Abstract
Subcutaneous nodular lesions in a patient with significantly elevated inflammatory markers always raise the possibility of infectious diagnoses such as atypical infection, pyogenic abscess, mycobacterial infection, and malignancy. Definitive diagnosis requires tissue biopsy and histopathologic examination. The atypical presentation of a foreign body granuloma with concurrent intraoperative findings supports the diagnosis of gouty arthritis.
We report a case of a 67-year-old man who presented with an inflammatory nodular lesion on the left elbow that was initially suspected to have an infectious cause. Histopathologic examination of the nodular tissue later revealed that the patient had a foreign body granuloma due to urate crystal deposition. The atypical appearance of the gouty arthritis, the low serum urate level, the negative crystal identification in the synovial fluid, and the markedly elevated inflammatory markers, which did not respond to the previous antibiotic and steroid therapy, raised the suspicion of atypical infection in this case.
Similar content being viewed by others
Introduction
A subcutaneous nodular lesion should always raise the suspicion of infection or malignancy, especially in the elderly and those at high risk. It is not always easy to make the diagnosis based on history and physical signs alone, as the differential diagnosis is broad. The gold standard for confirming the diagnosis of a nodular skin lesion is histopathologic examination [1].
Histopathologic findings of granulomatous inflammation would suggest infection such as Mycobacterium tuberculosis (MTB), atypical mycobacteria, fungi, and Brucella species. There are also inflammatory causes with granulomatous histopathologic findings such as sarcoidosis, crystal-associated arthritis, or foreign body reaction [2]. Granulomatous inflammation occurs in response to various infectious, autoimmune, toxic, allergic, and neoplastic conditions [3].
We report a case of a middle-aged man who presented with a subcutaneous nodular lesion associated with inflammatory joint pain. He was treated for infection and extensive investigations for atypical infection and malignancy were performed. Given the atypical presentation of gouty arthritis, low serum urate levels, and negative crystals in the synovial fluid, the diagnosis of gouty arthritis was not part of the initial differential diagnosis.
Case report
A 67-year-old man was admitted to our hospital with pain and swelling in the left elbow and forearm, associated with a small nodular formation on the posterior elbow for more than a week. He also complained of intermittent joint pain in his right knee and left ankle for a week. One month earlier, he was admitted with an episode of joint pain in the right wrist, right knee, and right first metatarsophalangeal joint (MTPJ), which was treated as reactive arthritis with oral prednisolone and non-steroidal anti-inflammatory drugs (NSAIDs), which were ineffective. Prior to the current hospitalization, there was no history of trauma or injury, no typical history of gout flares or inflammatory joint symptoms, no obvious precipitating causes, and no constitutional symptoms. There was no significant family history. He had diabetes mellitus, which was well controlled with basal-bolus insulin and metformin, and was also taking amlodipine and metoprolol for his hypertension. He denied taking alternative treatment.
On examination, he was afebrile. There was a painful, inflamed, firm, and diffuse nodule on the posterior left elbow (Fig. 1), and the left elbow and right knee joint were swollen and painful. The rest of the joint examination was normal, with no joint deformity or active synovitis. There were no signs suggestive of autoimmune connective tissue disease or psoriasis. His pulmonary and cardiovascular examination was normal. The rest of the clinical examination was unremarkable.
Laboratory tests showed a high total white blood cell count with normal platelet and hemoglobin levels, elevated inflammatory markers (ESR: > 120 mm/H, CRP: 189 mg/L), normal procalcitonin, hypoalbuminemia (serum albumin of 31 g/L), normal renal and liver function, and electrolyte levels. His serum uric acid (SUA) level was persistently normal (between 179 μmol/L and 281 μmol/L). Blood investigations looking for secondary causes of calcium pyrophosphate deposition disease (CPPD) including thyroid function (TSH: 1.634 mIU/L), parathyroid hormone (iPTH: 35.3 pg/mL), vitamin D3 (61.06 nmol/L), calcium (2.22 mmol/L), magnesium (0.94 mmol/L), and phosphate (0.85 mmol/L) were all normal.
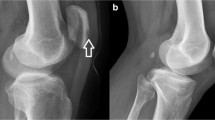
He had a low positive rheumatoid factor of 16 IU/ml, negative anticyclic citrullinated peptide antibodies and antinuclear antibodies, and normal complement levels. Plain radiograph of the left elbow showed subcutaneous fat streaks, which could represent edematous changes or cellulitis with possible elbow effusion. Plain radiograph of the right knee and left ankle showed no signs of chondrocalcinosis and degenerative changes in the femoral condyles and patella.
As septic arthritis was suspected, the right knee was aspirated and he was started on intravenous ampicillin/salbactam. He was also treated for cellulitis and abscess on the left elbow. Synovial fluid culture was negative for bacteria, fungi, and acid-fast bacilli (AFB). Synovial fluid sent to the laboratory for crystal identification using an automatic analyzer was negative.
However, the swelling and lump on his left elbow persisted throughout his hospitalization, and he had responded very poorly to antibiotics, with persistently elevated inflammatory parameters. His serial blood cultures were negative. Ultrasound of the left elbow showed no focal collection. Ultrasound of the right knee showed minimal suprapatellar effusion with synovial thickening but was negative for the double contour sign and power Doppler (PD) signal. Ultrasound of the left first MTPJ showed hyperechoic foci but was negative for PD signal.
Otherwise, the other imaging studies, including chest radiograph and abdominal ultrasound, were unremarkable. Extensive infectious investigations were all negative, including hepatitis, human immunodeficiency virus (HIV), syphilis, Coxiella burnettii, Brucella, and Bartonella henselae. Tumor markers including alpha-fetoprotein, carcinoembryonic antigen, CA 125, and CA 19–9 were normal.
Due to poor response to treatment and the possibility of chronic infections such as tuberculosis and malignancy, he was referred to an orthopedic surgeon for incisional biopsy and histopathologic examination of the nodule on the left elbow. The incisional biopsy of the left elbow was performed without complications. Intraoperatively, a 3 × 2-cm subcutaneous mass was found, consisting of adipose tissue surrounded by tophaceous material. Examination of the nodular tissue was negative for AFB, MTB, fungi, and neoplasm. Histopathologic examination revealed multiple nodular eosinophilic amorphous to feathery materials surrounded by histiocytes and some foreign body-like multinucleated giant cells, with negative periodic-acid Schiff (PAS), Gomori methenamine silver (GMS), and Ziehl–Neelsen stains suggestive of granulomatous inflammation consistent with gout tophi (Figs. 2, 3, and 4).
He was then treated for acute gouty arthritis with a tapering dose of prednisolone, colchicine, and allopurinol as a urate-lowering agent. His SUA level was only elevated when he was reviewed in the outpatient clinic 4 weeks later. In addition, his joint pain was well controlled with the current therapy.
Discussion
The formation of monosodium urate (MSU) crystals due to a persistent increase in SUA levels leading to tissue deposition is known to be the cause of gouty arthritis [4]. Although the typical presentation of gouty arthritis is joint inflammation and tophi formation, there is also evidence of subclinical involvement of the musculoskeletal system and other areas, including the external ears, nasal and thyroid cartilage, eyelids, articular cartilage, and tendons [5,6,7].
Subcutaneous nodular lesions presenting as periarticular masses have a wide spectrum of differential diagnoses, including pyogenic granulomas, atypical infections, gouty tophi, rheumatoid nodules, tumoral calcinosis, and paraneoplastic lesions [6]. A definitive diagnosis of gouty arthritis can be confirmed by the detection of MSU crystals in synovial fluid, which has a strong negative birefringence and is considered the gold standard [8]. However, subcutaneous nodules, which are difficult to distinguish from other possible diagnoses, require biopsy and histopathologic examination to confirm the diagnosis.
SUA levels are usually within the normal range during the acute phase of gout. The diagnosis of an acute gout attack should not be ruled out if the SUA values are normal. The low uric acid level correlates with increased inflammatory factors and urinary excretion of uric acid [9,10,11].
The initial concern with our patient was that his chief complaint was a nodule on his left posterior elbow that was inflamed and the absence of a typical history of gouty arthritis, elevated SUA level, and crystals in his synovial fluid. We also considered the possibility of a paraneoplastic lesion. The lack of response to intravenous antibiotics and previous steroid therapy in this patient prompted us to perform a comprehensive workup with tissue biopsy to confirm the diagnosis.
Granulomatous inflammation is characterized by the formation of distinct granulomas consisting of aggregates of epithelioid histiocytes with a peripheral cuff of lymphocytes and plasma cells. It is a chronic inflammatory response of the body to various causes such as infections, autoimmune diseases, toxins, allergies, and neoplasms [3]. Foreign body giant cell granulomas are histiocytic reactions to inert material without an adaptive immune response. A collection of histiocytes surrounds the foreign material, and as single histiocytes, they are unable to phagocytose the foreign material on their own. The foreign material can be visualized by light microscopy and often exhibits birefringence when polarized light is used [3].
When we received the first histopathologic finding of granulomatous inflammation from the nodular biopsy, we still suspected MTB infection. However, the negative MTB culture and Ziehl–Neelsen staining helped us to confirm the diagnosis of gout.
Conclusion
This case report illustrates an atypical form of gouty arthritis. Gouty arthritis must be clearly included in the differential diagnosis of a nodular skin lesion associated with joint inflammation. Gout remains one of the most common causes of oligoarthritis. Not all tophi have a hard nodule and a well-defined margin. Therefore, it is necessary to investigate extensively for gout in patients with oligoarthritis, including tissue biopsy, to establish the diagnosis.
Availability of data and materials
Not applicable.
Abbreviations
- AFB:
-
Acid-fast bacilli
- CPPD:
-
Calcium pyrophosphate deposition disease
- CRP:
-
C-reactive protein
- ESR:
-
Erythrocyte sedimentation rate
- GMS:
-
Gomori methenamine silver
- HIV:
-
Human immunodeficiency virus
- iPTH:
-
Intact parathyroid hormone
- MSU:
-
Monosodium urate
- MTPJ:
-
Metatarsophalangeal joint
- MTB:
-
Mycobacterium tuberculosis
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- PAS:
-
Periodic-acid Schiff
- PD:
-
Power doppler
- SUA:
-
Serum uric acid
References
Torraca PFS, Castro BC, Hans Filho G et al (2017) Cutaneous metastases in a patient with no previous diagnosis of cancer: diagnostic challenge. An Bras Dermatol. 92:47–9. https://doi.org/10.1590/abd1806-4841.20175870
Kostman JR, Rush P, Reginato AJ (1995) Granulomatous tophaceous gout mimicking tuberculous tenosynovitis: report of two cases. Clin Infect Dis 21:217–9. https://doi.org/10.1093/clinids/21.1.217
Shah KK, Pritt BS, Alexander MP (2017) Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis 7:1–12. https://doi.org/10.1016/j.jctube.2017.02.001
Ragab G, Elshahaly M, Bardin T (2017) Gout: an old disease in new perspective - a review. J Adv Res 8(5):495–511. https://doi.org/10.1016/j.jare.2017.04.008
Hussin P, Mawardi M, Nizlan NM (2014) The ‘Chalky Culprit’ of acute locked knee. G Chir 35:239–40
Abdel-Khalek A, Tariq A, White JA et al (2023) Atypical presentation of gout: a case report. Cureus 15:e36707. https://doi.org/10.7759/cureus.36707
Stark TW, Hirokawa RH (1982) Gout and its manifestations in the head and neck. Otolaryngol Clin North Am 15:659–64
Schlee S, Bollheimer LC, Bertsch T et al (2018) Crystal arthritides – gout and calcium pyrophosphate arthritis. Z Gerontol Geriat 51:579–84. https://doi.org/10.1007/s00391-017-1198-2
Schlesinger N, Norquist JM, Watson DJ (2009) Serum urate during acute gout. J Rheumatol 36:1287–9. https://doi.org/10.3899/jrheum.080938
Bădulescu M, Macovei L, Rezuş E (2014) Acute gout attack with normal serum uric acid levels. Rev Med Chir Soc Med Nat Iasi 118:942–5
Zhang J, Sun W, Gao F et al (2023) Changes of serum uric acid level during acute gout flare and related factors. Front Endocrinol 14:1077059. https://doi.org/10.3389/fendo.2023.1077059
Acknowledgements
We would like to thank the Director General of Health Malaysia for his permission to publish this article.
Funding
This study did not require any funding.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: 1. Study conception and idea: Abu Mansor Matardiah Nor Hashimah. 2. Writing (original draft): Farhana Omar, Abu Mansor Matardiah Nor Hashimah, Farhana Mohammad Mohaidin. 3. Writing (review and editing): Abu Mansor Matardiah Nor Hashimah, Ab Wahab Suhaila, Azman Ali Raymond. 4. Supervision: Azman Ali Raymond. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for this study was obtained from the Medical Research and Ethics Committee (MREC), Malaysia (NMRR-ID-23-02801-CAD).
Consent for publication
Written informed consent for the paper to be published (including images, case history, and data) was obtained from the patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Omar, F., Nor Hashimah, A.M.M., Suhaila, A.W. et al. Atypical gouty tophus masquerading as a foreign body granuloma. Egypt J Intern Med 36, 79 (2024). https://doi.org/10.1186/s43162-024-00347-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-024-00347-z