NDs, NAs, and NCs is important in understanding the functional differences that exist among the different types of therapeutic agents on the nanoscale. One example of a nano-carrier for targeted drug delivery is gold nanoparticles engineered to sense and deliver drugs to tumors (48). To date, there are four types of gold particles used in nanomedicine applications: gold with cationic side chains, with anionic side chains, PEG-coated gold, and peptide-linked gold. Important proteins (i.e., TNF-alpha and transferrin) that aid NDs in selective cellular intake into cancer cells have also been incorporated onto the gold particle surfaces (49).
The function of most NDs is also dependent on the active pharmaceutical ingredients and/or excipients within the therapeutic agent. For this reason, understanding the effects of NDs, NAs, and NCs requires the characterization of both active and inactive components. Such methods are specific for analyzing ingredients as either molecules or particles. On the molecular level, physical characterization is measured in several ways: melting and boiling points, solubility, pH, and electrical conductivity. Spectroscopy (e.g., circular dichroism, Raman, fluorescence, Fourier transform, mass, and photoelectron) is commonly used to characterize molecular components of a ND, NA, and NC (47,50). The aspects covered also have a profound impact on the stability of nanoparticles.
Physicochemical characterization of the particle requires the use of other methods, as well, including microscopy (e.g., optical, electron, atomic force, or scanning probe microscopy); scattering techniques (e.g., X-ray or light); thermal analysis (e.g., differential scanning calorimetry or thermogravimetric analysis); surface area and porosity measurements; flow characteristics (e.g., density, pourability, dustability, and viscosity); and storage conditions (e.g., partitioning coefficients, vapor pressure, and combustibility).
Chemical properties on the nanoscale can be significantly different from chemical properties of the same material on the micrometer or larger size scales; therefore, nanoparticles offer attractive and potentially useful applications that are unavailable when the particle is larger in size. A chemical property of a ND, NA, and NC is described as a characteristic of a particle that is observed during a reaction in which the chemical composition is changed (e.g., combustibility, solubility, redox capacity, and toxicity). Many studies have shown that substances on the nanoscale are more combustible and soluble/suspendable, have higher redox capacities, and exhibit differential toxicities when compared to the same substance but as a larger bulk material (44,51–53).
On the other hand, the measurement of a ND, NA, and NC morphological property gives information on the arrangement of matter in a sample. For instance, size and shape are morphological characteristics and, on the nanoscale, they are typically measured through microscopy, tomography, and light scattering techniques. Another physical property of importance is the surface of the particle, which includes the specific surface area, zeta potential as a measure of surface charge, interfacial reactions, solubility, and pH/iso-electric point. Both chemical and morphological properties provide unique insights into the composition and function of therapeutic agents on the nanoscale.
Another important property of NDs, NAs, and NCs is their tendency to aggregate. One way to measure this phenomenon is through photon correlation spectroscopy or quasi-elastic light scattering. The detector measures fluctuations in the scattered laser light due to the Brownian motion. This type of motion is the apparently a random motion of particles suspended in a gas or liquid. Light scattering methods are based on the assumption of sphere-like particles. The associated algorithm does not account for particles with an aspect ratio greater than 1.
Particle size, which is typically the first property measured when engineering or characterizing nanoparticles, is commonly performed through electron microscopy. Electron microscopy yields images that are a two-dimensional representation of a three-dimensional object. Scientists obtain information about the size, morphology, and aggregation of the nanoparticles. Electron microscopy with energy dispersive X-ray spectroscopy is the most widely used surface analytical technique that gives both morphological and elemental analyses.
The surface area of a particle is another property of interest. This area represents the interface between the particle and its environment. Particle surface area is inversely related to size in that the surface area per unit volume increases as particle size decreases. Surface area measurements also provide useful information about the porosity of the particle system. Pores in the particle’s surface can result in an extremely large surface area measurement. With regard to NDs, NAs, and NCs, specific surface area is related to dissolution rates, biological activity, electrostatic properties, opacity and coloring, and shelf life. Typically, surface area is measured in dry, powder form. The most common method for measuring this employs gas adsorption and applies the Brunauer-Emmett-Teller (BET) equation. BET theory describes the physical adsorption of gas molecules in a manner that allows the derivation of specific surface area (54).
Zeta potential (ζ-potential) is a measurement of charge and is useful in interpreting and predicting the behavior of NDs, NAs, and NCs. ζ-potential is defined as the potential difference that exists between the surface of a particle in a suspension (e.g., water) and the liquid. In nanoparticle characterization, ζ-potential is also known as the boundary of the diffuse layer of ions within which the particle acts as a single entity (55,56). The order of the three layers surrounding a charged nanoparticle, from closest to farthest away, is the following: surface (of particle), stern potential, and zeta potential. ζ-potential is often related to stability, which has several implications in biomedical research (57,58).
Therapeutic agents on the nanoscale can include NDs, which are designed as an active pharmaceutical ingredient (API), or NCs, as an inactive ingredient or excipient. In this context, an excipient is an inactive ingredient(s) that serves as the medium for an active ingredient in a pharmaceutical formulation. Excipients are important in drug formulations and delivery because they must facilitate and not hinder the function and distribution of the drug. One of the uses of nano-powders is excipient (59–61). In the case where the excipient is a solid particle (and not a molecule or suspension medium), the chemical composition, size, surface charge, and morphology of the particle can affect the properties of the therapeutic agent, such as product performance, ability to be processed, stability, toxicity, and appearance. In this regard, characterization of the nanoscale inactive excipient ingredient is just as important as that of the API.
THE PRODUCT LIFE CYCLE OF NANO-DRUGS, NANO-ADDITIVES, AND NANO-CARRIERS
As a rapidly expanding field that relies on new and emerging NDs, NAs, and NCs, nanomedicine ought to have the goal of developing metrics for the purpose of building a risk management methodology for therapeutic agents on the nanoscale. One way to do this is to devise different risk-benefit analyses, which allow the comparison of the benefits to the risk of therapeutic agent. The risk-benefit analyses should follow the four key steps in traditional risk assessment of hazardous materials (i.e., hazard identification, exposure assessment, toxicity assessment, and risk characterization) but should also include an analysis of product development risk, performance risk, and regulatory risk.
Traditional risk assessment, in the context of therapeutic agents on the nanoscale, includes comprehensive data acquisition probing-induced toxicities, unintended exposures, production of hazardous waste, and contamination of water supply.
In addition to a risk-benefit analysis, a life cycle assessment of NDs, NAs, and NCs should be conducted. From research and discovery to production and manufacturing, a life cycle assessment would provide the critical information needed regarding potential exposure and adverse health effects of the therapeutic agent to occupational workers and consumer groups. After reaching consumer hands, the human use of the therapeutic agent, including intake and excretion, should be considered to identify unwanted exposure or harmful side effects to peripheral populations along the product life cycle. In addition, the fate and transport of the therapeutic agent on the nanoscale may pose environmental threats to different organisms. Disposal strategies of NDs, NAs, and NCs should be researched and recommended in the absence of environmental regulations.
In the research and development stage, NDs, NAs, and NCs are engineered with specific functional properties. Researchers and developers study the therapeutic agent before it is used as drug products by characterizing its behavior in different biological and/or ecological systems. Toxicologists test the safety in a series of assays to ensure compliance with health and safety regulations before it is mass-produced into a commercial product.
The production and manufacturing stage accounts for the development of NDs, NAs, and NCs as commercially available products. Depending on the application, the addition of the nanomaterial is intended to improve some form or function of the development of the product or in the product itself.
The biological intake and excretion of NDs, NAs, and NCs is a growing area of research; safety and efficacy are tested in both human and non-human species. Adverse outcome pathway (AOP) is a framework developed under the emerging area of systems biology and have been used as a tool used to interpret the adverse biological responses in humans and other relevant receptors (62,63). The pathways are linked to each other through initiating events, other key events, and adverse outcome(s) along the systems biology continuum (i.e., a molecular, cellular, organ, organism, subpopulation, and community).
The disposal and eventual fate of NDs, NAs, and NCs, as well as the waste that is produced during the manufacturing process, is generally the same as the disposal process for other therapeutic agents. Most of these agents are disposed through landfills and waste incineration plants, To date, the eventual fate of these agents on the nanoscale is evaluated on an individual basis, but environmental emissions (through air, water, and soil) are generally observed through the leaching of contaminants from landfills or incinerators. Each of these stages, as well as the potential routes of exposures, is depicted in Fig. 3.
The three main routes of potential hazardous exposure along ND, NA, and NC product development value chain are occupational, consumer, and environmental. Occupational exposure is specific to individuals in the workplace. Rates of contact to the therapeutic agent or its waste products are typically greater both in frequency and dose than the contact rates of consumer and environmental exposures. The Occupational Safety and Health Administration (OSHA) provides strict safety regulations and protocols; companies must follow the guidelines to prevent toxic levels of exposure and minimize risk of adverse health effects in workers. Guidance for occupational health and safety in the field of nanotechnology can be found at https://www.osha.gov/dsg/nanotechnology/nanotechnology.html. Guidance for occupational health and safety in the field of pharmaceuticals and drugs can be found at https://www.osha.gov/SLTC/hazardousdrugs/.
Consumer exposure scenarios are typically intentional (i.e., consumers are purchasing products they intend to use or apply on themselves). However, unwanted exposure can occur through the misuse and/or overuse, or as secondary side effects. The Consumer Product Safety Commission (CPSC) has developed guidance for consumers interacting with nano-enabled products. In 2015, CPSC issued a technical report on scientific studies involving nanotechnology (64).
Environmental exposure scenarios focus on the release of NDs, NAs, NCs and the associated waste products into different environmental compartments (e.g., air, water, sediment, and biota). Release of contaminants into the environment has the potential to be the most widespread exposure. However, it may be the most complex and difficult-to-study exposure scenario due to the variability in disposal procedures and natural transformation processes that make it difficult to quantify release and evaluate exposure to organisms in the global environment.
CONCLUSION
Therapeutic agents on the nanoscale include nano-drugs, nano-additives, and nano-carriers. These agents can be highly beneficial when developed and used appropriately but may also pose risks to humans and the environment if misused or disposed of improperly. Several governmental agencies and non-governmental organizations have devoted efforts to establishing guidelines for innovative testing, characterization, control, and risk assessment of engineered nanomaterials, some of which are applicable to NDs, NAs, and NCs. However, there is much debate pertaining to the overgeneralization of guidelines and recommendations for safe handling of nanoscale objects since no two nanomaterials function or behave exactly the same in biological or ecological systems. Researchers and developers in the nanotechnology field ought to provide sufficient data on NDs, NAs, and NCs as opposed to non-specific data that may overlook the inherent hazards. The design of these nanoscale agents must be optimized in order to exploit the therapeutic qualities. This thoughtful design would also allow for a thorough understanding of potential risks associated with the therapeutic agent. The advent of nanomedicine research has moved the field of nanotechnology into a new age of product development; this requires greater scrutiny to existing drug development processes and different strategies addressing novel particle-based drugs to ensure safe and effective medical applications.
References
Etheridge ML et al. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomed: Nanotechnol, Biol Med. 2013;9(1):1–14.
Foldvari M, Bagonluri M. Carbon nanotubes as functional excipients for nanomedicines: I. Pharmaceutical properties. Nanomed: Nanotechnol, Biol Med. 2008;4(3):173–82.
Duncan R, Gaspar R. Nanomedicine (s) under the microscope. Mol Pharmaceutics. 2011;8(6):2101–41.
Weissig V, Pettinger TK, Murdock N. Nanopharmaceuticals (part 1): products on the market. Int J Nanomed. 2014;9:4357.
Peer D et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–60.
Barenholz YC. Doxil®—the first FDA-approved nano-drug: lessons learned. J Controlled Release. 2012;160(2):117–34.
Haley, B. and E. Frenkel. Nanoparticles for drug delivery in cancer treatment. in Urologic Oncology: Seminars and original investigations. Elsevier;2008.
Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Delivery Rev. 2004;56(11):1649–59.
Bawarski WE et al. Emerging nanopharmaceuticals. Nanomed: Nanotechnol, Biol Med. 2008;4(4):273–82.
Tyner, K. and N. Sadrieh, Considerations when submitting nanotherapeutics to FDA/CDER for regulatory review. Characterization of nanoparticles intended for drug delivery, 2011: p. 17–31.
De Jong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomed. 2008;3(2):133.
Horcajada P et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat Mater. 2010;9(2):172–8.
Hickey, A.J. and D. Ganderton, Statistical experimental design, in Pharmaceutical Process Engineering, Informa Healthcare: New York, NY;2010 p. 197–201.
Mansour HM, Hickey AJ. Raman characterization and chemical imaging of biocolloidal self-assemblies, drug delivery systems, and pulmonary inhalation aerosols: a review. AAPS PharmSciTech. 2007;8, E99.
Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24(1):1–16.
Torchilin VP. Multifunctional nanocarriers. Adv Drug Delivery Rev. 2012;64:302–15.
Warheit DB et al. Health effects related to nanoparticle exposures: environmental, health and safety considerations for assessing hazards and risks. Pharmacol Ther. 2008;120(1):35–42.
Stebounova LV et al. Health and safety implications of occupational exposure to engineered nanomaterials. Wiley Interdisciplinary Reviews. Nanomed Nanobiotechnol. 2012;4(3):310–21.
Wang X et al. A general strategy for nanocrystal synthesis. Nature. 2005;437(7055):121–4.
Grzelczak M et al. Shape control in gold nanoparticle synthesis. Chem Soc Rev. 2008;37(9):1783–91.
Wang Y, Xia Y. Bottom-up and top-down approaches to the synthesis of monodispersed spherical colloids of low melting-point metals. Nano Lett. 2004;4(10):2047–50.
Bartlett JA et al. Summary report of PQRI workshop on nanomaterial in drug products: current experience and management of potential risks. AAPS J. 2014;17(1):44–64.
Merisko-Liversidge E, Liversidge GG. Nanosizing for oral and parenteral drug delivery: a perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv Drug Deliv Rev. 2011;63(6):427–40.
Van Eerdenbrugh B et al. Characterization of physico-chemical properties and pharmaceutical performance of sucrose co-freeze–dried solid nanoparticulate powders of the anti-HIV agent loviride prepared by media milling. Int J Pharm. 2007;338(1):198–206.
He Q, Shi J. Mesoporous silica nanoparticle based nano drug delivery systems: synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J Mater Chem. 2011;21(16):5845–55.
Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro-and nanoscale drug delivery carriers. J Controlled Release. 2007;121(1):3–9.
Bemis GW, Murcko MA. The properties of known drugs. 1. Molecular frameworks. J Med Chem. 1996;39(15):2887–93.
Stöber W, Fink A, Bohn E. Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci. 1968;26(1):62–9.
Gaumet M et al. Nanoparticles for drug delivery: the need for precision in reporting particle size parameters. Eur J Pharm Biopharm. 2008;69(1):1–9.
Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discovery Today. 2007;12(23):1068–75.
Whitesides, G.M., J.P. Mathias, and C.T. Seto, Molecular self-assembly and nanochemistry: a chemical strategy for the synthesis of nanostructures, DTIC Document;1991.
Kim H et al. Controlled production of emulsion drops using an electric field in a flow-focusing microfluidic device. Appl Phys Lett. 2007;91(13):133106.
Mozafari, M., Nanoliposomes: preparation and analysis. Liposomes: methods and protocols, Volume 1: Pharmaceutical Nanocarriers, 2010: p. 29–50.
Lu Y, Park K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int J Pharm. 2013;453(1):198–214.
Reis CP et al. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomed: Nanotechnol, Biol Med. 2006;2(1):8–21.
Jones M-C, Leroux J-C. Polymeric micelles—a new generation of colloidal drug carriers. Eur J Pharm Biopharm. 1999;48(2):101–11.
Zanen P et al. The effect of the inhalation flow on the performance of a dry powder inhalation system. Int J Pharm. 1992;81(2):199–203.
Hickey AJ, Misra A, Fourie PB. Dry powder antibiotic aerosol product development: inhaled therapy for tuberculosis. J Pharm Sci. 2013;102(11):3900–7.
Hickey AJ. Back to the future: inhaled drug products. J Pharm Sci. 2013;102(4):1165–72.
Xu Z, Mansour HM, Hickey AJ. Particle interactions in dry powder inhaler unit processes: a review. J Adhes Sci Technol. 2011;25(4–5):451–82.
Ostraat ML et al. The Nanomaterial Registry: facilitating the sharing and analysis of data in the diverse nanomaterial community. Int J Nanomed. 2013;8 Suppl 1:7.
Gaheen S et al. caNanoLab: data sharing to expedite the use of nanotechnology in biomedicine. Comput Sci Dis. 2013;6(1):p. 014010.
Sayes CM et al. Correlating nanoscale titania structure with toxicity: a cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol Sci. 2006;92(1):174–85.
Sayes CM et al. Nano-C 60 cytotoxicity is due to lipid peroxidation. Biomaterials. 2005;26(36):7587–95.
Mills KC et al. Nanomaterial registry: database that captures the minimal information about nanomaterial physico-chemical characteristics. J Nanopart Res. 2014;16(2):1–9.
Guzan K et al. Integration of data: the Nanomaterial Registry project and data curation. Comput Sci Dis. 2013;6(1):014007.
Sayes CM, Reed KL, Warheit DB. Assessing toxicity of fine and nanoparticles: comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol Sci. 2007;97(1):163–80.
Alanazi FK, Radwan AA, Alsarra IA. Biopharmaceutical applications of nanogold. Saudi Pharm J. 2010;18(4):179–93.
Chen PC, Mwakwari SC, Oyelere AK. Gold nanoparticles: from nanomedicine to nanosensing. Nanotechnol Sci Appl. 2008;1:45–66.
Hickey AJ et al. Size reduction and classification. Pharmaceutical process engineering. New York: Marcel Dekker; 2001. p. p. 17.
Dobashi, R. Risk of dust explosions of combustible nanomaterials. in Journal of Physics: Conference Series. IOP Publishing;2009.
Berg JM et al. Internalization of carbon black and maghemite iron oxide nanoparticle mixtures leads to oxidant production. Chem Res Toxicol. 2010;23(12):1874–82.
Warheit DB et al. Pulmonary toxicity study in rats with three forms of ultrafine-TiO 2 particles: differential responses related to surface properties. Toxicology. 2007;230(1):90–104.
Wang TC et al. Ultrahigh surface area zirconium MOFs and insights into the applicability of the BET theory. J Am Chem Soc. 2015;137(10):3585–91.
Patil S et al. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials. 2007;28(31):4600–7.
Clogston, J.D. and A.K. Patri, Zeta potential measurement. Characterization of nanoparticles intended for drug delivery, 2011: p. 63–70.
Berg JM et al. The relationship between pH and zeta potential of 30 nm metal oxide nanoparticle suspensions relevant to in vitro toxicological evaluations. Nanotoxicology. 2009;3(4):276–83.
Riddick, T.M., Control of colloid stability through zeta potential. Blood, 1968. 10(1).
Sung JC, Pulliam BL, Edwards DA. Nanoparticles for drug delivery to the lungs. TRENDS Biotechnol. 2007;25(12):563–70.
Sham JO-H et al. Formulation and characterization of spray-dried powders containing nanoparticles for aerosol delivery to the lung. Int J Pharm. 2004;269(2):457–67.
Pilcer G, Amighi K. Formulation strategy and use of excipients in pulmonary drug delivery. Int J Pharm. 2010;392(1):1–19.
Ankley GT et al. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29(3):730–41.
Vinken M et al. Development of an adverse outcome pathway from drug-mediated bile salt export pump inhibition to cholestatic liver injury. Toxicol Sci. 2013;136(1):97–106.
Morita, S., Quantifying Exposure to Engineered Nanomaterials (QEEN) from manufactured products: addressing environmental, health, and safety implications, P. Johnson and G. Holdridge, Editors. Consumer Product Safety Commission (CPSC) and National Nanotechnology Initiative (NNI): Arlington, VA;2016.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Katherine Tyner, Sau (Larry) Lee, and Marc Wolfgang
Glossary
- Nano-additive (abbrev. NA)
-
An inactive ingredient or excipient; excipients on the nanoscale that are added to dosage forms; used as a transport module for a drug
- Nano-carrier (abbrev. NC)
-
Non-drug components (additives) prepared as nanoparticles in which the drug is either dispersed (in a single particle) or to which drug is added (particles of drug and carrier); used as a transport module for a drug
- Nanocrystal
-
An example of a nanoparticle and used as an active pharmaceutical ingredient (API) in nanomedicine; a nanoparticle of near-pure drug form; usually composed of atoms, molecules, or other ingredients in a crystalline arrangement
- Nano-drug (abbrev. ND)
-
An example of a therapeutic agent that is the drug alone on the nanoscale; can be a hard solid (e.g., tablets, capsules), soft solid (ointments, suppositories), liquid (solutions, suspensions), or gas (aerosol); a.k.a. nano-pharmaceutical
- Nano-enabled
-
An adjective that describes something as functionally enhanced as compared to its non-nano form; objects may only have one or two dimensions on a nanoscale
- Nanomedicine
-
The medical application of nanoscience; inclusive of drugs (or pharmaceuticals), therapeutic agents, devices, sensors, and machines
- Nanoparticle
-
A particle, either naturally occurring or anthropogenic, that exists with mass and density on the nanoscale; equivalent to nanoparticulate and includes examples such as nanocrystal and nanopowder
- Nanoparticle system
-
Population references for individual nanoparticle groups
- Nanopowder
-
An example of a nanoparticle that exists in the solid state and not suspended in a liquid or gas
- Nanoscale
-
Refers to the size of an agent on or within the nanometer size domain; usually reserved for materials between 1 and 1000 but sometimes defined as 1–100 for purposes outside pharmaceutical applications
- Nanoscience
-
The foundational discipline of nano-related discoveries; the study of materials on the scale of nanometers
- Nanotechnology
-
The application of nanoscience through engineering principles utilizing a material with dimensions of less than 100 nm or with a material engineered to exhibit properties unique from its bulk counterpart up to 1000 nm
Rights and permissions
About this article
Cite this article
Sayes, C.M., Aquino, G.V. & Hickey, A.J. Nanomaterial Drug Products: Manufacturing and Analytical Perspectives. AAPS J 19, 18–25 (2017). https://doi.org/10.1208/s12248-016-0008-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-0008-x