Abstract
Background
Rheumatoid arthritis (RA) is a chronic, inflammatory joint condition characterized by overproduction of pro-inflammatory cytokines. We aimed to assess TNF-α levels in both serum and synovial fluid in effusive knees in RA patients and find out if synovial fluid levels correlate with ultrasound (US)-detected local knee inflammatory and/or destructive changes in these patients.
Results
This study included 40 patients (20 with RA, 10 with systemic lupus erythematosus (SLE), and 10 with osteoarthritis (OA)) who had knee effusion (unilateral or bilateral) upon clinical examination. The mean age of RA patients was 48.4 years; most of them were females (80%), with a median (min–max) duration of knee effusion of 2 (1.5–3) months. Serum TNF-α was significantly higher in RA vs. non-RA and in OA cases (p = 0.052, 0.022, respectively), while in the synovial fluid, the difference was not statistically significant (3.73 ± 0.72 vs. 3.48 ± 0.58 U/ml, p = 0.252). Serum TNF-α at a cut point of > 3.24 U/ml can significantly discriminate RA from OA with 65% sensitivity and 90% specificity (AUC = 0.725, P = 0.018). There was no statistically significant correlation between synovial TNF-α and US parameters of the knee, either in RA or non-RA patients.
Conclusions
RA, OA, and SLE effusive joints share the presence of local articular joint inflammation, while systemic inflammation is more discriminative for RA patients regarding the level of TNF-α. The lack of correlation of TNF-α with ultrasonographic findings reflects the multifactorial complexity of these autoimmune diseases.
Similar content being viewed by others
Background
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by a chronic inflammatory process that can affect both joints and extra-articular organs, such as the heart, kidney, lung, digestive system, eye, skin, and nervous system. The incidence of RA is greater in women than in men, and it increases with age [2]. It is still unknown what causes RA; nevertheless, researchers have come to the conclusion that a combination of genetic and environmental variables is likely to be involved [3]. RA is primarily characterized by autoantibodies against immunoglobulin G (also known as rheumatoid factor [RF]) and antibodies against anti-citrullinated protein (anti-CCP) [4].
RA is a chronic autoimmune disease affecting the joints and characterized by a progressive symmetric inflammation of affected joints, resulting in cartilage destruction, bone erosion, and disability [5]. It primarily affects small joints, such as the proximal interphalangeal and metacarpophalangeal joints, but it can also affect large joints, such as the ankle, knee, elbow, and shoulder [2]. RA is characterized by synovial hyperplasia, which is the primary factor in the development of an invasive pannus [6].
In the management of individuals who have RA, there is an urgent need for biomarkers that can reliably identify specific diseases, predict disease progression, evaluate response to treatment, and predict medication responsiveness in individual patients. It is possible that the biomarkers found in synovial fluid provide the answer to both the correct diagnosis and effective treatment of knee arthritis. Synovial cells are responsible for the production of synovial fluid, which reflects chondrocyte metabolism and matrix turnover. One of the most important regulators in the development of RA is tumor necrosis factor alpha (TNF-α). Its expression is enhanced in those who suffer from RA, and excessive expression of TNF is what leads to autoimmune arthritis in transgenic mice [7]. TNF-α signaling is involved in multiple ways in the pathogenesis of RA. It activates endothelial cells and recruits proinflammatory cells such as synovial fibroblasts and macrophages, which then secrete proinflammatory cytokines including IL-6, IL-1, and TNF-α [8].
Musculoskeletal ultrasound (MSUS) can detect RA manifestations such as early bone erosions, synovitis, the degree of vascularization (Doppler activity), tendinopathy, and tenosynovitis with high sensitivity [9]. Additionally, it can discriminate between RA and OA [10]. As a result, it is widely utilized in clinical practice as well as research settings for the purpose of RA patient diagnosis, monitoring, and prognosis [11].
Even though TNF-α has been shown to play a significant role in the pathogenesis of RA, a comparison of the local and systemic levels of TNF-α in RA, SLE, and OA knees, as well as their relationship with MSUS findings, has not been investigated yet.
Thus, the aim of this study was to assess the level of TNF-α in serum and synovial fluid of the knee in RA and non-RA patients with knee effusion and to evaluate its correlation with US parameters of the effused knee joint.
Patients and methods
Study design and settings
This cross-sectional study was carried out at our rheumatology and immunology unit (inpatient and outpatient), during the period from 2018 to April 2020. The sample size was determined to be a convenient sample; all patients who met the inclusion criteria were offered the opportunity to participate in the study, unless they met any of the exclusion criteria or refused to participate.
Ethical consideration
The study was conducted according to the principles outlined in the Helsinki Declaration [8], and we used the STROBE cross-sectional checklist when writing our report [12]. The study was permitted to proceed after obtaining approval from the Institutional Research Board (MFM-IRB) (Approval No. MS.19.12.945). Participation in the study was entirely voluntary and kept confidential throughout. The purpose of the research was made crystal clear, and participants’ written consent was obtained before the study was carried out. Participants in the study who lacked the capacity to read and write or, for any other reason, were unable to offer their informed consent had to have their parents or legal guardians give consent before they could take part in the study.
Patient population
The inclusion criteria were the following: (a) patients with a confirmed diagnosis of RA according to ACR/EULAR 2010 rheumatoid arthritis classification criteria [13]; (b) age > 18 years; and (c) unilateral or bilateral knee effusion for more than one month detected by clinical examination and confirmed by MSUS.
Twenty age- and sex-matched non-RA patients with knee effusion were also included: 10 patients with a confirmed diagnosis of systemic lupus erythematosus (SLE) according to SLICC classification criteria for SLE [14] and 10 patients with a confirmed diagnosis of knee osteoarthritis (OA). Patients with a history of knee joint fracture, infection, malignancy, intra-articular administration of steroids for at least 3 months prior to evaluation, or a history of other autoimmune or rheumatic diseases were excluded from the study at the outset. Patients displaying clinical symptoms, ultrasound findings, or elevated serum uric acid levels indicative of gout were also excluded from the study.
Sample size calculation
The sample size was calculated using Power Analysis and Sample Size (PASS) software (version 15, 2017; NCSS, LLC., Kaysville, UT, USA). Based on previous research by Inam Illahi et al. [15], in which a significant correlation of serum TNF-α with DAS28-ESR in RA patients was reported, we hypothesized a statistically significant correlation of serum TNF-α with different grades of sonographic findings of RA with a Spearman’s correlation coefficient (rs) of moderate positive correlation (rs = 0.6) [16]. A sample size of 20 RA patients achieves 85% power to detect a Pearson correlation of 0.600 using a one-sided hypothesis test with a significance level of 0.050. These results are based on 5000 Monte-Carlo samples from the bivariate normal distribution under the alternative hypothesis.
Clinical assessment and laboratory testing
Sociodemographic data were collected from all patients, including gender, age, and occupation. For RA, a comprehensive medical and clinical examination was performed to assess the current status of the disease, which included counting the number of swollen and tender joints. The patient’s overall health was evaluated using a patient global assessment (PGA) score of 100 mm, with 0 indicating the best possible health and 100 indicating the worst possible health. For each RA patient, the disease activity score 28 (DAS28-ESR) was used to assess current disease activity [17] and the health assessment questionnaire (HAQ) to assess functional capacity [18]. Therapeutic data, including NSAIDs, corticosteroids, and disease-modifying antirheumatic drugs (DMARDs), were also collected.
We questioned the duration of the knee joint effusion. The visual analogue scale (VAS) was used to measure the intensity of the knee pain, with a score of 0 indicating no pain and a score of 10 indicating the most severe pain.
At the time of the clinical evaluation, a venous blood sample was collected from each RA patient, and relevant laboratory tests were evaluated with the assistance of an automated analyzer. The laboratory tests included rheumatoid factor (RF), C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR).
Serum and synovial fluid sampling and testing for TNF-α
The first thing that we did was make sure that there had been no intraarticular injections administered to the knee joint in the past 3 months. Then, under sterile conditions, venipuncture was used to collect 5 ml of venous blood and 5 ml of knee synovial fluid from each participant. Knee synovial fluid was obtained with the guidance of MSUS. A serum separator tube was used for the collection of the samples. After the formation of a clot, the samples were centrifuged at 2000 g for 10 min in order to extract the serum, which was then frozen at -20°C pending further investigation. TNF-α was analyzed in serum and synovial fluid samples using Biolegend’s ELISA MAX™ standard set, which is a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) [19].
Musculoskeletal ultrasound of the knee
The EDAN U2 ultrasound device (Shenzhen, China), equipped with a linear array transducer that ranged in frequency from 8 to 13.4 MHz, was used to conduct a real-time MSUS scan of the knee joint. The sonographic parameters were adjusted, and the frequency was set at 13 MHz in order to obtain the best US images of the knee structures. All sonographic examinations were performed by a rheumatologist with a minimum of 8 years of experience in the field of MSUS. During the evaluation, the operator was blinded to the clinical status of the patients. Knee joint scanning was performed in accordance with a predetermined protocol of the technical guidelines of the European Society of Musculoskeletal Radiology [20].
The suprapatellar and parapatellar joint recesses were examined for effusion and synovial hypertrophy as part of the knee US protocol. With full knee flexion, the V-shaped trochlea of the femur and the overlaying articular cartilage were inspected on the axial planes for any abnormalities. Additionally, the thickness of the medial and lateral femoral condylar cartilage was assessed. Measurements of the thickness of the femoral cartilage were taken, while the individuals were lying supine on the examination bed with their knees bent to their maximum flexion. Using the suprapatellar axial view, US imaging was performed, and measurements were acquired from the midpoints of the medial femoral cartilage and the lateral femoral cartilage [21].
Long- and short-axis planes were used to map the quadriceps and patellar tendons from their cranial origin to their distal insertion. MSUS assessment of both the patellar and quadriceps tendons was conducted on a supine patient with the knee slightly flexed at 20°. Tendinosis was identified by a hypoechoic appearance of the tendon and a disruption of its fibrillar pattern. The quadriceps and patellar entheses were also inspected for structural abnormalities, bursitis, bone erosions, or calcification indicating enthesopathy [22]. In order to determine whether or not a Baker’s cyst was present, we checked the region located medially between the semimembranosus tendon and the medial head of the gastrocnemius.
The MSUS images of each patient were collected, and then, using proprietary software, they were reviewed later on. The US findings were analyzed and interpreted using the criteria established by the Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) US group [23]. Knee effusion, synovial hypertrophy, and power Doppler were scored on a semiquantitative scale (where 0 = absence, 1 = mild, 2 = moderate, and 3 = severe) [24].
Statistical analysis
Data were entered and analyzed using IBM-SPSS software (IBM Corp., Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.). Qualitative data were expressed as absolute frequency (N) and relative frequency (%, percentage). Quantitative data were initially tested for normality using Shapiro–Wilk’s test, with data being normally distributed if p > 0.050. Quantitative data were expressed as mean ± standard deviation (SD) if normally distributed without significant outliers, or median (25th–75th percentiles) if not normally distributed and/or with significant outliers. Chi-square test was used to test the association between two nominal variables when the expected count in all cells was ≥ 5. Otherwise, Fisher’s exact test was used. One-way ANOVA (with Tukey’s post-hoc tests for statistically significant results) was used if homogeneity of variances was met with. Welsh ANOVA (with Games-Howell post-hoc tests for statistically significant results) was used if homogeneity of variances was violated. Pearson’s correlation, or Spearman’s correlation, was used as appropriate to assess the strength and direction of the association/relationship between variables. Results were considered statistically significant if the p value was ≤ 0.050. Receiver operating characteristic (ROC) curve analysis was performed for the serum TNF-α to determine the cutoff point above which RA is likely.
Results
Twenty RA patients (16 females and 4 males), and 20 sex- and age-matched non-RA patients (10 SLE and 10 OA) were successfully recruited to participate in the study. The non-RA group included 5 males and 15 females. The flowchart of the study is illustrated in Figure 1. The mean age of the studied RA patients was 48.4 ± 14.1 years, with a median disease duration of 3 years. The administered DMARDs included methotrexate (80%), leflunomide (80%), and hydroxychloroquine (95%). The median (min–max) duration of knee effusion was 2 (1.5–3) months. The mean of synovial fluid TNF-α was 3.73 ± 0.72 U/ml while in serum was 3.66 ± 0.76 U/ml. Other clinical and laboratory data are illustrated in Table 1.
As shown in Fig. 2, serum TNF-α was statistically significantly different between RA, SLE, and OA patients. Games-Howell post-hoc test revealed a statistically significantly higher serum TNF-α in RA vs. OA (p = 0.022) but not in SLE.
On the other hand, there was no statistically significant difference between RA patients, SLE patients, and OA patients in the level of TNF-α in their synovial fluid, as seen in Fig. 3.
Figure 4 demonstrates that a serum TNF-α level of > 3.24 U/ml could significantly differentiate between RA and OA with a sensitivity of 65% and a specificity of 90% (AUC = 0.725, p = 0.018).
MSUS findings of the knee in the RA and non-RA groups are summarized in Table 2. In RA patients, knee effusion was moderate in 7 (35%) patients and severe in 13 (65%) patients, while synovial hypertrophy was moderate in 8 (40%) patients and severe in 7 (35%) patients. There was no statistically significant difference between RA and non-RA patients regarding the examined MSUS parameters except for synovial hypertrophy. Severe synovial hypertrophy was significantly higher in RA patients in comparison to non-RA patients (7 (35%) versus 1 (5%), respectively, p = 0.028).
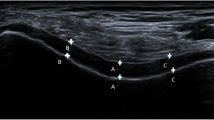
As shown in Table 3, there was no statistically significant correlation between TNF-α levels in both serum and synovial fluid and US parameters of the knee joint in RA patients. Demonstrative MSUS findings of the effused knee joints in RA patients are illustrated in Fig. 5.
Musculoskeletal ultrasound findings of the knee joint in RA group. a Sagittal scan of the suprapatellar recess shows a well-defined anechoic collection with no internal color flow Doppler signal suggestive of severe knee effusion in a 45-year-old female patient with RA (synovial fluid TNF-α = 3.52 U/ml, serum TNF-α = 3.32). b Transverse scan at the end of the femur shows decreased thickness of the cartilage at the medial condyle in a 40-year-old female patient with RA (synovial fluid TNF-α = 2.53, serum TNF-α = 2.65)
Discussion
In this cross-sectional study, we tried to assess the levels of TNF-α in the synovial fluid and serum of RA and non-RA patients who had knee effusion and to determine the extent to which these levels correlated with MSUS findings of the effused knee joint. Even though the serum level of TNF-α in patients with RA was higher than in individuals without RA, the level of TNF-α in synovial fluid did not differ between the two groups. There was no correlation found between the levels of TNF-α in both serum and synovial fluid and the MSUS parameters of the knee joint. To the best of our knowledge, this is the first study to assess TNF-α in RA synovial fluid and connect it with MSUS characteristics.
In fact, TNF-α is an essential component of a normal immune response. Nevertheless, excessive or improper TNF-α production can be damaging and lead to a disease. It is considered a crucial factor in the pathological development of RA [25]. Patients with RA have abundant TNF-α, which is a key and important cytokine that upsets the normal balance between pro-inflammatory and anti-inflammatory cytokines [26]. TNF-α is known to effectively stimulate the production of other proinflammatory mediators such as IL-1b, chemokines, and proteases [27].
As a result of this, we found that it would be beneficial to investigate this cytokine in patients who have RA and compare their results to those of patients who do not have RA. Considering SLE, which is an inflammatory form of arthritis, and OA, which is a non-inflammatory form of arthritis, it seems like they may be a reasonable candidate for making this comparison.
In this study, we demonstrated that serum TNF-α levels were statistically significantly higher in RA patients versus non-RA patients, and a post-hoc test revealed a statistically significantly higher serum TNF-α level in RA vs. OA but not SLE. This can be explained by the high level of circulating TNF-α in SLE and its association with lupus disease activity [28,29,30]. However, in OA, TNF-α levels in plasma in RA patients are 10–20 times higher than in OA patients and normal people [31]. TNF-α has shown conflicting outcomes when used as a disease burden marker [32, 33]. TNF-α has demonstrated qualities as an indicator of treatment efficacy in OA patients [34, 35]. Additional research is required to understand its role and determine whether it is effective as a biochemical marker of OA.
Synovial fluid functions primarily as a biological lubricant of the joint, minimizing friction between synovial joint articular cartilage surfaces, but also as a nutrient pool for surrounding tissues and a conduit for cytokine movement [36, 37]. In the current study, there was no statistically significant difference between RA patients, SLE patients, and OA patients when it came to the level of TNF-α in their synovial fluid. In a study conducted by Alaaeddine et al., increased TNF-α receptors were discovered in chondrocytes and synoviocytes produced from OA joints when compared to nonarthritic tissues. When OA chondrocytes were cultured with normal human chondrocytes, TNF-α receptors increased [38, 39]. TNF-α receptors were bound by a 100 µg (99 m) Tc human anti-TNF-α antibody, and higher levels of TNF-α were observed in arthritic joints utilizing scintigraphy in OA and RA patients. Tc-infliximab scintigraphy shows a correlation with acute phase reactants, clinical indicators, and imaging signs of synovitis, suggesting that this approach has the potential to evaluate TNF-α-associated synovitis [40]. Synovial cells cultured from human OA synovium showed that the inflammatory and damaging responses are cytokine-driven by TNF-α [41]. In our study, TNF-α has been identified in the synovial fluid in OA patients and may be linked to the pathophysiological processes involved in OA. It could also help determine the severity of the disease.
The TNF-α concentration in the synovial fluid of RA patients is approximately twice that of OA patients. When immunohistochemistry performed on RA synovium, it revealed that TNF-α was present in the synovial lining cell layer, particularly at the pannus and cartilage junction [42]. In the current study, we found that a serum TNF-α level of > 3.24 U/ml could significantly differentiate between RA and OA with a sensitivity of 65% and a specificity of 90%. Knee involvement is one of the major consequences of RA, which is one of the primary contributors to persistent pain and disabilities [43]. So, serum TNF-α levels may be more useful in patients with knee effusion who do not meet a specific diagnostic criterion, particularly those with serum anti-CCP and/or RF test results. Since early and intensive treatment of undifferentiated arthritis significantly reduces the frequency of progressions to RA and provides an effective strategy for slowing radiologic progression [44], it has been shown that it is possible to delay radiologic progression. As a result, identifying high-risk patients and initiating therapy sooner would be highly desirable.
In our RA cohort, there was no statistically significant correlation between TNF-α levels in both serum and synovial fluid and US parameters of the knee joint in RA patients. In this regard, power Doppler vascularity was positively linked with the presence of inflammatory cytokines and growth factors in synovial fluid, with larger coefficient values in both untreated and treated individuals with RA. There is also a significant relationship between the findings of the US synovial examination and the levels of serum vascular endothelial growth factor (VEGF) [45], YKL-40 [46], and calprotectin [47] in RA patients.
Regarding the lack of statistical difference in the synovial fluid of effusive joints in OA compared to RA and SLE patients could indicate that the local inflammation in OA knees could be as inflammatory as the RA/SLE joints; however, the systemic inflammation is much more evident by the lower values of OA serum levels compared with the RA patients, which could be discriminative of RA compared with OA.
A possible limitation of this study is that no healthy control group was involved, as it is difficult to attain healthy subjects with knee effusion. In addition, the number of patients is relatively small because of the high expense of testing for TNF-α, which is not carried out on a routine basis. It would be better to exclude patients on steroids, as it might affect the results of TNF-α. Finally, comparing cartilage thickness between RA patients and both SLE and OA patients would be more appropriate, as it is expected to be lower in OA patients compared with SLE. To our knowledge, there are no reports that correlate TNF-α in both serum and synovial fluid to MSUS parameters in RA patients. The expression of serum TNF-α may play a role in the intensification of inflammatory activity in RA, screening for this cytokine in RA patients in order to monitor the disease activity could prove to be beneficial for patients who are undergoing anti-TNF therapy. Furthermore, its high value in knee effusion may be indicative of a better response to intraarticular administration of anti-TNF agents. Further prospective studies are required to ascertain the diagnostic and prognostic value of serum and synovial fluid TNF-α in RA.
RA, OA, and SLE effusive joints share the presence of local articular joint inflammation, while systemic inflammation is more discriminative for RA patients regarding the level of TNF-α. The lack of correlation of TNF-α with ultrasonographic findings reflects the multifactorial complexity of these autoimmune diseases.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Anti-CCP:
-
Anti-citrullinated protein
- CRP:
-
C-reactive protein
- DAS28:
-
Disease activity score 28
- DMARDs:
-
Disease-modifying antirheumatic drugs
- ESR:
-
Erythrocyte sedimentation rate
- HAQ:
-
Health assessment questionnaire
- MSUS:
-
Musculoskeletal ultrasound
- OA:
-
Osteoarthritis
- OMERACT:
-
Outcome Measures in Rheumatoid Arthritis Clinical Trials
- PGA:
-
Patient global assessment
- RA:
-
Rheumatoid arthritis
- RF:
-
Rheumatoid factor
- SLE:
-
Systemic lupus erythematosus
- TNF-α:
-
Tumor necrosis factor-α
- US:
-
Ultrasound
- VAS:
-
Visual analogue scale
References
Hassaany AHE, Tharwat S, Mansour M, Enein AF (2022) AB0099 knee synovial fluid and serum TNF-α in rheumatoid arthritis: correlation with sonographic parameters. Ann Rheum Dis 81:1180–1. Available from: https://ard.bmj.com/content/81/Suppl_1/1180.2. Cited 2024 Mar 18.
Scott DL, Wolfe F, Huizinga TWJ (2010) Rheumatoid arthritis. Lancet Lond Engl 376:1094–1108
Catrina AI, Joshua V, Klareskog L, Malmström V (2016) Mechanisms involved in triggering rheumatoid arthritis. Immunol Rev 269:162–174
Abbasi M, Mousavi MJ, Jamalzehi S, Alimohammadi R, Bezvan MH, Mohammadi H et al (2019) Strategies toward rheumatoid arthritis therapy; the old and the new. J Cell Physiol 234:10018–10031
Aletaha D, Smolen JS (2018) Diagnosis and management of rheumatoid arthritis: a review. JAMA 320:1360–1372
Scherer HU, Häupl T, Burmester GR (2020) The etiology of rheumatoid arthritis. J Autoimmun 110:102400
McInnes IB, Schett G (2017) Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 389:2328–37. Available from: https://www.sciencedirect.com/science/article/pii/S0140673617314721. Cited 2023 Apr 17.
General Assembly of the World Medical Association (2014) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent 81:14–18
Østergaard M, Pedersen SJ, Døhn UM (2008) Imaging in rheumatoid arthritis–status and recent advances for magnetic resonance imaging, ultrasonography, computed tomography and conventional radiography. Best Pract Res Clin Rheumatol 22:1019–1044
GadAllah N, Elhossieny M, Abaza N, Yousry S, El-Bakry SA (2015) Verification of an ultrasonographic scoring system in discriminating rheumatoid arthritis from osteoarthritic and normal joints in an Egyptian cohorts. Egypt Rheumatol Rehabil 42:19–26
Carstensen SMD, Terslev L, Jensen MP, Østergaard M (2020) Future use of musculoskeletal ultrasonography and magnetic resonance imaging in rheumatoid arthritis. Curr Opin Rheumatol 32:264–272
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP et al (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet Lond Engl 370:1453–1457
2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative -PubMed. Available from: https://pubmed.ncbi.nlm.nih.gov/20872595/. Cited 2023 Apr 20.
Petri M, Orbai A-M, Alarcón GS, Gordon C, Merrill JT, Fortin PR et al (2012) Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64:2677–2686
Inam Illahi M, Amjad S, Alam SM, Ahmed ST, Fatima M, Shahid MA (2021) Serum tumor necrosis factor-alpha as a competent biomarker for evaluation of disease activity in early rheumatoid arthritis. Cureus 13:e15314
Hinkle DE, Wiersma W, Jurs SG. Applied statistics for the behavioral sciences. 5th ed. Boston, Mass., [London]: Houghton Mifflin ; [Hi Marketing] (distributor); 2003. Available from: http://catalog.hathitrust.org/api/volumes/oclc/50716608.html/. Cited 2024 Feb 23.
Wells G, Becker J-C, Teng J, Dougados M, Schiff M, Smolen J et al (2009) Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis 68:954–960
Uhlig T, Haavardsholm EA, Kvien TK (2006) Comparison of the Health Assessment Questionnaire (HAQ) and the modified HAQ (MHAQ) in patients with rheumatoid arthritis. Rheumatol Oxf Engl 45:454–458
Gamel EB, Hashim NT, Satti A, Gismalla BG (2017) Salivary TNFα levels in groups of subjects with rheumatoid arthritis and chronic periodontitis. BMC Res Notes 10:34
Martinoli C (2010) Musculoskeletal ultrasound: technical guidelines. Insights. Imaging 1:99–141
Tuna S, Balcı N, Özçakar L (2016) The relationship between femoral cartilage thickness and muscle strength in knee osteoarthritis. Clin Rheumatol 35:2073–2077
Alves TI, Girish G, Kalume Brigido M, Jacobson JA (2016) US of the knee: scanning techniques, pitfalls, and pathologic conditions. Radiogr Rev Publ Radiol Soc N Am Inc 36:1759–75
Wakefield RJ, Balint PV, Szkudlarek M, Filippucci E, Backhaus M, D’Agostino M-A et al (2005) Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol 32:2485–2487
D’Agostino M-A, Terslev L, Aegerter P, Backhaus M, Balint P, Bruyn GA et al (2017) Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR-OMERACT ultrasound taskforce-Part 1: definition and development of a standardised, consensus-based scoring system. RMD Open 3:e000428
Jang D-I, Lee A-H, Shin H-Y, Song H-R, Park J-H, Kang T-B et al (2021) The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int J Mol Sci 22:2719
Edwards CJ (2005) Immunological therapies for rheumatoid arthritis. Br Med Bull 73–74:71–82
Moelants EAV, Mortier A, Van Damme J, Proost P (2013) Regulation of TNF-α with a focus on rheumatoid arthritis. Immunol Cell Biol 91:393–401
Idborg H, Eketjäll S, Pettersson S, Gustafsson JT, Zickert A, Kvarnström M et al (2018) TNF-α and plasma albumin as biomarkers of disease activity in systemic lupus erythematosus. Lupus Sci Med 5:e000260
McCarthy EM, Smith S, Lee RZ, Cunnane G, Doran MF, Donnelly S et al (2014) The association of cytokines with disease activity and damage scores in systemic lupus erythematosus patients. Rheumatol Oxf Engl 53:1586–1594
Postal M, Lapa AT, Sinicato NA, de Oliveira PK, Peres FA, Costallat LTL et al (2016) Depressive symptoms are associated with tumor necrosis factor alpha in systemic lupus erythematosus. J Neuroinflammation 13:5
Stanczyk J, Pedrioli DML, Brentano F, Sanchez-Pernaute O, Kolling C, Gay RE et al (2008) Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum 58:1001–1009
Penninx BWJH, Abbas H, Ambrosius W, Nicklas BJ, Davis C, Messier SP et al (2004) Inflammatory markers and physical function among older adults with knee osteoarthritis. J Rheumatol 31:2027–2031
Kammermann JR, Kincaid SA, Rumph PF, Baird DK, Visco DM (1996) Tumor necrosis factor-alpha (TNF-alpha) in canine osteoarthritis: immunolocalization of TNF-alpha, stromelysin and TNF receptors in canine osteoarthritic cartilage. Osteoarthritis Cartilage 4:23–34
Mabey T, Honsawek S (2015) Cytokines as biochemical markers for knee osteoarthritis. World J Orthop 6:95–105
Vincent HK, Percival SS, Conrad BP, Seay AN, Montero C, Vincent KR (2013) Hyaluronic acid (HA) viscosupplementation on synovial fluid inflammation in knee osteoarthritis: a pilot study. Open Orthop J 7:378–384
Blewis ME, Nugent-Derfus GE, Schmidt TA, Schumacher BL, Sah RL (2007) A model of synovial fluid lubricant composition in normal and injured joints. Eur Cell Mater 13:26–39
Tamer TM (2013) Hyaluronan and synovial joint: function, distribution and healing. Interdiscip Toxicol 6:111–125
Alaaeddine N, Hilal G, Baddoura R, Antoniou J, Di Battista JA (2011) CCL20 stimulates proinflammatory mediator synthesis in human fibroblast-like synoviocytes through a MAP kinase-dependent process with transcriptional and posttranscriptional control. J Rheumatol 38:1858–1865
Alaaeddine N, Di Battista JA, Pelletier JP, Kiansa K, Cloutier JM, Martel-Pelletier J (1999) Differential effects of IL-8, LIF (pro-inflammatory) and IL-11 (anti-inflammatory) on TNF-alpha-induced PGE(2)release and on signalling pathways in human OA synovial fibroblasts. Cytokine 11:1020–1030
Hermann J, Lipp RW, Dunzinger A, Spreizer C, Schaffler G, Kvaternik H et al (2014) Anti-TNF scintigraphy to assess TNF-α-associated joint inflammation in rheumatoid arthritis and osteoarthritis. Clin Exp Rheumatol 32:614
Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE (2006) The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther 8:R187
Ulfgren AK, Gröndal L, Lindblad S, Khademi M, Johnell O, Klareskog L et al (2000) Interindividual and intra-articular variation of proinflammatory cytokines in patients with rheumatoid arthritis: potential implications for treatment. Ann Rheum Dis 59:439–447
Shadmanfar S, Labibzadeh N, Emadedin M, Jaroughi N, Azimian V, Mardpour S et al (2018) Intra-articular knee implantation of autologous bone marrow-derived mesenchymal stromal cells in rheumatoid arthritis patients with knee involvement: results of a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy 20:499–506
Verpoort KN, van Dongen H, Allaart CF, Toes REM, Breedveld FC, Huizinga TWJ (2004) Undifferentiated arthritis–disease course assessed in several inception cohorts. Clin Exp Rheumatol 22:S12–17
Zhang Y-F, Gao S-S, Li J-L, Zuo W-S, Qiu Y-W, Xiao Y-C (2022) Comparison and correlation study of synovial ultrasound indices and serum VEGF in rheumatoid wrist arthritis before and after treatment. Clin Rheumatol 41:2677–2683
Kazakova M, Batalov A, Deneva T, Mateva N, Kolarov Z, Sarafian V (2013) Relationship between sonographic parameters and YKL-40 levels in rheumatoid arthritis. Rheumatol Int 33:341–346
Inciarte-Mundo J, Ramirez J, Hernández MV, Ruiz-Esquide V, Cuervo A, Cabrera-Villalba SR et al (2016) Calprotectin and TNF trough serum levels identify power Doppler ultrasound synovitis in rheumatoid arthritis and psoriatic arthritis patients in remission or with low disease activity. Arthritis Res Ther 18:160. Available from: https://doi.org/10.1186/s13075-016-1032-z. Cited 2024 Feb 25.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: AE, ST. Investigation: all authors. Data curation, formal analysis: MM, AE. Writing–original draft: ST. Writing–review and editing: all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the principles outlined in the Helsinki Declaration. The study was permitted to proceed after obtaining approval from the Mansoura Faculty of Medicine Institutional Research Board (MFM-IRB) (Approval No. MS.19.12.945.).
Consent for publication
Not applicable.
Competing interests
All authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassany, A.E., Tharwat, S., Mansour, M. et al. TNF-α in the serum and synovial fluid of patients with rheumatoid arthritis: correlation with sonographic parameters: a cross-sectional study. Egypt Rheumatol Rehabil 51, 22 (2024). https://doi.org/10.1186/s43166-024-00256-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43166-024-00256-7